Histopathological Chromogranin A-Positivity Is Associated with Right-Sided Colorectal Cancers and Worse Prognosis
Abstract
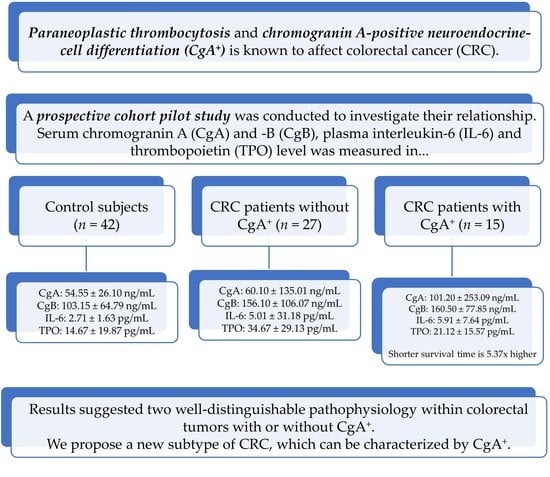
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. Preoperative Measurements
2.2. Measurement of Chromogranins, Interleukin-6 and Thrombopoietin
2.3. Analysis of Conventional and Personalized Indicator Thrombocytosis (PIT)-Based Thrombocytosis
2.4. Postoperative Measurements
2.5. Survival Analysis
3. Discussion
Limitations of the Study
4. Materials and Methods
4.1. Patients and Study Design
4.2. Clinical and Laboratory Data Measurements
4.3. Chromogranin A-Specific Histopathological Analysis
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A


References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Institute of Oncology. National Cancer Registry: Cancer Statistics Reports for Hungary. Available online: http://stat.nrr.hu/ (accessed on 10 June 2020).
- Winkler, H.; Fischer-Colbrie, R. The chromogranins A and B: The first 25 years and future perspectives. Neuroscience 1992, 49, 497–528. [Google Scholar] [CrossRef]
- Vinik, A.I.; Silva, M.P.; Woltering, E.A.; Go, V.L.; Warner, R.; Caplin, M. Biochemical testing for neuroendocrine tumors. Pancreas 2009, 38, 876–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.; Zheng-Pywell, R.; Chen, H.A.; Bibb, J.A.; Chen, H.; Rose, J.B. Management of gastrointestinal neuroendocrine tumors. Clin. Med. Insights Endocrinol. Diabetes 2019, 12, 1179551419884058. [Google Scholar] [CrossRef]
- Yang, S.; Chung, H.C. Novel biomarker candidates for gastric cancer. Oncol. Rep. 2008, 19, 675–680. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Shen, B.; Sun, X.F. Chromogranin-A expression as a novel biomarker for early diagnosis of colon cancer patients. Int. J. Mol. Sci. 2019, 20, 2919. [Google Scholar] [CrossRef] [Green Version]
- Glinicki, P.; Jeske, W. Chromogranin A (CgA)—The influence of various factors in vivo and in vitro, and existing disorders on it’s concentration in blood. Endokrynol. Pol. 2010, 61, 384–387. [Google Scholar]
- Miki, M.; Ito, T.; Hijioka, M.; Lee, L.; Yasunaga, K.; Ueda, K.; Fujiyama, T.; Tachibana, Y.; Kawabe, K.; Jensen, R.T.; et al. Utility of chromogranin B compared with chromogranin A as a biomarker in Japanese patients with pancreatic neuroendocrine tumors. Jpn. J. Clin. Oncol. 2017, 47, 520–528. [Google Scholar] [CrossRef] [Green Version]
- Stridsberg, M.; Eriksson, B.; Fellstrom, B.; Kristiansson, G.; Tiensuu Janson, E. Measurements of chromogranin B can serve as a complement to chromogranin A. Regul. Pept. 2007, 139, 80–83. [Google Scholar] [CrossRef]
- Baranyai, Z.; Josa, V.; Toth, A.; Szilasi, Z.; Tihanyi, B.; Zarand, A.; Harsanyi, L.; Szallasi, Z. Paraneoplastic thrombocytosis in gastrointestinal cancer. Platelets 2016, 27, 269–275. [Google Scholar] [CrossRef] [Green Version]
- Gulubova, M.; Vlaykova, T. Chromogranin A-, serotonin-, synaptophysin- and vascular endothelial growth factor-positive endocrine cells and the prognosis of colorectal cancer: An immunohistochemical and ultrastructural study. J. Gastroenterol. Hepatol. 2008, 23, 1574–1585. [Google Scholar] [CrossRef]
- Cho, Y.B.; Yang, S.S.; Lee, W.Y.; Song, S.Y.; Kim, S.H.; Shin, H.J.; Yun, S.H.; Chun, H.K. The clinical significance of neuroendocrine differentiation in T3-T4 node-negative colorectal cancer. Int. J. Surg. Pathol. 2010, 18, 201–206. [Google Scholar] [CrossRef]
- Liu, Y.; He, J.; Xu, J.; Li, J.; Jiao, Y.; Bei, D.; Hu, Y.; Chen, H.; Xiao, Q.; Ding, K. Neuroendocrine differentiation is predictive of poor survival in patients with stage II colorectal cancer. Oncol. Lett. 2017, 13, 2230–2236. [Google Scholar] [CrossRef] [Green Version]
- Ogimi, T.; Sadahiro, S.; Kamei, Y.; Chan, L.F.; Miyakita, H.; Saito, G.; Okada, K.; Suzuki, T.; Kajiwara, H. Distribution of neuroendocrine marker-positive cells in colorectal cancer tissue and normal mucosal tissue: Consideration of histogenesis of neuroendocrine cancer. Oncology 2019, 97, 294–300. [Google Scholar] [CrossRef]
- Jessup, J.; Goldberg, R.; Asare, E.; Benson, A.; Brierley, J.; Chang, G.; Chen, V.; Compton, C.; De Nardi, P.; Goodman, K.; et al. Colon and Rectum. In AJCC Cancer Staging Manual, 8th ed.; Amin, M., Edge, S., Greene, F., Byrd, D., Brookland, R., Washington, M., Gershenwald, J., Compton, C., Hess, K., Sullivan, D., et al., Eds.; Springer International Publishing: Chicago, IL, USA, 2018; pp. 251–274. [Google Scholar]
- Herold, Z.; Herold, M.; Lohinszky, J.; Dank, M.; Somogyi, A. Personalized indicator thrombocytosis shows connection to staging and indicates shorter survival in colorectal cancer patients with or without type 2 diabetes. Cancers (Basel) 2020, 12, 556. [Google Scholar] [CrossRef]
- De Bruine, A.P.; Wiggers, T.; Beek, C.; Volovics, A.; von Meyenfeldt, M.; Arends, J.W.; Bosman, F.T. Endocrine cells in colorectal adenocarcinomas: Incidence, hormone profile and prognostic relevance. Int. J. Cancer 1993, 54, 765–771. [Google Scholar] [CrossRef]
- Kleist, B.; Poetsch, M. Neuroendocrine differentiation: The mysterious fellow of colorectal cancer. World J. Gastroenterol. 2015, 21, 11740–11747. [Google Scholar] [CrossRef]
- Josa, V.; Krzystanek, M.; Eklund, A.C.; Salamon, F.; Zarand, A.; Szallasi, Z.; Baranyai, Z. Relationship of postoperative thrombocytosis and survival of patients with colorectal cancer. Int. J. Surg. 2015, 18, 1–6. [Google Scholar] [CrossRef]
- Stone, R.L.; Nick, A.M.; McNeish, I.A.; Balkwill, F.; Han, H.D.; Bottsford-Miller, J.; Rupairmoole, R.; Armaiz-Pena, G.N.; Pecot, C.V.; Coward, J.; et al. Paraneoplastic thrombocytosis in ovarian cancer. N. Engl. J. Med. 2012, 366, 610–618. [Google Scholar] [CrossRef] [Green Version]
- Young, S.; Golzarian, J. Primary tumor location in colorectal cancer: Comparison of right- and left-sided colorectal cancer characteristics for the interventional radiologist. Cardiovasc. Intervent. Radiol. 2018, 41, 1819–1825. [Google Scholar] [CrossRef]
- Kanellos, D.; Kitsios, G.; Kanellos, I.; Demetriades, H.; Pramateftakis, M.G.; Angelopoulos, S.; Betsis, D. Anaemia as a symptom of right colon cancer. Tech. Coloproctol. 2004, 8, S62–S64. [Google Scholar] [CrossRef]
- Baran, B.; Mert Ozupek, N.; Yerli Tetik, N.; Acar, E.; Bekcioglu, O.; Baskin, Y. Difference between left-sided and right-sided colorectal cancer: A focused review of literature. Gastroenterol. Res. 2018, 11, 264–273. [Google Scholar] [CrossRef] [Green Version]
- Lee, G.H.; Malietzis, G.; Askari, A.; Bernardo, D.; Al-Hassi, H.O.; Clark, S.K. Is right-sided colon cancer different to left-sided colorectal cancer?—A systematic review. Eur. J. Surg. Oncol. 2015, 41, 300–308. [Google Scholar] [CrossRef]
- Petrelli, F.; Tomasello, G.; Borgonovo, K.; Ghidini, M.; Turati, L.; Dallera, P.; Passalacqua, R.; Sgroi, G.; Barni, S. Prognostic survival associated with left-sided vs. right-sided colon cancer: A systematic review and meta-analysis. JAMA Oncol. 2017, 3, 211–219. [Google Scholar] [CrossRef]
- Xu, G.H.; Zou, H.W.; Grossman, A.B. Difference in survival between right- versus left-sided colorectal neuroendocrine neoplasms. J. Zhejiang Univ. Sci. B 2019, 20, 933–939. [Google Scholar] [CrossRef]
- Baranyai, Z.; Krzystanek, M.; Josa, V.; Dede, K.; Agoston, E.; Szasz, A.M.; Sinko, D.; Szarvas, V.; Salamon, F.; Eklund, A.C.; et al. The comparison of thrombocytosis and platelet-lymphocyte ratio as potential prognostic markers in colorectal cancer. Thromb. Haemost. 2014, 111, 483–490. [Google Scholar] [CrossRef]
- Bishop, A.E.; Sekiya, K.; Salahuddin, M.J.; Carlei, F.; Rindi, G.; Fahey, M.; Steel, J.H.; Hedges, M.; Domoto, T.; Fischer-Colbrie, R.; et al. The distribution of GAWK-like immunoreactivity in neuroendocrine cells of the human gut, pancreas, adrenal and pituitary glands and its co-localisation with chromogranin B. Histochemistry 1989, 90, 475–483. [Google Scholar] [CrossRef]
- Facer, P.; Bishop, A.E.; Lloyd, R.V.; Wilson, B.S.; Hennessy, R.J.; Polak, J.M. Chromogranin: A newly recognized marker for endocrine cells of the human gastrointestinal tract. Gastroenterology 1985, 89, 1366–1373. [Google Scholar] [CrossRef]
- Reinecke, M.; Hoog, A.; Ostenson, C.G.; Efendic, S.; Grimelius, L.; Falkmer, S. Phylogenetic aspects of pancreastatin- and chromogranin-like immunoreactive cells in the gastro-entero-pancreatic neuroendocrine system of vertebrates. Gen. Comp. Endocrinol. 1991, 83, 167–182. [Google Scholar] [CrossRef]
- Curnis, F.; Dallatomasina, A.; Bianco, M.; Gasparri, A.; Sacchi, A.; Colombo, B.; Fiocchi, M.; Perani, L.; Venturini, M.; Tacchetti, C.; et al. Regulation of tumor growth by circulating full-length chromogranin A. Oncotarget 2016, 7, 72716–72732. [Google Scholar] [CrossRef] [Green Version]
- Ding, X.; Zhang, W.; Li, S.; Yang, H. The role of cholesterol metabolism in cancer. Am. J. Cancer Res. 2019, 9, 219–227. [Google Scholar]
- Wang, C.; Li, P.; Xuan, J.; Zhu, C.; Liu, J.; Shan, L.; Du, Q.; Ren, Y.; Ye, J. Cholesterol enhances colorectal cancer progression via ROS elevation and MAPK signaling pathway activation. Cell. Physiol. Biochem. 2017, 42, 729–742. [Google Scholar] [CrossRef]
- Zhang, D.; Lavaux, T.; Sapin, R.; Lavigne, T.; Castelain, V.; Aunis, D.; Metz-Boutigue, M.H.; Schneider, F. Serum concentration of chromogranin A at admission: An early biomarker of severity in critically ill patients. Ann. Med. 2009, 41, 38–44. [Google Scholar] [CrossRef]
- Corti, A.; Marcucci, F.; Bachetti, T. Circulating chromogranin A and its fragments as diagnostic and prognostic disease markers. Pflug. Arch. 2018, 470, 199–210. [Google Scholar] [CrossRef]
- Rosjo, H.; Husberg, C.; Dahl, M.B.; Stridsberg, M.; Sjaastad, I.; Finsen, A.V.; Carlson, C.R.; Oie, E.; Omland, T.; Christensen, G. Chromogranin B in heart failure: A putative cardiac biomarker expressed in the failing myocardium. Circ. Heart Fail 2010, 3, 503–511. [Google Scholar] [CrossRef] [Green Version]
- Wiedenmann, B.; Waldherr, R.; Buhr, H.; Hille, A.; Rosa, P.; Huttner, W.B. Identification of gastroenteropancreatic neuroendocrine cells in normal and neoplastic human tissue with antibodies against synaptophysin, chromogranin A, secretogranin I (chromogranin B), and secretogranin II. Gastroenterology 1988, 95, 1364–1374. [Google Scholar] [CrossRef]
- Stridsberg, M.; Eriksson, B.; Oberg, K.; Janson, E.T. A comparison between three commercial kits for chromogranin a measurements. J. Endocrinol. 2003, 177, 337–341. [Google Scholar] [CrossRef] [Green Version]
- Schwandt, A.; Denkinger, M.; Fasching, P.; Pfeifer, M.; Wagner, C.; Weiland, J.; Zeyfang, A.; Holl, R.W. Comparison of MDRD, CKD-EPI, and Cockcroft-Gault equation in relation to measured glomerular filtration rate among a large cohort with diabetes. J. Diabetes Complicat. 2017, 31, 1376–1383. [Google Scholar] [CrossRef]
- Helle, K.B.; Metz-Boutigue, M.H.; Cerra, M.C.; Angelone, T. Chromogranins: From discovery to current times. Pflug. Arch. 2018, 470, 143–154. [Google Scholar] [CrossRef]
- Shen, H.; Yang, J.; Huang, Q.; Jiang, M.J.; Tan, Y.N.; Fu, J.F.; Zhu, L.Z.; Fang, X.F.; Yuan, Y. Different treatment strategies and molecular features between right-sided and left-sided colon cancers. World J. Gastroenterol. 2015, 21, 6470–6478. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 22 June 2020).



| Parameter | CgA− Group (n = 27) | CgA+ Group (n = 15) | Uncorrected p-Value 1 | FDR-Corrected p-Value |
|---|---|---|---|---|
| Age (years) | 67.60 ± 7.90 | 69.00 ± 10.74 | 0.9113 | |
| Sex (male: female) | 17: 10 | 9: 6 | 1.0000 | |
| Staging (AJCC [16]) | ||||
| Stage I | 7 | 0 | ||
| Stage II | 9 | 6 | 0.0838 | 0.3831 |
| Stage III | 3 | 2 | ||
| Stage IV | 8 | 7 | ||
| Side of CRC | ||||
| Left-sided | 22 | 7 | 0.0352 | 0.2462 |
| Right-sided | 5 | 8 | ||
| Chemotherapy | ||||
| Adjuvant | 6 | 3 | ||
| First-line | 4 | 3 | 1.0000 | |
| Second-line | 2 | 2 | ||
| Third-line | 1 | 0 | ||
| Usage of biological therapy | 5 | 2 | 1.0000 | |
| Comorbidities | ||||
| Diabetes | 5 | 4 | 1.0000 | |
| Hypertension | 20 | 10 | 1.0000 | |
| Major cardiovascular event prior CRC | 4 | 6 | 0.3831 | |
| Thyroid diseases | 2 | 1 | 1.0000 | |
| Drug treatment | ||||
| Aggregation inhibition | 2 | 7 | 0.0055 | 0.0765 |
| Statin | 5 | 6 | 0.3831 | |
| Agents acting on the renin-angiotensin system | 18 | 8 | 0.8947 | |
| Antacid therapy | 7 | 6 | 0.8947 |
| Parameter | CgA− Group (n = 27) | CgA+ Group (n = 15) | Uncorrected p-Value 1 | FDR-Corrected p-Value |
|---|---|---|---|---|
| Waist circumference (cm) | 103.00 ± 11.11 | 94.50 ± 9.97 | 0.0222 | |
| Hip circumference (cm) | 108.00 ± 7.88 | 101.50 ± 5.81 | 0.0099 | |
| Body mass index (kg/m2) | 28.70 ± 3.98 | 27.00 ± 3.23 | 0.0465 | |
| White blood cell count (109/L) | 8.37 ± 7.02 | 8.26 ± 1.89 | 0.9113 | |
| Neutrophil count (109/L) | 5.46 ± 5.14 | 6.14 ± 1.72 | 0.3613 | |
| Eosinophil count (109/L) | 0.14 ± 1.68 | 0.14 ± 0.18 | 0.9903 | |
| Basophil count (109/L) | 0.05 ± 0.05 | 0.06 ± 0.03 | 0.9113 | |
| Monocyte count (109/L) | 0.54 ± 0.40 | 0.51 ± 0.20 | 0.9442 | |
| Lymphocyte count (109/L) | 1.67 ± 0.56 | 1.48 ± 0.43 | 0.0426 | |
| Red blood cell count (1012/L) | 4.75 ± 0.41 | 4.52 ± 0.49 | 0.3118 | |
| Hemoglobin (g/L) | 137.00 ± 20.51 | 118.00 ± 17.58 | 0.0099 | |
| Hematocrit (L/L) | 0.41 ± 0.05 | 0.37 ± 0.04 | 0.0119 | |
| Mean corpuscular volume (fL) | 85.50 ± 6.16 | 82.70 ± 8.96 | 0.2660 | |
| Mean corpuscular hemoglobin (pg) | 28.10 ± 2.76 | 26.10 ± 4.09 | 0.0582 | 0.1212 |
| Mean corpuscular hemoglobin concentration (g/L) | 330.00 ± 16.04 | 316.00 ± 17.09 | 0.0426 | |
| Red blood cell distribution width (%) | 13.20 ± 2.94 | 14.40 ± 5.20 | 0.0522 | 0.1186 |
| Platelet count (109/L) | 292.00 ± 98.94 | 286.00 ± 128.38 | 0.3804 | |
| Lactate dehydrogenase (U/L) | 202.00 ± 120.25 | 204.00 ± 669.73 | 0.9113 | |
| Estimated glomerular filtration rate | 90.60 ± 14.27 | 92.70 ± 25.19 | 0.9113 | |
| High-density cholesterol (mmol/L) | 1.29 ± 0.36 | 1.36 ± 2.94 | 0.9113 | |
| Low-density cholesterol (mmol/L) | 3.79 ± 0.93 | 2.88 ± 0.79 | 0.0119 | |
| High sensitivity C reactive protein (mg/L) | 6.40 ± 50.46 | 7.10 ± 47.79 | 0.9113 | |
| Serum total protein (g/L) | 74.00 ± 4.79 | 67.90 ± 4.68 | 0.0256 | |
| Serum albumin (g/L) | 42.70 ± 5.02 | 38.10 ± 4.64 | 0.0222 | |
| Carcinoembryonic antigen (ng/mL) | 3.70 ± 257.75 | 6.20 ± 958.29 | 0.7094 | |
| Carbohydrate antigen 19-9 (U/mL) | 7.04 ± 1684 | 10.98 ± 12591 | 0.9055 |
| Parameter | CgA− Group (n = 21) | CgA+ Group (n = 10) | Uncorrected p-Value 1 | FDR-Corrected p-Value |
|---|---|---|---|---|
| Waist circumference (cm) | 103.00 ± 9.19 | 93.00 ± 4.55 | 0.0010 | |
| Hip circumference (cm) | 111.00 ± 8.25 | 103.50 ± 4.81 | 0.0082 | |
| Body mass index (kg/m2) | 28.40 ± 4.15 | 25.50 ± 2.13 | 0.0046 | |
| White blood cell count (109/L) | 7.00 ± 1.80 | 7.37 ± 1.78 | 0.8506 | |
| Neutrophil count (109/L) | 4.32 ± 1.47 | 5.95 ± 1.74 | 0.8506 | |
| Eosinophil count (109/L) | 0.17 ± 0.11 | 0.12 ± 0.16 | 1.0000 | |
| Basophil count (109/L) | 0.05 ± 0.04 | 0.05 ± 0.03 | 0.8516 | |
| Monocyte count (109/L) | 0.45 ± 0.18 | 0.48 ± 0.11 | 0.8506 | |
| Lymphocyte count (109/L) | 1.61 ± 0.62 | 1.61 ± 0.48 | 0.8506 | |
| Red blood cell count (1012/L) | 4.64 ± 0.50 | 4.75 ± 0.63 | 0.8506 | |
| Hemoglobin (g/L) | 136.00 ± 18.52 | 126.50 ± 15.23 | 0.0346 | 0.1125 |
| Hematocrit (L/L) | 0.41 ± 0.05 | 0.40 ± 0.05 | 0.8506 | |
| Mean corpuscular volume (fL) | 87.00 ± 5.78 | 83.00 ± 7.18 | 0.0151 | 0.0561 |
| Mean corpuscular hemoglobin (pg) | 29.80 ± 2.70 | 26.55 ± 2.95 | 0.0046 | |
| Mean corpuscular hemoglobin concentration (g/L) | 330.00 ± 13.39 | 316.50 ± 10.78 | 0.0041 | |
| Red blood cell distribution width (%) | 13.60 ± 2.41 | 16.50 ± 3.40 | 0.0041 | |
| Platelet count (109/L) | 290.00 ± 83.74 | 287.50 ± 128.95 | 1.0000 | |
| Lactate dehydrogenase (U/L) | 182.00 ±57.81 | 195.00 ± 1063.21 | 0.7870 | |
| 84.80 ± 13.42 | 89.85 ± 15.37 | 0.6855 | ||
| High density cholesterol (mmol/L) | 1.43 ± 0.35 | 1.40 ± 0.30 | 0.8891 | |
| Low density cholesterol (mmol/L) | 3.42 ± 0.82 | 3.49 ± 1.12 | 0.8506 | |
| High sensitivity C reactive protein (mg/L) | 3.90 ± 10.90 | 2.15 ± 61.25 | 0.8506 | |
| Serum total protein (g/L) | 73.40 ± 4.27 | 75.70 ± 5.18 | 0.8506 | |
| Serum albumin (g/L) | 42.75 ± 4.48 | 41.10 ± 5.35 | 0.8506 |
| Group | Measurement | Interleukin-6 (pg/mL) | Thrombopoietin (pg/mL) |
|---|---|---|---|
| CgA− group (n = 21) | Preoperative | 4.83 ± 17.29 | 36.62 ± 28.63 |
| Postoperative | 3.70 ± 3.35 | 30.10 ± 24.60 | |
| CgA+ group (n = 10) | Preoperative | 5.01 ± 9.06 | 21.02 ± 12.51 |
| Postoperative | 4.59 ± 15.13 | 28.15 ± 16.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herold, Z.; Dank, M.; Herold, M.; Nagy, P.; Rosta, K.; Somogyi, A. Histopathological Chromogranin A-Positivity Is Associated with Right-Sided Colorectal Cancers and Worse Prognosis. Cancers 2021, 13, 67. https://doi.org/10.3390/cancers13010067
Herold Z, Dank M, Herold M, Nagy P, Rosta K, Somogyi A. Histopathological Chromogranin A-Positivity Is Associated with Right-Sided Colorectal Cancers and Worse Prognosis. Cancers. 2021; 13(1):67. https://doi.org/10.3390/cancers13010067
Chicago/Turabian StyleHerold, Zoltan, Magdolna Dank, Magdolna Herold, Peter Nagy, Klara Rosta, and Aniko Somogyi. 2021. "Histopathological Chromogranin A-Positivity Is Associated with Right-Sided Colorectal Cancers and Worse Prognosis" Cancers 13, no. 1: 67. https://doi.org/10.3390/cancers13010067
APA StyleHerold, Z., Dank, M., Herold, M., Nagy, P., Rosta, K., & Somogyi, A. (2021). Histopathological Chromogranin A-Positivity Is Associated with Right-Sided Colorectal Cancers and Worse Prognosis. Cancers, 13(1), 67. https://doi.org/10.3390/cancers13010067