Vitamin A Rich Diet Diminishes Early Urothelial Carcinogenesis by Altering Retinoic Acid Signaling
Abstract
:1. Introduction
2. Results
2.1. Vitamin A Protects Against BBN-Induced Urothelial Atypia
2.2. Effects of Vitamin A-Rich Diet and BBN Treatment on Urothelial Cell Differentiation
2.3. A Vitamin A Rich Diet Decreases Apoptosis and Preserves Proliferation of Urothelial Cells During Early Bladder Carcinogenesis
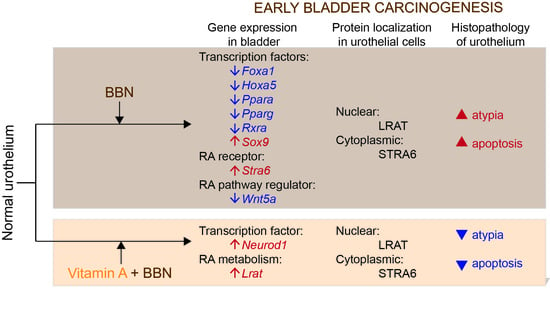
2.4. Retinoic Acid (RA) Signaling Is Altered during Early Bladder Carcinogenesis, and a Diet Rich in Vitamin A Reduces These Alterations
2.5. LRAT Localization in Urothelial Cells Changes during Early Carcinogenesis Induced by BBN, While STRA6 Localization Is Unaltered
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Animals
4.3. Serum Vitamin A Measurement
4.4. Characterization of Urothelial Lesions
4.5. Immunohistochemistry
4.6. Immunofluorescence
4.7. Scanning Electron Microscopy
4.8. PCR Array and qPCR Experiments
4.9. Statistical Analysis of Urothelial Changes and Bioinformatics
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2018, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegel, R.L.; Naishadham, D.; Jemal, A. Cancer statistics, 2013. CA A Cancer J. Clin. 2013, 63, 11–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, G.; Zhou, G.; Schapira, M.; Sun, T.T.; Kong, X.-P. Structural basis of urothelial permeability barrier function as revealed by Cryo-EM studies of the 16 nm uroplakin particle. J. Cell Sci. 2003, 116, 4087–4094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jost, S.P.; Gosling, J.A.; Dixon, J.S. The morphology of normal human bladder urothelium. J. Anat. 1989, 167, 103–115. [Google Scholar]
- Romih, R.; Veranic, P.; Jezernik, K. Appraisal of differentiation markers in urothelial cells. Appl. Immunohistochem. Mol. Morphol. 2002, 10, 339–343. [Google Scholar] [CrossRef]
- Veraniča, P.; Romiha, R.; Jezernika, K. What determines differentiation of urothelial umbrella cells? Eur. J. Cell Boil. 2004, 83, 27–34. [Google Scholar] [CrossRef]
- Kachar, B.; Liang, F.; Lins, U.; Ding, M.; Wu, X.-R.; Stoffler, D.; Aebi, U.; Sun, T.T. Three-dimensional analysis of the 16 nm urothelial plaque particle: Luminal surface exposure, preferential head-to-head interaction, and hinge formation 1 1Edited by W. Baumeisser. J. Mol. Boil. 1999, 285, 595–608. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.R.; Manabe, M.; Yu, J.; Sun, T.T. Large scale purification and immunolocalization of bovine uroplakins I, II, and III. Molecular markers of urothelial differentiation. J. Boil. Chem. 1990, 265, 19170–19179. [Google Scholar]
- Huang, H.-Y.; Shariat, S.F.; Sun, T.T.; Lepor, H.; Shapiro, E.; Hsieh, J.-T.; Ashfaq, R.; Lotan, Y.; Wu, X.-R. Persistent uroplakin expression in advanced urothelial carcinomas: Implications in urothelial tumor progression and clinical outcome. Hum. Pathol. 2007, 38, 1703–1713. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, K.; John, M.S.; de Oliveira, M.L.; Arnold, L.; Shirai, T.; Sun, T.T.; Cohen, S.M. Comparison of Uroplakin Expression During Urothelial Carcinogenesis Induced by N-Butyl-N-(4-Hydroxybutyl)Nitrosamine in Rats and Mice. Toxicol. Pathol. 1999, 27, 645–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zupančič, D.; Ovčak, Z.; Vidmar, G.; Romih, R. Altered expression of UPIa, UPIb, UPII, and UPIIIa during urothelial carcinogenesis induced by N-butyl-N-(4-hydroxybutyl)nitrosamine in rats. Virchows Arch. 2011, 458, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Zupančič, D.; Romih, R. Heterogeneity of uroplakin localization in human normal urothelium, papilloma and papillary carcinoma. Radiol. Oncol. 2013, 47, 338–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arai, M.; Kani, T.; Sugihara, S.; Matsumura, K.; Miyata, Y.; Shinohara, Y.; Ito, N. Scanning and transmission electron microscopy of changes in the urinary bladder in rats treated with N-butyl-N-(4-hydroxybutyl)nitrosoamine. Gan 1974, 65, 529–540. [Google Scholar] [PubMed]
- Zupančič, D.; Zakrajšek, M.; Zhou, G.; Romih, R. Expression and localization of four uroplakins in urothelial preneoplastic lesions. Histochem. Cell Boil. 2011, 136, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.B.; Arai, M.; Cohen, S.M.; Friedell, G.H. Early lesions in experimental bladder cancer: Scanning electron microscopy of cell surface markers. Cancer Res. 1976, 36, 2512–2517. [Google Scholar] [PubMed]
- Waliszewski, P.; Waliszewska, M.; Gordon, N.; Hurst, R.; Benbrook, D.M.; Dhar, A.; Hemstreet, G.P. Retinoid signaling in immortalized and carcinoma-derived human uroepithelial cells. Mol. Cell. Endocrinol. 1999, 148, 55–65. [Google Scholar] [CrossRef]
- Taniai, E.; Yafune, A.; Kimura, M.; Morita, R.; Nakane, F.; Suzuki, K.; Mitsumori, K.; Shibutani, M. Fluctuations in cell proliferation, apoptosis, and cell cycle regulation at the early stage of tumor promotion in rat two-stage carcinogenesis models. J. Toxicol. Sci. 2012, 37, 1113–1126. [Google Scholar] [CrossRef] [Green Version]
- Doldo, E.; Costanza, G.; Agostinelli, S.; Tarquini, C.; Ferlosio, A.; Arcuri, G.; Passeri, D.; Scioli, M.G.; Orlandi, A. Vitamin A, Cancer Treatment and Prevention: The New Role of Cellular Retinol Binding Proteins. BioMed Res. Int. 2015, 2015, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Mongan, N.P.; Gudas, L.J. Diverse actions of retinoid receptors in cancer prevention and treatment. Differentiation 2007, 75, 853–870. [Google Scholar] [CrossRef]
- Tang, J.-E.; Wang, R.-J.; Zhong, H.; Yu, B.; Chen, Y. Vitamin A and risk of bladder cancer: A meta-analysis of epidemiological studies. World J. Surg. Oncol. 2014, 12, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmoud, L.A.N.; Robinson, W.A. Vitamin A levels in human bladder cancer. Int. J. Cancer 1982, 30, 143–145. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.; von Lintig, J. STRA6: Role in cellular retinol uptake and efflux. HepatoBiliary Surg. Nutr. 2015, 4, 229–242. [Google Scholar]
- Kawaguchi, R.; Yu, J.; Honda, J.; Hu, J.; Whitelegge, J.; Ping, P.; Wiita, P.; Bok, D.; Sun, H. A Membrane Receptor for Retinol Binding Protein Mediates Cellular Uptake of Vitamin A. Science 2007, 315, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Das, B.C.; Thapa, P.; Karki, R.; Das, S.; Mahapatra, S.; Liu, T.-C.; Torregroza, I.; Wallace, D.P.; Kambhampati, S.; van Veldhuizen, P.; et al. Retinoic acid signaling pathways in development and diseases. Bioorg. Med. Chem. 2013, 22, 673–683. [Google Scholar] [CrossRef] [Green Version]
- Duester, G. Retinoic Acid Synthesis and Signaling during Early Organogenesis. Cell 2008, 134, 921–931. [Google Scholar] [CrossRef] [Green Version]
- Chopra, B.; Hinley, J.; Oleksiewicz, M.B.; Southgate, J. Trans-Species Comparison of PPAR and RXR Expression by Rat and Human Urothelial Tissues. Toxicol. Pathol. 2008, 36, 485–495. [Google Scholar] [CrossRef] [Green Version]
- Kawaguchi, R.; Zhong, M.; Kassai, M.; Ter-Stepanian, M.; Sun, H. Vitamin A Transport Mechanism of the Multitransmembrane Cell-Surface Receptor STRA6. Membranes 2015, 5, 425–453. [Google Scholar] [CrossRef] [Green Version]
- Chambon, P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996, 10, 940–954. [Google Scholar] [CrossRef]
- De Graff, D.J.; Cates, J.M.M.; Mauney, J.R.; Clark, P.E.; Matusik, R.J.; Adam, R.M. When urothelial differentiation pathways go wrong: Implications for bladder cancer development and progression. Urol. Oncol. Semin. Orig. Investig. 2011, 31, 802–811. [Google Scholar] [CrossRef] [Green Version]
- Blomhoff, R.; Blomhoff, H.K. Overview of retinoid metabolism and function. J. Neurobiol. 2006, 66, 606–630. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Knudsen, B.S.; Peehl, D.M.; Ruiz, A.; Bok, D.; Rando, R.R.; Rhim, J.S.; Nanus, D.M.; Gudas, L.J. Retinol metabolism and lecithin: Retinol acyltransferase levels are reduced in cultured human prostate cancer cells and tissue specimens. Cancer Res. 2002, 62, 1654–1661. [Google Scholar] [PubMed]
- Guo, X.; Ruiz, A.; Rando, R.R.; Bok, D.; Gudas, L.J. Esterification of all-trans-retinol in normal human epithelial cell strains and carcinoma lines from oral cavity, skin and breast: Reduced expression of lecithin: Retinol acyltransferase in carcinoma lines. Carcinognesis 2000, 21, 1925–1933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreola, F.; Giandomenico, V.; Spero, R.; de Luca, L.M. Expression of a Smaller Lecithin: Retinol Acyl Transferase Transcript and Reduced Retinol Esterification in MCF-7 Cells. Biochem. Biophys. Res. Commun. 2000, 279, 920–924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boorjian, S. Reduced Lecithin: Retinol Acyltransferase Expression Correlates with Increased Pathologic Tumor Stage in Bladder Cancer. Clin. Cancer Res. 2004, 10, 3429–3437. [Google Scholar] [CrossRef] [Green Version]
- Gandhi, D.; Molotkov, A.; Batourina, E.; Schneider, K.; Dan, H.; Reiley, M.; Laufer, E.; Metzger, D.; Liang, F.; Liao, Y.; et al. Retinoid signaling in progenitors controls specification and regeneration of the urothelium. Dev. Cell 2013, 26, 469–482. [Google Scholar] [CrossRef] [Green Version]
- Liang, F.-X.; Bosland, M.C.; Huang, H.; Romih, R.; Baptiste, S.; Deng, F.-M.; Wu, X.-R.; Shapiro, E.; Sun, T.T. Cellular basis of urothelial squamous metaplasia. J. Cell Boil. 2005, 171, 835–844. [Google Scholar] [CrossRef] [Green Version]
- Ariel, I.; Ayesh, S.; Gofrit, O.; Ayesh, B.; Abdul-Ghani, R.; Pizov, G.; Smith, Y.; Sidi, A.A.; Birman, T.; Schneider, T.; et al. Gene expression in the bladder carcinoma rat model. Mol. Carcinog. 2004, 41, 69–76. [Google Scholar] [CrossRef]
- Gofrit, O.N.; Birman, T.; Dinaburg, A.; Ayesh, S.; Ohana, P.; Hochberg, A. Chemically induced bladder cancer—A sonographic and morphologic description. Urology 2006, 68, 231–235. [Google Scholar] [CrossRef]
- Fantini, D.; Glaser, A.P.; Rimar, K.J.; Wang, Y.; Schipma, M.; Varghese, N.; Rademaker, A.; Behdad, A.; Yellapa, A.; Yu, Y.; et al. A Carcinogen-induced mouse model recapitulates the molecular alterations of human muscle invasive bladder cancer. Oncogene 2018, 37, 1911–1925. [Google Scholar] [CrossRef]
- Fantini, D.; Meeks, J.J. The BBN model: A mouse bladder cancer model featuring basalsubtype gene expression and MLL3/MLL4 genetic disruption. Oncoscience 2018, 5, 172–173. [Google Scholar] [CrossRef] [PubMed]
- Degoricija, M.; Korac-Prlic, J.; Vilovic, K.; Ivanisevic, T.; Haupt, B.; Palada, V.; Petkovic, M.; Karaman, I.; Terzić, J. The dynamics of the inflammatory response during BBN-induced bladder carcinogenesis in mice. J. Transl. Med. 2019, 17, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Felipe, F.; Mercader, J.; Ribot, J.; Palou, A.; Bonet, M.L. Effects of retinoic acid administration and dietary vitamin A supplementation on leptin expression in mice: lack of correlation with changes of adipose tissue mass and food intake. Biochim. Biophys. Acta Mol. Basis Dis. 2005, 1740, 258–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jost, S.P. Cell cycle of normal bladder urothelium in developing and adult mice. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 1989, 57, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Thompson, H.J.; Becci, P.J.; Grubbs, C.J.; Shealy, Y.F.; Stanek, E.J.; Brown, C.C.; Sporn, M.B.; Moon, R.C. Inhibition of urinary bladder cancer by N-(ethyl)-all-trans-retinamide and N-(2-hydroxyethyl)-all-trans-retinamide in rats and mice. Cancer Res. 1981, 41, 933–936. [Google Scholar]
- Ganguly, S.; Chandra, A.; Chattopadhyay, D.J.; Chatterjee, I.B. p-Benzoquinone initiates non-invasive urothelial cancer through aberrant tyrosine phosphorylation of EGFR, MAP kinase activation and cell cycle deregulation: Prevention by vitamin C. Toxicol. Rep. 2017, 4, 296–305. [Google Scholar] [CrossRef]
- Mauney, J.R.; Ramachandran, A.; Yu, R.N.; Daley, G.Q.; Adam, R.M.; Estrada, C.R. All-Trans Retinoic Acid Directs Urothelial Specification of Murine Embryonic Stem Cells via GATA4/6 Signaling Mechanisms. PLoS ONE 2010, 5, e11513. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, H.; Amponsa, V.O.; Warrick, J.I.; Zheng, Z.; Clark, P.E.; Raman, J.D.; Wu, X.-R.; Mendelsohn, C.; de Graff, D.J. On a FOX hunt: Functions of FOX transcriptional regulators in bladder cancer. Nat. Rev. Urol. 2016, 14, 98–106. [Google Scholar] [CrossRef]
- Eriksson, P.; Aine, M.; Veerla, S.; Liedberg, F.; Sjödahl, G.; Höglund, M. Molecular subtypes of urothelial carcinoma are defined by specific gene regulatory systems. BMC Med Genom. 2015, 8, 25. [Google Scholar] [CrossRef] [Green Version]
- Varley, C.L.; Bacon, E.J.; Holder, J.C.; Southgate, J. FOXA1 and IRF-1 intermediary transcriptional regulators of PPARγ-induced urothelial cytodifferentiation. Cell Death Differ. 2008, 16, 103–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malgor, R.; Crouser, S.; Greco, D.; Brockett, C.; Coschigano, K.; Nakazawa, M.; Jenkinson, S. Correlation of Wnt5a expression with histopathological grade/stage in urothelial carcinoma of the bladder. Diagn. Pathol. 2013, 8, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Zhao, J.; Yang, C.; Kuang, R.; Kazobinka, G.; Pang, Z.; Hou, T. Prognostic Implication of Urothelial Stem Cell Markers Differs According to Primary Tumour Location in Non-Muscle-Invasive Bladder Cancer. Cell. Physiol. Biochem. 2018, 48, 2364–2373. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.C.; Levi, L.; Noy, N. Holo-retinol-binding protein and its receptor STRA6 drive oncogenic transformation. Cancer Res. 2014, 74, 6341–6351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karunanithi, S.; Levi, L.; de Vecchio, J.; Karagkounis, G.; Reizes, O.; Lathia, J.D.; Kalady, M.F.; Noy, N. RBP4-STRA6 Pathway Drives Cancer Stem Cell Maintenance and Mediates High-Fat Diet-Induced Colon Carcinogenesis. Stem Cell Rep. 2017, 9, 438–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrera, S.; Cuadrado-Castano, S.; Samuel, J.; Jones, G.; Villar, E.; Lee, S.W.; Macip, S. Stra6, a retinoic acid-responsive gene, participates in p53-induced apoptosis after DNA damage. Cell Death Differ. 2013, 20, 910–919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, A.C. Retinoid Production and Catabolism: Role of Diet in Regulating Retinol Esterification and Retinoic Acid Oxidation. J. Nutr. 2003, 133, 291S–296S. [Google Scholar] [CrossRef] [Green Version]
- Simmons, D.P.; Peach, M.L.; Friedman, J.; Green, M.M.; Nicklaus, M.C.; de Luca, L.M. Evidence that sequence homologous region in LRAT-like proteins possesses anti-proliferative activity and DNA binding properties: Translational implications and mechanism of action. Carcinogenesis 2005, 27, 693–707. [Google Scholar] [CrossRef]
- Ruiz, A.; Kuehn, M.H.; Andorf, J.L.; Stone, E.; Hageman, G.S.; Bok, D. Genomic organization and mutation analysis of the gene encoding lecithin retinol acyltransferase in human retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 2001, 42, 31–37. [Google Scholar]
- Mehmood, R.; Yasuhara, N.; Oe, S.; Nagai, M.; Yoneda, Y. Synergistic nuclear import of NeuroD1 and its partner transcription factor, E47, via heterodimerization. Exp. Cell Res. 2009, 315, 1639–1652. [Google Scholar] [CrossRef]







| Differential Expression | Gene | Fold Change | p-Value | Gene | Fold Change | p-Value |
|---|---|---|---|---|---|---|
| BBN vs. NT. | VitA vs. NT | |||||
| Upregulated | Stra6 | 6.61 | 0.0092 | Cyp26b1 | 7.72 | 0.0176 |
| Adh1a3 | 3.41 | 0.0022 | Lrat | 5.42 | 0.0329 | |
| Cd38 | 3.02 | 0.0063 | ||||
| Sox9 | 2.53 | 0.0003 | BBN + VitA vs. BBN | |||
| Plat | 2.42 | 0.0009 | Neurod1 | 4.51 | 0.0177 | |
| Tubb3 | 2.26 | 0.0445 | Lrat | 10.64 | 0.0276 | |
| Chd7 | 2.06 | 0.0199 | ||||
| Bhlhe40 | 2.05 | 0.0216 | BBN + VitA vs. NT | |||
| Rdh10 | 2.01 | 0.0014 | Lrat | 11.31 | 0.0276 | |
| Downregulated | Hoxa5 | −2.08 | 0.0011 | Foxa1 | −5.96 | 0.0023 |
| Srebf1 | −2.23 | 0.0074 | Msx2 | −8.02 | 0.0489 | |
| Rxra | −2.43 | 0.0004 | ||||
| Bmp2 | −2.52 | 0.0298 | ||||
| Hnf1b | −2.93 | 0.0006 | ||||
| Ucp1 | −2.94 | 0.0148 | ||||
| Gli1 | −3.23 | 0.0002 | ||||
| Foxa1 | −3.39 | 0.0018 | ||||
| Rbp4 | −3.61 | 0.0058 | ||||
| Wnt5a | −4.22 | 0.0005 | ||||
| Pparg | −4.29 | 0.0003 | ||||
| Adh1 | −4.77 | 0.0007 | ||||
| Ppara | −5.41 | 0.0001 | ||||
| Shh | −10.14 | 0.0006 | ||||
| Hsd17b2 | −20.35 | 0.0001 | ||||
| Primary Antibody | Dilution | Cat. No. | Provider |
|---|---|---|---|
| polyclonal rabbit anti-cleaved caspase-3 | 1:200 | 9661 | Cell Signaling Technology® |
| polyclonal rabbit anti-uroplakins (UPs) 1 | 1:10,000 | – | Prof. Tung-Tien Sun |
| monoclonal mouse anti-KRT20 | 1:200 | M7019 | Dako |
| polyclonal rabbit anti-Ki-67 | 1:200 | ab15580 | Abcam |
| polyclonal rabbit anti-STRA6 | 1:500 | bs-12351R | Bioss Antibodies |
| polyclonal rabbit anti-LRAT | 1:50 | PA5-38556 | Thermo Fisher Scientific |
| Secondary Antibody | |||
| anti-rabbit HRP | 1:500 | P0448 | Dako |
| anti-rabbit Alexa Fluor 555 | 1:400 | A21428 | Thermo Fisher Scientific |
| anti-rabbit Alexa Fluor 488 | 1:400 | A11008 | Invitrogen |
| anti-mouse Alexa Fluor 488 | 1:400 | A11001 | Thermo Fisher Scientific |
| Gene | Gene ID | Primer Sequence |
|---|---|---|
| Upk1a | NM_026815 | F 5'TTTGGTGTAGGAGCTGCG3' |
| R 5'GTTATCAGGGATGGGTTGGAC3' | ||
| Upk1b | NM_178924 | F 5'GGGTGGAGAATAACGATGCTG3' |
| R 5'GGTCCAGAGATCAGTTCATAGC3' | ||
| Upk2 | NM_009476 | F 5'GCGCATATCAGGTGACAAAC3' |
| R 5'CCATTCCTAACCCAATAGACTCC3' | ||
| Upk3 | NM_023478 | F 5'GGAGTGGAGGCATGATTGTC3' |
| R 5'GGTGATCTGTGAGTCGTGTG3' | ||
| Upk3b | NM_175309 | F 5'TCCAACCCCATTTATCTCCAC3' |
| R 5'GACGATCATACAGCCGCTC3' |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zupančič, D.; Korać-Prlić, J.; Kreft, M.E.; Franković, L.; Vilović, K.; Jeruc, J.; Romih, R.; Terzić, J. Vitamin A Rich Diet Diminishes Early Urothelial Carcinogenesis by Altering Retinoic Acid Signaling. Cancers 2020, 12, 1712. https://doi.org/10.3390/cancers12071712
Zupančič D, Korać-Prlić J, Kreft ME, Franković L, Vilović K, Jeruc J, Romih R, Terzić J. Vitamin A Rich Diet Diminishes Early Urothelial Carcinogenesis by Altering Retinoic Acid Signaling. Cancers. 2020; 12(7):1712. https://doi.org/10.3390/cancers12071712
Chicago/Turabian StyleZupančič, Daša, Jelena Korać-Prlić, Mateja Erdani Kreft, Lucija Franković, Katarina Vilović, Jera Jeruc, Rok Romih, and Janoš Terzić. 2020. "Vitamin A Rich Diet Diminishes Early Urothelial Carcinogenesis by Altering Retinoic Acid Signaling" Cancers 12, no. 7: 1712. https://doi.org/10.3390/cancers12071712
APA StyleZupančič, D., Korać-Prlić, J., Kreft, M. E., Franković, L., Vilović, K., Jeruc, J., Romih, R., & Terzić, J. (2020). Vitamin A Rich Diet Diminishes Early Urothelial Carcinogenesis by Altering Retinoic Acid Signaling. Cancers, 12(7), 1712. https://doi.org/10.3390/cancers12071712