Twelve-Year Single Center Experience Shows Safe Implementation of Developed Peritoneal Surface Malignancy Treatment Protocols for Gastrointestinal and Gynecological Primary Tumors
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection Criteria
2.2. Indications
- Deemed cytoreducable on laparoscopy;
- Response to systemic chemotherapy;
- PCI cut-offs (gastric: 6–10; colorectal/appendix <16; Mesothelioma/PMP/Ovarian: no cut-off).
2.3. Cytoreductive Surgery
2.4. HIPEC
2.5. Statistics
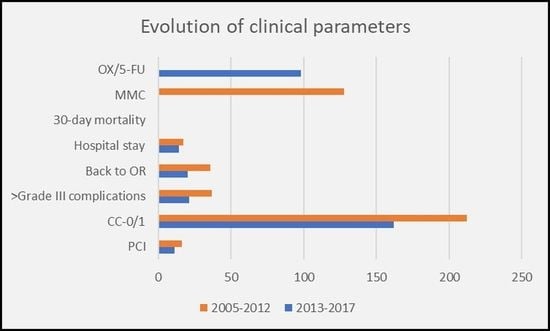
3. Results
3.1. Morbidity and Mortality
3.2. Survival Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Quénet, F.; Elias, D.; Roca, L.; Goéré, D.; Ghouti, L.; Pocard, M.; Facy, O.; Arvieux, C.; Lorimier, G.; Pezet, D.; et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 256–266. [Google Scholar] [CrossRef]
- Goere, D.; Glehen, O.; Quenet, F.; Guilloit, J.M.; Bereder, J.M.; Lorimier, G. BIG-RENAPE group: Second-look surgery plus hyper-thermic intraperitoneal chemotherapy versus surveillance in patients at high risk of developing colorectal peritoneal metas-tases (PROPHYLOCHIP-PRODIGE 15): A randomised, phase 3 study. Lancet Oncol. 2020, 21, 1147–1154. [Google Scholar] [CrossRef]
- Klaver, C.E.L.; Wisselink, D.D.; Punt, C.J.A.; Snaebjornsson, P.; Crezee, J.; Aalbers, A.G.J. COLOPEC collaborators group: Adjuvant hyperthermic intraperitoneal chemotherapy in patients with locally advanced colon cancer (COLOPEC): A multicentre, open-label, randomised trial. Lancet Gastroenterol. Hepatol. 2019, 4, 761–770. [Google Scholar] [CrossRef]
- Ceelen, W. HIPEC with oxaliplatin for colorectal peritoneal metastasis: The end of the road? Eur. J. Surg. Oncol. (EJSO) 2019, 45, 400–402. [Google Scholar] [CrossRef] [PubMed]
- Tanis, P.J.; Tuynman, J.B.; De Hingh, I.H.J.T. Results from the PROPHYLOCHIP-PRODIGE 15 trial. Lancet Oncol. 2020, 21, e496. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Q.; Wei, M.; Deng, X.; Gu, C.; Wang, Z. Oxaliplatin versus mitomycin C in HIPEC for peritoneal metastasis from colorectal cancer: A systematic review and meta-analysis of comparative studies. Int. J. Color. Dis. 2020, 35, 1831–1839. [Google Scholar] [CrossRef]
- Horvath, P.; Beckert, S.; Königsrainer, A.; Nadalin, S.; Königsrainer, I. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy combined with liver resection for concurrent peritoneal and hepatic metastases of gastrointestinal and gyne-cological primary tumors. J. Visc. Surg. 2019, 156, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Goéré, D.; Souadka, A.; Faron, M.; Cloutier, A.S.; Viana, B.; Honoré, C.; Dumont, F.; Elias, D. Extent of Colorectal Peritoneal Carcinomatosis: Attempt to Define a Threshold Above Which HIPEC Does Not Offer Survival Benefit: A Comparative Study. Ann. Surg. Oncol. 2015, 22, 2958–2964. [Google Scholar] [CrossRef]
- Glehen, O.; Gilly, F.N.; Arvieux, C.; Cotte, E.; Boutitie, F.; Mansvelt, B.; Bereder, J.M.; Lorimier, G.; Quenet, F.; Elias, D. Peritoneal carci-nomatosis from gastric cancer: A multi-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann. Surg. Oncol. 2010, 17, 2370–2377. [Google Scholar] [CrossRef] [Green Version]
- Zanon, C.; Clara, R.; Chiappino, I.; Bortolini, M.; Cornaglia, S.; Simone, P.; Bruno, F.; De Riu, L.; Airoldi, M.; Pedani, F. Cytoreductive Surgery and Intraperitoneal Chemohyperthermia for Recurrent Peritoneal Carcinomatosis from Ovarian Cancer. World J. Surg. 2004, 28, 1040–1045. [Google Scholar] [CrossRef]
- Di Giorgio, A.; Naticchioni, E.; Biacchi, D.; Sibio, S.; Accarpio, F.; Rocco, M.; Tarquini, S.; Di Seri, M.; Ciardi, A.; Montruccoli, D.; et al. Cytoreductive surgery (peritonectomy procedures) combined with hyperthermic intraperitoneal chemotherapy (HIPEC) in the treatment of diffuse peritoneal carcinomatosis from ovarian cancer. Cancer 2008, 113, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Cotte, E.; Glehen, O.; Mohamed, F.; Lamy, F.; Falandry, C.; Golfier, F.; Gilly, F.N. Cytoreductive surgery and intraperitoneal chemo-hyperthermia for chemo-resistant and recurrent advanced epithelial ovarian cancer: Prospective study of 81 patients. World J. Surg. 2007, 31, 1813–1820. [Google Scholar] [CrossRef]
- Van Oudheusden, T.R.; Braam, H.J.; Nienhuijs, S.W.; Wiezer, M.J.; van Ramshorst, B.; Luyer, P.; de Hingh, I.H. Poor outcome after cy-toreductive surgery and HIPEC for colorectal peritoneal carcinomatosis with signet ring cell histology. J. Surg. Oncol. 2015, 111, 237–242. [Google Scholar] [CrossRef]
- Solomon, D.; DeNicola, N.; Ba, D.F.; Liu, P.H.; Pa, S.A.; Golas, B.J.; Sarpel, U.; Labow, D.M.; Magge, D.R. Signet ring cell features with peritoneal carcinomatosis in patients undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy are associated with poor overall survival. J. Surg. Oncol. 2019, 119, 758–765. [Google Scholar] [CrossRef]
- Überrück, L.; Nadiradze, G.; Yurttas, C.; Königsrainer, A.; Königsrainer, I.; Horvath, P. In-Hospital Mortality and Complication Rates According to Health Insurance Data in Patients Undergoing Hyperthermic Intraperitoneal Chemotherapy for Peritoneal Surface Malignancies in Germany. Ann. Surg. Oncol. 2020, 1–8. [Google Scholar] [CrossRef]
- Elias, D.; Gilly, F.; Boutitie, F.; Quenet, F.; Bereder, J.M.; Mansvelt, B.; Lorimier, G.; Dubè, P.; Glehen, O. Peritoneal colorectal carcino-matosis treated with surgery and perioperative intraperitoneal chemotherapy: Retrospective analysis of 523 patients from a multicentric French study. J. Clin. Oncol. 2010, 28, 63–68. [Google Scholar] [CrossRef] [Green Version]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, P.; Sugarbaker, P.H. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomato-sis. Cancer Treat. Res. 1996, 82, 359–374. [Google Scholar]
- Spratt, J.S.; Adcock, R.A.; Muskovin, M.; Sherrill, W.; McKeown, J. Clinical delivery system for intraperitoneal hyperthermic chem-otherapy. Cancer Res. 1980, 40, 256–260. [Google Scholar]
- Kusamura, S.; Baratti, D.; Deraco, M. Multidimensional analysis of the learning curve for cytoreductive surgery and hyperther-mic intraperitoneal chemotherapy in peritoneal surface malignancies. Ann. Surg. 2012, 255, 348–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Alzahrani, N.A.; Liauw, W.; Morris, D.L. Learning curve for cytoreductive surgery and perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis. ANZ J. Surg. 2017, 87, 49–54. [Google Scholar] [CrossRef]
- Hentzen, J.E.K.R.; Constansia, R.D.N.; Been, L.B.; Hoogwater, F.J.H.; Van Ginkel, R.J.; Van Dam, G.M.; Hemmer, P.H.J.; Kruijff, S. Diagnostic Laparoscopy as a Selection Tool for Patients with Colorectal Peritoneal Metastases to Prevent a Non-therapeutic Laparotomy During Cytoreductive Surgery. Ann. Surg. Oncol. 2019, 27, 1084–1093. [Google Scholar] [CrossRef] [Green Version]
- Iversen, L.H.; Rasmussen, P.C.; Laurberg, S. Value of laparoscopy before cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis. BJS 2012, 100, 285–292. [Google Scholar] [CrossRef]
- Glehen, O.; Gilly, F.N.; Boutitie, F.; Bereder, J.M.; Quenet, F.; Sideris, L.; Mansvelt, B.; Lorimier, G.; Msika, S.; Elias, D. Toward curative treatment of peritoneal carcinomatosis from nonovarian origin by cytoreductive surgery combined with perioperative intra-peritoneal chemotherapy: A multi-institutional study of 1290 patients. Cancer 2010, 116, 5608–5618. [Google Scholar] [CrossRef] [PubMed]
- Baratti, D.; Kusamura, S.; Mingrone, E.; Balestra, M.R.; Laterza, B.; Deraco, M. Identification of a Subgroup of Patients at Highest Risk for Complications after Surgical Cytoreduction and Hyperthermic Intraperitoneal Chemotherapy. Ann. Surg. 2012, 256, 334–341. [Google Scholar] [CrossRef]
- Chua, T.C.; Moran, B.J.; Sugarbaker, P.H.; Levine, E.A.; Glehen, O.; Gilly, F.N.; Baratti, D.; Deraco, M.; Elias, D.; Sardi, A.; et al. Early- and Long-Term Outcome Data of Patients With Pseudomyxoma Peritonei From Appendiceal Origin Treated by a Strategy of Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. J. Clin. Oncol. 2012, 30, 2449–2456. [Google Scholar] [CrossRef]
- Bakrin, N.; Bereder, J.; Decullier, E.; Classe, J.; Msika, S.; Lorimier, G.; Abboud, K.; Meeus, P.; Ferron, G.; Quenet, F.; et al. Peritoneal carcinomatosis treated with cytoreductive surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for advanced ovarian carcinoma: A French multicentre retrospective cohort study of 566 patients. Eur. J. Surg. Oncol. (EJSO) 2013, 39, 1435–1443. [Google Scholar] [CrossRef]
- Macrì, A.; Arcoraci, V.; Belgrano, V.; Caldana, M.; Cioppa, T.; Costantini, B.; Cucinotta, E.; De Cian, F.; De Iaco, P.; De Manzoni, G.; et al. Short-term outcome of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: Preliminary analysis of a multicentre study. Anticancer. Res. 2014, 34, 5689–5693. [Google Scholar] [PubMed]
- Saxena, A.; Yan, T.D.; Chua, T.C.; Morris, D.L. Critical Assessment of Risk Factors for Complications After Cytoreductive Surgery and Perioperative Intraperitoneal Chemotherapy for Pseudomyxoma Peritonei. Ann. Surg. Oncol. 2009, 17, 1291–1301. [Google Scholar] [CrossRef]
- Yang, X.-J.; Huang, C.-Q.; Suo, T.; Mei, L.-J.; Yang, G.-L.; Cheng, F.-L.; Zhou, Y.-F.; Xiong, B.; Yonemura, Y.; Li, Y. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy Improves Survival of Patients with Peritoneal Carcinomatosis from Gastric Cancer: Final Results of a Phase III Randomized Clinical Trial. Ann. Surg. Oncol. 2011, 18, 1575–1581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonnot, P.M.; Piessen, G.; Kepenekian, V.; Decullier, E.; Pocard, M.; Meunier, B.; Bereder, J.M.; Abboud, K.; Marchal, F.; Quenet, F.; et al. FREGAT and BIG-RENAPE Networks. Cytoreductive Surgery with or without Hyperther-mic Intraperitoneal Chemotherapy for Gastric Cancer with Peritoneal Metastases (CYTO-CHIP Study): A Propensity Score Analysis. J. Clin. Oncol. 2019, 37, 2028–2040. [Google Scholar] [CrossRef] [PubMed]
- Witkamp, A.J.; de Bree, E.; Kaag, M.M.; Boot, H.; Beijnen, J.H.; van Slooten, G.W.; van Coevorden, F.; Zoetmulder, F.A. Extensive cy-toreductive surgery followed by intra-operative hyperthermic intraperitoneal chemotherapy with mitomycin-C in patients with peritoneal carcinomatosis of colorectal origin. Eur. J. Cancer 2001, 37, 979–984. [Google Scholar] [CrossRef]
- Lambert, L.A.; Armstrong, T.S.; Lee, J.J.; Liu, S.; Katz, M.H.; Eng, C.; Wolff, R.A.; Tortorice, M.L.; Tansey, P.; Gonzalez-Moreno, S.; et al. Incidence, risk factors, and impact of severe neutropenia after hyperthermic intraperitoneal mitomycin C. Ann. Surg. Oncol. 2009, 16, 2181–2187. [Google Scholar] [CrossRef] [Green Version]
- Adileh, M.; Mor, E.; Assaf, D.; Benvenisti, H.; Laks, S.; Ben-Yaacov, A.; Schtrechman, G.; Hazzan, D.; Shacham-Shmueli, E.; Margalit, O.; et al. Perioperative and Oncological Outcomes of Combined Hepatec-tomy with Complete Cytoreduction and Hyperthermic Intraperitoneal Chemotherapy for Metastatic Colorectal Cancer. Ann. Surg. Oncol. 2021, 28, 3320–3329. [Google Scholar] [CrossRef]
- Hall, B.; Padussis, J.; Foster, J.M. Cytoreduction and Hyperthermic Intraperitoneal Chemotherapy in the Management of Colo-rectal Peritoneal Metastasis. Surg. Clin. N. Am. 2017, 97, 671–682. [Google Scholar] [CrossRef]
- Franko, J.; Shi, Q.; Meyers, J.P.; Maughan, T.S.; Adams, R.A.; Seymour, M.T.; Saltz, L.; Punt, C.J.A.; Koopman, M.; Tournigand, C.; et al. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: An analysis of individual patient data from prospective randomised trials from the Analysis and Research in Cancers of the Digestive System (ARCAD) database. Lancet Oncol. 2016, 17, 1709–1719. [Google Scholar] [CrossRef]
- Schneider, M.A.; Eden, J.; Pache, B.; Laminger, F.; Lopez-Lopez, V.; Steffen, T.; Hübner, M.; Kober, F.; Roka, S.; Campos, P.C.; et al. Mutations of RAS/RAF Pro-to-oncogenes Impair Survival After Cytoreductive Surgery and HIPEC for Peritoneal Metastasis of Colorectal Origin. Ann Surg. 2018, 268, 845–853. [Google Scholar] [CrossRef] [Green Version]
- Arjona-Sanchez, A.; Rodriguez-Ortiz, L.; Baratti, D.; Schneider, M.A.; Gutiérrez-Calvo, A.; García-Fadrique, A.; Tuynman, J.B.; Cascales-Campos, P.A.; Martín, V.C.; Morales, R.; et al. RAS Mutation Decreases Overall Sur-vival After Optimal Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy of Colorectal Peritoneal Me-tastasis: A Modification Proposal of the Peritoneal Surface Disease Severity Score. Ann. Surg. Oncol. 2019, 26, 2595–2604. [Google Scholar] [CrossRef]
- Morgan, Z.; Chow, B.E.; Strong, E.A.; Tsai, S.; Christians, K.; Mogal, H.; Gamblin, T.C.; Clarke, C.N. RAS Mutation Status Confers Prognostic Relevance in Patients Treated With Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Colorectal Cancer. J. Surg. Res. 2019, 240, 130–135. [Google Scholar] [CrossRef]
- Baratti, D.; Kusamura, S.; Niger, M.; Perrone, F.; Milione, M.; Cattaneo, L.; Guaglio, M.; Bartolini, V.; Pietrantonio, F.; Deraco, M. Prog-nostic Impact of Primary Side and RAS/RAF Mutations in a Surgical Series of Colorectal Cancer with Peritoneal Metastases. Ann. Surg. Oncol. 2021, 28, 3332–3342. [Google Scholar] [CrossRef]
- Graf, W.; Cashin, P.H.; Ghanipour, L.; Enblad, M.; Botling, J.; Terman, A.; Birgisson, H. Prognostic Impact of BRAF and KRAS Muta-tion in Patients with Colorectal and Appendiceal Peritoneal Metastases Scheduled for CRS and HIPEC. Ann. Surg. Oncol. 2020, 27, 293–300. [Google Scholar] [CrossRef] [Green Version]
- Rau, B.; Brandl, A.; Piso, P.; Pelz, J.; Busch, P.; Demtröder, C.; Schüle, S.; Schlitt, H.J.; Roitman, M.; Tepel, J.; et al. Peritoneum Surface Oncology Group and members of the StuDoQ|Peritoneum Registry of the German Society for General and Visceral Surgery (DGAV). Peritoneal metastasis in gastric cancer: Results from the German database. Gastric Cancer 2020, 23, 11–22. [Google Scholar] [CrossRef]
- Spiliotis, J.; Halkia, E.; Lianos, E.; Kalantzi, N.; Grivas, A.; Efstathiou, E.; Giassas, S. Cytoreductive Surgery and HIPEC in Recurrent Epithelial Ovarian Cancer: A Prospective Randomized Phase III Study. Ann. Surg. Oncol. 2015, 22, 1570–1575. [Google Scholar] [CrossRef] [PubMed]
- Fagotti, A.; Costantini, B.; Petrillo, M.; Vizzielli, G.; Fanfani, F.; Margariti, P.A.; Turco, L.C.; Piovano, E.; Scambia, G. Cytoreductive sur-gery plus HIPEC in platinum-sensitive recurrent ovarian cancer patients: A case-control study on survival in patients with two year follow-up. Gynecol. Oncol. 2012, 127, 502–505. [Google Scholar] [CrossRef]
- Van Driel, W.J.; Koole, S.N.; Sikorska, K.; Schagen van Leeuwen, J.H.; Schreuder, H.W.R.; Hermans, R.H.M.; de Hingh, I.H.J.T.; van der Velden, J.; Arts, H.J.; Massuger, L.F.A.G.; et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N. Engl. J. Med. 2018, 378, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.L.; Spirtos, N.M.; Enserro, D.; Herzog, T.J.; Sabbatini, P.; Armstrong, D.K.; Kim, J.-W.; Park, S.-Y.; Kim, B.-G.; Nam, J.-H.; et al. Secondary Surgical Cytoreduction for Recurrent Ovarian Cancer. N. Engl. J. Med. 2019, 381, 1929–1939. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, F.; Chen, P.; Liu, S.; Song, Z.; Ma, X. Effects of CytoReductive surgery plus hyperthermic IntraPEritoneal chemo-therapy (HIPEC) versus CytoReductive surgery for ovarian cancer patients: A systematic review and meta-analysis. Eur. J. Surg. Oncol. 2019, 45, 301–309. [Google Scholar] [CrossRef]
- Wu, Q.; Wu, Q.; Xu, J.; Cheng, X.; Wang, X.; Lu, W.; Li, X. Efficacy of hyperthermic intraperitoneal chemotherapy in patients with epithelial ovarian cancer: A meta-analysis. Int. J. Hyperth. 2019, 36, 561–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.I.; Cho, J.; Lee, E.J.; Park, S.; Park, S.J.; Seol, A.; Lee, N.; Yim, G.W.; Lee, M.; Lim, W.; et al. Selection of patients with ovarian cancer who may show survival benefit from hyperthermic intraperitoneal chemotherapy: A system-atic review and meta-analysis. Medicine 2019, 98, e18355. [Google Scholar] [CrossRef]
- Cianci, S.; Riemma, G.; Ronsini, C.; De Franciscis, P.; Torella, M.; Schiattarella, A.; La Verde, M.; Colacurci, N. Hyperthermic intraper-itoneal chemotherapy (HIPEC) for ovarian cancer recurrence: Systematic review and meta-analysis. Gland Surg. 2020, 9, 1140–1148. [Google Scholar] [CrossRef]


| Parameter | Group 1 (n = 237) | Group 2 (n = 162) | p-Value |
|---|---|---|---|
| Median Age (range) | 55.3 (14–75) | 54.2 (19–79) | 0.3 |
| Sex (male) %(n) | 34 (81) | 42 (68) | 0.07 |
| Tumor etiology %(n) | |||
| CRC | 28 (67) | 32 (51) | 0.268 |
| Ovarian | 27 (64) | 6 (10) | <0.0001 |
| Gastric | 12 (28) | 12 (19) | 0.979 |
| Appendix | 9 (21) | 17 (28) | 0.011 |
| Mesothelioma | 5 (12) | 3 (5) | 0.337 |
| PMP | 11 (26) | 17 (28) | 0.075 |
| Others | 8 (19) | 13 (21) | 0.648 |
| Median PCI (range) | 15.5 (1–39) | 11 (1–39) | 0.002 |
| Operative times (min) | 541 (107–1076) | 315.5 (66–770) | <0.001 |
| CC-score % (n) | 0.010 | ||
| CC-0 | 63 (150) | 69 (112) | |
| CC-1 | 30 (71) | 31 (50) | |
| CC-2 | 4 (9) | - | |
| CC-3 | 3 (7) | - | |
| HIPEC technique | open | closed | |
| HIPEC compound % (n) | |||
| MMC | 54 (128) | - | |
| Cisplatin | 41 (97) | - | |
| MMC/Cisplatin | 3 (8) | - | |
| OX (i.p.)/5-FU (i.v.) | - | 61 (98) | |
| OX | - | 3 (6) | |
| Cisplatin/Doxorubicin | - | 30 (48) | |
| Others | 2 (4) | 6 (10) | |
| HIPEC duration | |||
| OX-based | |||
| Cisplatin-based | 60 min | 30 min | |
| MMC-based | 90 min | ||
| Resections % (n) | |||
| Omentectomy | 49 (116) | 64 (104) | 0.003 |
| Appendectomy | 11 (26) | 20 (32) | 0.339 |
| Splenectomy | 31 (73) | 7 (11) | <0.0001 |
| Rectum | 25 (59) | 12 (19) | 0.007 |
| Small bowel | 23 (55) | 13 (21) | 0.014 |
| Internal genitals | 23 (55) | 20 (32) | 0.323 |
| Right colon | 17 (40) | 14 (23) | 0.078 |
| Gastric | 17 (40) | 19 (31) | 0.562 |
| Complication rate % (n) | |||
| Total | 60 (142) | 62 (101) | 0.21 |
| >Grade IIIa | 16 (37) | 13 (21) | 0.461 |
| Complication type % (n) | |||
| Leucopenia | 34 (81) | 16 (26) | <0.0001 |
| Anastomotic | 4 (9) | 6 (10) | 0.332 |
| insufficiency | |||
| Pleural effusion | 10 (24) | 23 (37) | 0.091 |
| Pneumonia | 4 (9) | 3 (5) | 0.704 |
| Pulmonary embolism | 6 (14) | 3 (5) | 0.152 |
| Fascial rupture | 3 (7) | 6 (10) | 0.588 |
| SSI | 10 (24) | 6 (10) | 0.079 |
| Back to theatre | 15 (36) | 12 (20) | 0.421 |
| 30-day mortality | 0% | 0% | |
| Hospital stay (days (range)) | 17 (3–105) | 14 (6–74) | <0.0001 |
| Tumor Etiology | Median OS (Months (Range)) |
|---|---|
| CRC | Group 1: 34 (1–85) |
| Group 2: 25 (3–42) | |
| Ovarian | Group 1: 45 (10–142) |
| Group 2: n.r. | |
| Gastric | Group 1: 30 (9–117) |
| Group 2: 16 (5–32) | |
| Appendix | Group 1: 39 (32–61) |
| Group 2: n.r. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horvath, P.; Yurttas, C.; Beckert, S.; Königsrainer, A.; Königsrainer, I. Twelve-Year Single Center Experience Shows Safe Implementation of Developed Peritoneal Surface Malignancy Treatment Protocols for Gastrointestinal and Gynecological Primary Tumors. Cancers 2021, 13, 2471. https://doi.org/10.3390/cancers13102471
Horvath P, Yurttas C, Beckert S, Königsrainer A, Königsrainer I. Twelve-Year Single Center Experience Shows Safe Implementation of Developed Peritoneal Surface Malignancy Treatment Protocols for Gastrointestinal and Gynecological Primary Tumors. Cancers. 2021; 13(10):2471. https://doi.org/10.3390/cancers13102471
Chicago/Turabian StyleHorvath, Philipp, Can Yurttas, Stefan Beckert, Alfred Königsrainer, and Ingmar Königsrainer. 2021. "Twelve-Year Single Center Experience Shows Safe Implementation of Developed Peritoneal Surface Malignancy Treatment Protocols for Gastrointestinal and Gynecological Primary Tumors" Cancers 13, no. 10: 2471. https://doi.org/10.3390/cancers13102471
APA StyleHorvath, P., Yurttas, C., Beckert, S., Königsrainer, A., & Königsrainer, I. (2021). Twelve-Year Single Center Experience Shows Safe Implementation of Developed Peritoneal Surface Malignancy Treatment Protocols for Gastrointestinal and Gynecological Primary Tumors. Cancers, 13(10), 2471. https://doi.org/10.3390/cancers13102471