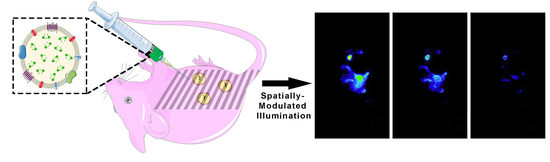
Near Infrared Fluorescence Imaging of Intraperitoneal Ovarian Tumors in Mice Using Erythrocyte-Derived Optical Nanoparticles and Spatially-Modulated Illumination
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication of nRBCs-ICG
2.2. Size and Optical Characterization of nRBCs-ICG
2.3. SMI System
2.4. Animal Study
2.5. Statistical Analysis
3. Results and Discussion
3.1. Size and Optical Characteristics of nRBCs-ICG
3.2. Animal Imaging
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Auersperg, N.; Wong, A.S.; Choi, K.C.; Kang, S.K.; Leung, P.C. Ovarian surface epithelium: Biology, endocrinology, and pathology. Endocr. Rev. 2001, 22, 255–288. [Google Scholar] [CrossRef] [PubMed]
- Ushijima, K.; Kawano, K.; Tsuda, N.; Nishio, S.; Terada, A.; Kato, H.; Tasaki, K.; Matsukuma, K. Epithelial borderline ovarian tumor: Diagnosis and treatment strategy. Obstet. Gynecol. Sci. 2015, 58, 183–187. [Google Scholar] [CrossRef] [Green Version]
- Jemal, A.; Siegel, R.; Ward, E.; Hao, Y.; Xu, J.; Murray, T.; Thun, M.J. Cancer Statistics, 2008. CA Cancer J. Clin. 2008, 58, 71–96. [Google Scholar] [CrossRef]
- Bristow, R.E.; Tomacruz, R.S.; Armstrong, D.K.; Trimble, E.L.; Montz, F.J. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: A meta-analysis. J. Clin. Oncol. 2002, 20, 1248–1259. [Google Scholar] [CrossRef]
- Vergote, I.; Van Gorp, T.; Amant, F.; Leunen, K.; Neven, P.; Berteloot, P. Timing of debulking surgery in advanced ovarian cancer. Int. J. Gynecol. Cancer 2008, 18, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Zivanovic, O.; Aldini, A.; Carlson, J.W.; Chi, D.S. Advanced cytoreductive surgery: American perspective. Gynecol. Oncol. 2009, 114, S3–S9. [Google Scholar] [CrossRef] [PubMed]
- Fader, A.N.; Rose, P.G. Role of surgery in ovarian carcinoma. J. Clin. Oncol. 2007, 25, 2873–2883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chi, D.S.; Eisenhauer, E.L.; Zivanovic, O.; Sonoda, Y.; Abu-Rustum, N.R.; Levine, D.A.; Guile, M.W.; Bristow, R.E.; Aghajanian, C.; Barakat, R.R. Improved progression-free and overall survival in advanced ovarian cancer as a result of a change in surgical paradigm. Gynecol. Oncol. 2009, 114, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, G.M.; Themelis, G.; Crane, L.M.A.; Harlaar, N.J.; Pleijhuis, R.G.; Kelder, W.; Sarantopoulos, A.; De Jong, J.S.; Arts, H.J.G.; Van Der Zee, A.G.J.; et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-α targeting: First in-human results. Nat. Med. 2011, 17, 1315–1319. [Google Scholar] [CrossRef]
- Frangioni, J.V. In vivo near-infrared fluorescence imaging. Curr. Opin. Chem. Biol. 2003, 7, 626–634. [Google Scholar] [CrossRef]
- Marshall, M.V.; Rasmussen, J.C.; Tan, I.-C.; Aldrich, M.B.; Adams, K.E.; Wang, X.; Fife, C.E.; Maus, E.A.; Smith, L.A.; Sevick-Muraca, E.M. Near-infrared fluorescence imaging in humans with indocyanine green: A review and update. Open Surg. Oncol. J. 2010, 2, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Hadjipanayis, C.G.; Jiang, H.; Roberts, D.W.; Yang, L. Current and future clinical applications for optical imaging of cancer: From intraoperative surgical guidance to cancer screening. Semin. Oncol. 2011, 38, 109–118. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.-M.; Shi, R.; Hou, J.-C.; Liu, Z.-R.; Cui, Z.-L.; Li, Y.; Wu, D.; Shi, Y.; Shen, Z.-Y. Liver tumor boundaries identified intraoperatively using real-time indocyanine green fluorescence imaging. J. Cancer Res. Clin. Oncol. 2017, 143, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Tummers, Q.R.J.G.; Hoogstins, C.E.S.; Peters, A.A.W.; De Kroon, C.D.; Trimbos, J.B.M.Z.; Van De Velde, C.J.H.; Frangioni, J.V.; Vahrmeijer, A.L.; Gaarenstroom, K.N. The value of intraoperative near-infrared fluorescence imaging based on enhanced permeability and retention of indocyanine green: Feasibility and false-positives in ovarian cancer. PLoS ONE 2015, 10, e0129766. [Google Scholar] [CrossRef]
- Desmettre, T.; Devoisselle, J.M.; Mordon, S. Fluorescence properties and metabolic features of indocyanine green (ICG) as related to angiography. Surv. Ophthalmol. 2000, 45, 15–27. [Google Scholar] [CrossRef]
- Chijiiwa, K.; Watanabe, M.; Nakano, K.; Noshiro, H.; Tanaka, M. Biliary indocyanine green excretion as a predictor of hepatic adenosine triphosphate levels in patients with obstructive jaundice. Am. J. Surg. 2000, 179, 161–166. [Google Scholar] [CrossRef]
- Wu, L.; Fang, S.; Shi, S.; Deng, J.; Liu, B.; Cai, L. Hybrid polypeptide micelles loading indocyanine green for tumor imaging and photothermal effect study. Biomacromolecules 2013, 14, 3027–3033. [Google Scholar] [CrossRef]
- Toyota, T.; Fujito, H.; Suganami, A.; Ouchi, T.; Ooishi, A.; Aoki, A.; Onoue, K.; Muraki, Y.; Madono, T.; Fujinami, M.; et al. Near-infrared-fluorescence imaging of lymph nodes by using liposomally formulated indocyanine green derivatives. Bioorg. Med. Chem. 2014, 22, 721–727. [Google Scholar] [CrossRef]
- Bahmani, B.; Lytle, C.Y.; Walker, A.M.; Gupta, S.; Vullev, V.I.; Anvari, B. Effects of nanoencapsulation and PEGylation on biodistribution of indocyanine green in healthy mice: Quantitative fluorescence imaging and analysis of organs. Int. J. Nanomed. 2013, 8, 1609–1620. [Google Scholar]
- Yaseen, M.A.; Yu, J.; Wong, M.S.; Anvari, B. In-vivo fluorescence imaging of mammalian organs using charge-assembled mesocapsule constructs containing indocyanine green. Opt. Express 2008, 16, 20577–20587. [Google Scholar] [CrossRef]
- Sharma, P.; Bengtsson, N.E.; Walter, G.A.; Sohn, H.-B.; Zhou, G.; Iwakuma, N.; Zeng, H.; Grobmyer, S.R.; Scott, E.W.; Moudgil, B.M. Gadolinium-doped silica nanoparticles encapsulating indocyanine green for near infrared and magnetic resonance imaging. Small 2012, 8, 2856–2868. [Google Scholar] [CrossRef]
- Yoo, J.-W.; Irvine, D.J.; Discher, D.E.; Mitragotri, S. Bio-inspired, bioengineered and biomimetic drug delivery carriers. Nat. Rev. Drug Discov. 2011, 10, 521–535. [Google Scholar] [CrossRef]
- Pierigè, F.; Serafini, S.; Rossi, L.; Magnani, M. Cell-based drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 286–295. [Google Scholar] [CrossRef]
- Muzykantov, V.R. Drug delivery by red blood cells: Vascular carriers designed by Mother Nature. Expert Opin. Drug Deliv. 2010, 7, 403–427. [Google Scholar] [CrossRef] [Green Version]
- Villa, C.H.; Anselmo, A.C.; Mitragotrib, S.; Muzykantov, V.; Mitragotri, S.; Muzykantov, V. Red blood cells: Supercarriers for drugs, biologicals, and nanoparticles and inspiration for advanced delivery systems. Adv. Drug Deliv. Rev. 2016, 106, 88–103. [Google Scholar] [CrossRef] [Green Version]
- Oldenborg, P.A. CD47: A cell surface glycoprotein which regulates multiple functions of hematopoietic cells in health and disease. ISRN Hematol. 2013, 2013, 614619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahmani, B.; Bacon, D.; Anvari, B. Erythrocyte-derived photo-theranostic agents: Hybrid nano-vesicles containing indocyanine green for near infrared imaging and therapeutic applications. Sci. Rep. 2013, 3, 2180. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.M.; Vankayala, R.; Mac, J.T.; Anvari, B. Erythrocyte-derived theranostic nanoplatforms for near infrared fluorescence imaging and photodestruction of tumors. ACS Appl. Mater. Interfaces 2018, 10, 27621–27630. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Burns, J.M.; Villantay, B.; Tang, J.C.; Vankayala, R.; Lertsakdadet, B.; Choi, B.; Nelson, J.S.; Anvari, B. Intravital vascular phototheranostics and real-time circulation dynamics of micro- And nanosized erythrocyte-derived carriers. ACS Appl. Mater. Interfaces 2020, 12, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Cuccia, D.J.; Bevilacqua, F.; Durkin, A.J.; Ayers, F.R.; Tromberg, B.J. Quantitation and mapping of tissue optical properties using modulated imaging. J. Biomed. Opt. 2009, 14, 024012. [Google Scholar] [CrossRef]
- Cuccia, D.J.; Bevilacqua, F.; Durkin, A.J.; Tromberg, B.J. Modulated imaging: Quantitative analysis and tomography of turbid media in the spatial-frequency domain. Opt. Lett. 2005, 30, 1354–1356. [Google Scholar] [CrossRef]
- Konecky, S.D.; Owen, C.M.; Rice, T.; Valdés, P.A.; Kolste, K.; Wilson, B.C.; Leblond, F.; Roberts, D.W.; Paulsen, K.D.; Tromberg, B.J. Spatial frequency domain tomography of protoporphyrin IX fluorescence in preclinical glioma models. J. Biomed. Opt. 2012, 17, 056008. [Google Scholar] [CrossRef] [Green Version]
- Sibai, M.; Fisher, C.; Veilleux, I.; Elliott, J.T.; Leblond, F.; Roberts, D.W.; Wilson, B.C. Preclinical evaluation of spatial frequency domain-enabled wide-field quantitative imaging for enhanced glioma resection. J. Biomed. Opt. 2017, 22, 076007. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Miller, J.P.; Akers, W.J.; Sun, J.; Miller, J.P.; Hathi, D.; Zhou, H.; Achilefu, S.; Shokeen, M. Enhancing in vivo tumor boundary delineation with structured illumination fluorescence molecular imaging and spatial gradient mapping. J. Biomed. Opt. 2016, 21, 080502. [Google Scholar] [CrossRef] [Green Version]
- Gathje, J.; Steuer, R.R.; Nicholes, K.R. Stability studies on indocyanine green dye. J. Appl. Physiol. 1970, 29, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.M.; Sekelj, P. Studies on the determination of dye concentration in nonhemolyzed blood. J. Lab. Clin. Med. 1968, 72, 705–713. [Google Scholar]
- Tang, J.C.; Partono, A.; Anvari, B. Near-infrared-fluorescent erythrocyte-mimicking particles: Physical and optical characteristics. IEEE Trans. Biomed. Eng. 2019, 66, 1034–1044. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.C.; Vankayala, R.; Mac, J.T.; Anvari, B. RBC-derived optical nanoparticles remain stable after a freeze-thaw cycle. Langmuir 2020, 36, 10003–10011. [Google Scholar] [CrossRef] [PubMed]
- Mac, J.T.; Nuñez, V.; Burns, J.M.; Guerrero, Y.A.; Vullev, V.I.; Anvari, B. Erythrocyte-derived nano-probes functionalized with antibodies for targeted near infrared fluorescence imaging of cancer cells. Biomed. Opt. Express 2016, 7, 1311. [Google Scholar] [CrossRef] [Green Version]
- Vankayala, R.; Mac, J.T.; Burns, J.M.; Dunn, E.; Carroll, S.; Bahena, E.M.; Patel, D.K.; Griffey, S.; Anvari, B. Biodistribution and toxicological evaluation of micron- and nano-sized erythrocyte-derived optical particles in healthy Swiss Webster mice. Biomater. Sci. 2019, 7, 2123–2133. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Jun, F.; Maeda, H. Development of next-generation macromolecular drugs based on the EPR effect: Challenges and pitfalls. Expert Opin. Drug Deliv. 2015, 12, 53–64. [Google Scholar] [CrossRef]
- Torchilin, V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv. Drug Deliv. Rev. 2011, 63, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Lukas, G.; Brindle, S.D.; Greengard, P. The route of absorption of intraperitoneally administered compounds. J. Pharmacol. Exp. Ther. 1971, 178, 562–566. [Google Scholar]
- Zhang, Y.N.; Poon, W.; Tavares, A.J.; McGilvray, I.D.; Chan, W.C.W. Nanoparticle–liver interactions: Cellular uptake and hepatobiliary elimination. J. Control. Release 2016, 240, 332–348. [Google Scholar] [CrossRef] [PubMed]
- Wisse, E.; Braet, F.; Luo, D.; De Zanger, R.; Jans, D.; Crabbé, E.; Vermoesen, A. Structure and function of sinusoidal cells in the liver. Toxicol. Pathol. 1996, 24, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Davies-Venn, C.; Sevick-Muraca, E.M. In vivo dynamic imaging of intestinal motions using diet-related autofluorescence. J. Neurogastroenterol. Motil. 2012, 24, 494–497. [Google Scholar] [CrossRef] [Green Version]
- Gioux, S.; Mazhar, A.; Cuccia, D.J. Spatial frequency domain imaging in 2019: Principles, applications, and perspectives. J. Biomed. Opt. 2019, 24, 071613. [Google Scholar] [CrossRef] [Green Version]
- Jacques, S.L. Optical properties of biological tissues: A review. Phys. Med. Biol. 2013, 58, R37–R61. [Google Scholar] [CrossRef]
- O’Sullivan, T.D.; Cerussi, A.E.; Cuccia, D.J.; Tromberg, B.J. Diffuse optical imaging using spatially and temporally modulated light. J. Biomed. Opt. 2012, 17, 071311. [Google Scholar] [CrossRef] [Green Version]
- Mazhar, A.; Cuccia, D.J.; Gioux, S.; Durkin, A.J.; Frangioni, J.V.; Tromberg, B.J. Structured illumination enhances resolution and contrast in thick tissue fluorescence imaging. J. Biomed. Opt. 2010, 15, 010506. [Google Scholar] [CrossRef] [PubMed]
- Nandy, S.; Mostafa, A.; Kumavor, P.D.; Sanders, M.; Brewer, M.; Zhu, Q. Characterizing optical properties and spatial heterogeneity of human ovarian tissue using spatial frequency domain imaging. J. Biomed. Opt. 2016, 21, 101402. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.M.; Saager, R.; Majaron, B.; Jia, W.; Anvari, B. Optical properties of biomimetic probes engineered from erythrocytes. Nanotechnology 2017, 28, 035101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanley, T.; Yin, R.; Mac, J.T.; Tan, W.; Anvari, B. Functionalized erythrocyte-derived optical nanoparticles to target ephrin-B2 ligands. J. Biomed. Opt. 2019, 24, 1. [Google Scholar] [CrossRef]
- Hanley, T.M.; Vankayala, R.; Mac, J.T.; Lo, D.D.; Anvari, B. Acute immune response of micro- and nanosized erythrocyte-derived optical particles in healthy mice. Mol. Pharm. 2020, 17, 3900–3914. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Giulia, F.; Luffer, S.; Kumar, R.; Wu, B.; Tavallai, M.; Bekele, R.T.; Birrer, M.J. How can molecular abnormalities influence our clinical approach. Ann. Oncol. 2017, 28, viii16–viii24. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burns, J.M.; Shafer, E.; Vankayala, R.; Kundra, V.; Anvari, B. Near Infrared Fluorescence Imaging of Intraperitoneal Ovarian Tumors in Mice Using Erythrocyte-Derived Optical Nanoparticles and Spatially-Modulated Illumination. Cancers 2021, 13, 2544. https://doi.org/10.3390/cancers13112544
Burns JM, Shafer E, Vankayala R, Kundra V, Anvari B. Near Infrared Fluorescence Imaging of Intraperitoneal Ovarian Tumors in Mice Using Erythrocyte-Derived Optical Nanoparticles and Spatially-Modulated Illumination. Cancers. 2021; 13(11):2544. https://doi.org/10.3390/cancers13112544
Chicago/Turabian StyleBurns, Joshua M., Elise Shafer, Raviraj Vankayala, Vikas Kundra, and Bahman Anvari. 2021. "Near Infrared Fluorescence Imaging of Intraperitoneal Ovarian Tumors in Mice Using Erythrocyte-Derived Optical Nanoparticles and Spatially-Modulated Illumination" Cancers 13, no. 11: 2544. https://doi.org/10.3390/cancers13112544
APA StyleBurns, J. M., Shafer, E., Vankayala, R., Kundra, V., & Anvari, B. (2021). Near Infrared Fluorescence Imaging of Intraperitoneal Ovarian Tumors in Mice Using Erythrocyte-Derived Optical Nanoparticles and Spatially-Modulated Illumination. Cancers, 13(11), 2544. https://doi.org/10.3390/cancers13112544