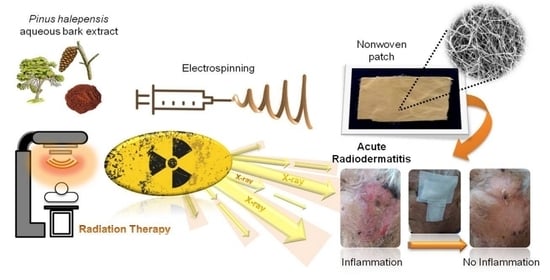
Management of Acute Radiodermatitis in Non-Melanoma Skin Cancer Patients Using Electrospun Nanofibrous Patches Loaded with Pinus halepensis Bark Extract
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Electrospun Micro/Nanofibrous Patch
2.3. Characterization of the Micro/Nanofibrous Patch
2.4. Study Design and Patient Selection
2.5. Radiotherapy Schedules
2.6. Clinical Assessment
2.7. Skin Analysis
2.8. Measurements of the Skin’s Biophysical Parameters
2.9. Patient Self-Report
2.10. Statistical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Da Silva, E.S.; Tavares, R.; Paulitsch, F.D.S.; Zhang, L. Use of sunscreen and risk of melanoma and non-melanoma skin cancer: A systematic review and meta-analysis. Eur. J. Dermatol. EJD 2018, 28, 186–201. [Google Scholar] [CrossRef] [PubMed]
- Linares, M.A.; Zakaria, A.; Nizran, P. Skin Cancer. Prim. Care Clin. Off. Pr. 2015, 42, 645–659. [Google Scholar] [CrossRef] [PubMed]
- Marinho, S.A.; Lima, N.L.; Verli, F.D.; De Miranda, J.L. Basosquamous carcinoma: Histopathological features. Indian J. Dermatol. 2012, 57, 382–383. [Google Scholar] [CrossRef] [PubMed]
- Liu-Smith, F.; Jia, J.; Zheng, Y. UV-Induced Molecular Signaling Differences in Melanoma and Non-melanoma Skin Cancer. Adv. Exp. Med. Biol. 2017, 996, 27–40. [Google Scholar] [CrossRef]
- Madan, V.; Lear, J.T.; Szeimies, R.-M. Non-melanoma skin cancer. Lancet 2010, 375, 673–685. [Google Scholar] [CrossRef] [Green Version]
- Miller, D.L.; Weinstock, M.A. Nonmelanoma skin cancer in the United States: Incidence. J. Am. Acad. Dermatol. 1994, 30, 774–778. [Google Scholar] [CrossRef]
- Peris, K.; Fargnoli, M.C.; Garbe, C.; Kaufmann, R.; Bastholt, L.; Seguin, N.B.; Bataille, V.; Del Marmol, V.; Dummer, R.; Harwood, C.A.; et al. Diagnosis and treatment of basal cell carcinoma: European consensus–based interdisciplinary guidelines. Eur. J. Cancer 2019, 118, 10–34. [Google Scholar] [CrossRef] [Green Version]
- Stratigos, A.J.; Garbe, C.; Dessinioti, C.; Lebbe, C.; Bataille, V.; Bastholt, L.; Dreno, B.; Fargnoli, M.C.; Forsea, A.M.; Frenard, C.; et al. European interdisciplinary guideline on invasive squamous cell carcinoma of the skin: Part 1. epidemiology, diagnostics and prevention. Eur. J. Cancer 2020, 128, 60–82. [Google Scholar] [CrossRef] [Green Version]
- Verkouteren, J.; Ramdas, K.; Wakkee, M.; Nijsten, T. Epidemiology of basal cell carcinoma: Scholarly review. Br. J. Dermatol. 2017, 177, 359–372. [Google Scholar] [CrossRef]
- Kallini, J.R.; Hamed, N.; Khachemoune, A. Squamous cell carcinoma of the skin: Epidemiology, classification, management, and novel trends. Int. J. Dermatol. 2014, 54, 130–140. [Google Scholar] [CrossRef]
- Rong, Y.; Zuo, L.; Shang, L.; Bazan, J.G. Radiotherapy treatment for nonmelanoma skin cancer. Expert Rev. Anticancer. Ther. 2015, 15, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Bichakjian, C.K.; Olencki, T.; Aasi, S.Z.; Alam, M.; Andersen, J.S.; Berg, D.; Bowen, G.M.; Cheney, R.T.; Daniels, G.A.; Glass, L.F.; et al. Basal Cell Skin Cancer, Version 1.2016, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2016, 14, 574–597. [Google Scholar] [CrossRef]
- Bray, F.N.; Simmons, B.J.; Wolfson, A.H.; Nouri, K. Acute and Chronic Cutaneous Reactions to Ionizing Radiation Therapy. Dermatol. Ther. 2016, 6, 185–206. [Google Scholar] [CrossRef] [Green Version]
- Salvo, N.; Barnes, E.; van Draanen, J.; Stacey, E.; Mitera, G.; Breen, D.; Giotis, A.; Czarnota, G.; Pang, J.; De Angelis, C. Prophylaxis and Management of Acute Radiation-Induced Skin Reactions: A Systematic Review of the Literature. Curr. Oncol. 2010, 17, 94–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kole, A.J.; Kole, L.; Moran, M.S. Acute radiation dermatitis in breast cancer patients: Challenges and solutions. Breast Cancer: Targets Ther. 2017, 9, 313–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meimeti, E.; Kafanas, A.; Pavlou, P.; Evangelatou, A.; Tsouparelou, P.; Kanellopoulos, S.; Kipouros, P.; Koliarakis, N.; Leonis, G.; Ioannou, E.; et al. Topical Treatment of Skin Injury Inflicted in Mice by X-Ray Irradiation. Ski. Pharmacol. Physiol. 2018, 31, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-J.; Hou, M.-F.; Luo, K.-H.; Wei, S.-Y.; Huang, M.-Y.; Su, S.-J.; Kuo, H.-Y.; Yuan, S.-S.F.; Chen, G.-S.; Hu, S.C.-S.; et al. RTOG, CTCAE and WHO criteria for acute radiation dermatitis correlate with cutaneous blood flow measurements. Breast 2015, 24, 230–236. [Google Scholar] [CrossRef]
- Singh, M.; Alavi, A.; Wong, R.; Akita, S. Radiodermatitis: A Review of Our Current Understanding. Am. J. Clin. Dermatol. 2016, 17, 277–292. [Google Scholar] [CrossRef]
- Wong, R.K.S.; Bensadoun, R.-J.; Boers-Doets, C.B.; Bryce, J.; Chan, A.; Epstein, J.B.; Eaby-Sandy, B.; Lacouture, M.E. Clinical practice guidelines for the prevention and treatment of acute and late radiation reactions from the MASCC Skin Toxicity Study Group. Support. Care Cancer 2013, 21, 2933–2948. [Google Scholar] [CrossRef]
- McQuestion, M. Evidence-Based Skin Care Management in Radiation Therapy: Clinical Update. Semin. Oncol. Nurs. 2011, 27, e1–e17. [Google Scholar] [CrossRef]
- Harris, R.; Probst, H.; Beardmore, C.; James, S.; Dumbleton, C.; Bolderston, A.; Faithfull, S.; Wells, M.; Southgate, E. Radiotherapy skin care: A survey of practice in the UK. Radiogr. 2012, 18, 21–27. [Google Scholar] [CrossRef]
- Kavanagh, S.; De Jong, A. Care of burn patients in the hospital. Burn. 2004, 30, A2–A6. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Kenawy, E.-R.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef]
- Mogoşanu, G.D.; Grumezescu, A.M. Natural and synthetic polymers for wounds and burns dressing. Int. J. Pharm. 2014, 463, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Romano, I.; Summa, M.; Heredia-Guerrero, J.A.; Spano’, R.; Ceseracciu, L.; Pignatelli, C.; Bertorelli, R.; Mele, E.; Athanassiou, A. Fumarate-loaded electrospun nanofibers with anti-inflammatory activity for fast recovery of mild skin burns. Biomed. Mater. 2016, 11, 041001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotroni, E.; Simirioti, E.; Kikionis, S.; Sfiniadakis, I.; Siamidi, A.; Karalis, V.; Vitsos, A.; Vlachou, M.; Ioannou, E.; Roussis, V.; et al. In Vivo Evaluation of the Anti-Inflammatory Activity of Electrospun Micro/Nanofibrous Patches Loaded with Pinus halepensis Bark Extract on Hairless Mice Skin. Materials 2019, 12, 2596. [Google Scholar] [CrossRef] [Green Version]
- Hajiali, H.; Summa, M.; Russo, D.; Armirotti, A.; Brunetti, V.; Bertorelli, R.; Athanassiou, A.; Mele, E. Alginate–lavender nanofibers with antibacterial and anti-inflammatory activity to effectively promote burn healing. J. Mater. Chem. B 2016, 4, 1686–1695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konwarh, R.; Karak, N.; Misra, M. Electrospun cellulose acetate nanofibers: The present status and gamut of biotechnological applications. Biotechnol. Adv. 2013, 31, 421–437. [Google Scholar] [CrossRef]
- Weng, L. Smart Electrospun Nanofibers for Controlled Drug Release: Recent Advances and New Perspectives. Curr. Pharm. Des. 2015, 21, 1944–1959. [Google Scholar] [CrossRef] [Green Version]
- Kikionis, S.; Ioannou, E.; Toskas, G.; Roussis, V. Electrospun biocomposite nanofibers of ulvan/PCL and ulvan/PEO. J. Appl. Polym. Sci. 2015, 132, 42153. [Google Scholar] [CrossRef]
- Thenmozhi, S.; Dharmaraj, N.; Kadirvelu, K.; Kim, H.Y. Electrospun nanofibers: New generation materials for advanced applications. Mater. Sci. Eng. B 2017, 217, 36–48. [Google Scholar] [CrossRef]
- Anstey, A.; Chang, E.; Kim, E.S.; Rizvi, A.; Kakroodi, A.R.; Park, C.B.; Lee, P.C. Nanofibrillated polymer systems: Design, application, and current state of the art. Prog. Polym. Sci. 2021, 113, 101346. [Google Scholar] [CrossRef]
- Teo, W.E.; Ramakrishna, S. A review on electrospinning design and nanofibre assemblies. Nanotechnology 2006, 17, R89–R106. [Google Scholar] [CrossRef] [PubMed]
- Greiner, A.; Wendorff, J.H. Electrospinning: A Fascinating Method for the Preparation of Ultrathin Fibers. Angew. Chem. Int. Ed. 2007, 46, 5670–5703. [Google Scholar] [CrossRef] [PubMed]
- Vlachou, M.; Kikionis, S.; Siamidi, A.; Kyriakou, S.; Tsotinis, A.; Ioannou, E.; Roussis, V. Development and Characterization of Eudragit®-Based Electrospun Nanofibrous Mats and Their Formulation into Nanofiber Tablets for the Modified Release of Furosemide. Pharmaceuticals 2019, 11, 480. [Google Scholar] [CrossRef] [Green Version]
- Kikionis, S.; Ioannou, E.; Andrén, O.C.J.; Chronakis, I.S.; Fahmi, A.; Malkoch, M.; Toskas, G.; Roussis, V. Nanofibrous nonwovens based on dendritic-linear-dendritic poly (ethylene glycol) hybrids. J. Appl. Polym. Sci. 2017, 135, 45949. [Google Scholar] [CrossRef]
- Cheng, H.; Yang, X.; Che, X.; Yang, M.; Zhai, G. Biomedical application and controlled drug release of electrospun fibrous materials. Mater. Sci. Eng. C 2018, 90, 750–763. [Google Scholar] [CrossRef]
- Kikionis, S.; Ioannou, E.; Konstantopoulou, M.; Roussis, V. Electrospun Micro/Nanofibers as Controlled Release Systems for Pheromones of Bactrocera oleae and Prays oleae. J. Chem. Ecol. 2017, 43, 254–262. [Google Scholar] [CrossRef]
- Liu, M.; Duan, X.P.; Li, Y.M.; Yang, D.P.; Long, Y.Z. Electrospun nanofibers for wound healing. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 1413–1423. [Google Scholar] [CrossRef]
- Schiller, G. Therapeutic use of Aleppo Pine (Pinus halepensis Mill.). In Springer-Medicinal and Aromatic Plants of the Middle-East, 1st ed.; Yaniv, Z., Dudai, N., Eds.; Springer: Dordrecht, The Netherlands, 2014; Volume 2, pp. 215–224. [Google Scholar]
- Süntar, I.; Tumen, I.; Ustün, O.; Keleş, H.; Akkol, E.K. Appraisal on the wound healing and anti-inflammatory activities of the essential oils obtained from the cones and needles of Pinus species by in vivo and in vitro experimental models. J. Ethnopharmacol. 2012, 139, 533–540. [Google Scholar] [CrossRef]
- Ioannou, E.; Koutsaviti, A.; Tzakou, O.; Roussis, V. The genus Pinus: A comparative study on the needle essential oil composition of 46 pine species. Phytochem. Rev. 2014, 13, 741–768. [Google Scholar] [CrossRef]
- Zoumpliou, V.; Stamatiadi, M.; Vassiliadis, C.; Rallis, M.; Papaioannou, G.T.; Liakos, S.; Angelou, A.; Daskalaki, S.; Kyriazi, M.; Roussis, V.; et al. Effect of Cigarette Smoke on Diabetic Skin and Protection with Topical Administration of Pinus halepensis Extract. Am. J. Plant. Sci. 2014, 5, 3964–3973. [Google Scholar] [CrossRef] [Green Version]
- Guri, A.; Kefalas, P.; Roussis, V. Antioxidant potential of six pine species. Phytother. Res. 2006, 20, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Fekiha, N.; Allalia, H.; Merghachea, S.; Chaïb, F.; Merghachec, D.; El Amine, M.; Djabou, N.; Musellib, A.; Tabtia, B.; Costab, J. Chemical composition and antibacterial activity of Pinus halepensis Miller growing in West Northern of Algeria. Asian Pac. J. Trop. Dis. 2014, 4, 97–103. [Google Scholar] [CrossRef]
- Petri, A.; Alexandratou, E.; Kyriazi, M.; Rallis, M.; Roussis, V.; Yova, D. Combination of Fospeg-IPDT and a natural antioxidant compound prevents photosensitivity in a murine prostate cancer tumour model. Photodiagn. Photodyn. Ther. 2012, 9, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Hashemikia, S.; Farhangpazhouh, F.; Parsa, M.; Hasan, M.; Hassanzadeh, A.; Hamidi, M. Fabrication of ciprofloxacin-loaded chitosan/polyethylene oxide/silica nanofibers for wound dressing application: In vitro and in vivo evaluations. Int. J. Pharm. 2021, 597, 120313. [Google Scholar] [CrossRef]
- Mokhena, T.C.; Mochane, M.J.; Mtibe, A.; John, M.J.; Sadiku, E.R.; Sefadi, J.S. Electrospun Alginate Nanofibers Toward Various Applications: A Review. Materials 2020, 13, 934. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, M.A.; Paiva, M.C.; Amorim, M.T.P.; Felgueiras, A.H.P. Electrospun Nanocomposites Containing Cellulose and Its Derivatives Modified with Specialized Biomolecules for an Enhanced Wound Healing. Nanomaterials 2020, 10, 557. [Google Scholar] [CrossRef] [Green Version]
- Schmeel, L.C.; Koch, D.; Stumpf, S.; Leitzen, C.; Simon, B.; Schüller, H.; Vornholt, S.; Schoroth, F.; Müdder, T.; Röhner, F.; et al. Prophylactically applied Hydrofilm polyurethane film dressings reduce radiation dermatitis in adjuvant radiation therapy of breast cancer patients. Acta Oncol. 2018, 57, 908–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmeel, L.C.; Koch, D.; Schmeel, F.C.; Bücheler, B.; Leitzen, C.; Mahlmann, B.; Kunze, D.; Heimann, M.; Brüser, D.; Abramian, A.-V.; et al. Hydrofilm Polyurethane Films Reduce Radiation Dermatitis Severity in Hypofractionated Whole-Breast Irradiation: An Objective, Intra-Patient Randomized Dual-Center Assessment. Polymer 2019, 11, 2112. [Google Scholar] [CrossRef] [Green Version]
- Herst, P.M.; Bennett, N.C.; Sutherland, A.E.; Peszynski, R.I.; Paterson, D.B.; Jasperse, M.L. Prophylactic use of Mepitel Film prevents radiation-induced moist desquamation in an intra-patient randomised controlled clinical trial of 78 breast cancer patients. Radiother. Oncol. 2014, 110, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Yuan, L.; Wang, J.; Li, S.; Yao, M.; Wang, K.; Herst, P.M. Mepitel Film is superior to Biafine cream in managing acute radiation-induced skin reactions in head and neck cancer patients: A randomised intra-patient controlled clinical trial. J. Med. Radiat. Sci. 2020, 67, 208–216. [Google Scholar] [CrossRef] [PubMed]









| Characteristics of Patients | PHBE Patch Group (N = 6) | Reference Product Group (N = 6) | Total Patients (N = 12) |
|---|---|---|---|
| Demographics | |||
| Median age (interquartile range)—year | 86 (80–93) | 75 (51–90) | 80 (51–93) |
| Gender—% | |||
| Male | 66.7 | 83.3 | 75.0 |
| Female | 33.3 | 16.7 | 25.0 |
| Body mass index—% | |||
| >25 (overweight) | 33.3 | 50 | 41.7 |
| <25 (healthy weight) | 66.7 | 50 | 58.3 |
| Fitzpatrick skin type—% | |||
| I | 16.7 | 33.3 | 25.0 |
| II | 50.0 | 33.3 | 41.7 |
| III | 33.3 | 33.3 | 33.3 |
| Unprotected sun exposure—% | 100.0 | 83.3 | 91.7 |
| Current Smoker—% | 0.0 | 16.7 | 8.3 |
| Characteristics of non-melanoma skin cancer (NMSC) | |||
| Cancer type—% | |||
| Basal cell carcinoma (BCC) | 50.0 | 50.0 | 50.0 |
| Squamous cell carcinoma (SCC) | 33.3 | 16.7 | 25.0 |
| Basosquamous carcinoma (BSC) | 16.7 | 33.3 | 25.0 |
| Tumor size (cm2)—% | |||
| >20 | 50.0 | 50.0 | 50.0 |
| <20 | 50.0 | 50.0 | 50.0 |
| Primary—% | 83.3 | 50.0 | 66.7 |
| Recurring—% | 16.7 | 50.0 | 33.3 |
| Surgery—% | 100.0 | 66.7 | 83.3 |
| Radiation therapy (RT) | |||
| Total dose (cGy)—% | |||
| 5750 | 50.0 | 16.7 | 33.3 |
| 5500 | 33.3 | 66.7 | 50.0 |
| 4800 | 16.7 | 16.7 | 16.7 |
| Fraction dose (cGy)—% | |||
| 250 | 66.7 | 50.0 | 58.3 |
| 200/400 1 | 16.7 | 0.0 | 8.3 |
| 250/300 1 | 16.7 | 16.7 | 16.7 |
| 250/308 1 | 0.0 | 16.7 | 8.3 |
| 300/400 1 | 0.0 | 16.7 | 8.3 |
| Fractions—% | |||
| 15 | 0.0 | 16.7 | 8.3 |
| 22 | 33.3 | 66.7 | 50.0 |
| 23 | 66.7 | 16.7 | 41.7 |
| Characteristics of Patients | p-Value |
|---|---|
| Demographics | |
| Age | 0.394 |
| Gender | 0.699 |
| Body mass index | 0.132 |
| Fitzpatrick skin type | 0.818 |
| Sun exposure | 0.699 |
| Smoking | 0.699 |
| Characteristics of non-melanoma skin cancer (NMSC) | |
| Cancer type | 0.818 |
| Tumor size | 0.394 |
| Primary or recurring | 0.394 |
| Surgery or not | 0.394 |
| Radiation therapy (RT) | |
| Total dose | 0.310 |
| Fraction dose | 0.394 |
| Fractions | 0.132 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kyritsi, A.; Kikionis, S.; Tagka, A.; Koliarakis, N.; Evangelatou, A.; Papagiannis, P.; Stratigos, A.; Karalis, V.; Dallas, P.; Vitsos, A.; et al. Management of Acute Radiodermatitis in Non-Melanoma Skin Cancer Patients Using Electrospun Nanofibrous Patches Loaded with Pinus halepensis Bark Extract. Cancers 2021, 13, 2596. https://doi.org/10.3390/cancers13112596
Kyritsi A, Kikionis S, Tagka A, Koliarakis N, Evangelatou A, Papagiannis P, Stratigos A, Karalis V, Dallas P, Vitsos A, et al. Management of Acute Radiodermatitis in Non-Melanoma Skin Cancer Patients Using Electrospun Nanofibrous Patches Loaded with Pinus halepensis Bark Extract. Cancers. 2021; 13(11):2596. https://doi.org/10.3390/cancers13112596
Chicago/Turabian StyleKyritsi, Aikaterini, Stefanos Kikionis, Anna Tagka, Nikolaos Koliarakis, Antonia Evangelatou, Panagiotis Papagiannis, Alexandros Stratigos, Vangelis Karalis, Paraskevas Dallas, Andreas Vitsos, and et al. 2021. "Management of Acute Radiodermatitis in Non-Melanoma Skin Cancer Patients Using Electrospun Nanofibrous Patches Loaded with Pinus halepensis Bark Extract" Cancers 13, no. 11: 2596. https://doi.org/10.3390/cancers13112596
APA StyleKyritsi, A., Kikionis, S., Tagka, A., Koliarakis, N., Evangelatou, A., Papagiannis, P., Stratigos, A., Karalis, V., Dallas, P., Vitsos, A., Ioannou, E., Roussis, V., & Rallis, M. (2021). Management of Acute Radiodermatitis in Non-Melanoma Skin Cancer Patients Using Electrospun Nanofibrous Patches Loaded with Pinus halepensis Bark Extract. Cancers, 13(11), 2596. https://doi.org/10.3390/cancers13112596