DLX Genes: Roles in Development and Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Structure and Origin of the DLX Gene Clusters
3. Dlx Genes in Normal and Aberrant Development
4. DLX Genes in Normal Hematopoiesis
5. DLX Genes in Aberrant Hematopoiesis
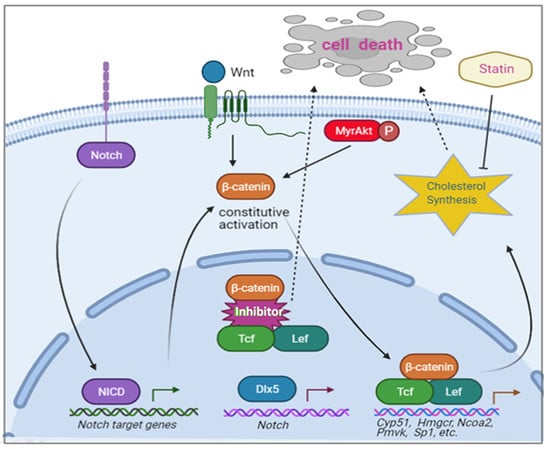
6. Involvement of DLX Genes in Other Malignancies
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gehring, W.J. Homeo boxes in the study of development. Science 1987, 236, 1245–1252. [Google Scholar] [CrossRef]
- Holland, P.W. Evolution of homeobox genes. Wiley Interdiscip. Rev. Dev. Biol. 2013, 2, 31–45. [Google Scholar] [CrossRef]
- Burglin, T.R.; Affolter, M. Homeodomain proteins: An update. Chromosoma 2016, 125, 497–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gehring, W.J.; Affolter, M.; Burglin, T. Homeodomain proteins. Annu. Rev. Biochem. 1994, 63, 487–526. [Google Scholar] [CrossRef]
- Gehring, W.J.; Muller, M.; Affolter, M.; Percival-Smith, A.; Billeter, M.; Qian, Y.Q.; Otting, G.; Wuthrich, K. The structure of the homeodomain and its functional implications. Trends Genet. 1990, 6, 323–329. [Google Scholar] [CrossRef]
- Scott, M.P.; Tamkun, J.W.; Hartzell, G.W., 3rd. The structure and function of the homeodomain. Biochim. Biophys. Acta 1989, 989, 25–48. [Google Scholar] [CrossRef]
- Luke, G.N.; Castro, L.F.; McLay, K.; Bird, C.; Coulson, A.; Holland, P.W. Dispersal of NK homeobox gene clusters in amphioxus and humans. Proc. Natl. Acad. Sci. USA 2003, 100, 5292–5295. [Google Scholar] [CrossRef] [Green Version]
- Homminga, I.; Pieters, R.; Meijerink, J.P. NKL homeobox genes in leukemia. Leukemia 2012, 26, 572–581. [Google Scholar] [CrossRef] [Green Version]
- Holland, P.W.; Booth, H.A.; Bruford, E.A. Classification and nomenclature of all human homeobox genes. BMC Biol. 2007, 5, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagel, S.; Venturini, L.; Przybylski, G.K.; Grabarczyk, P.; Meyer, C.; Kaufmann, M.; Battmer, K.; Schmidt, C.A.; Drexler, H.G.; Scherr, M.; et al. NK-like homeodomain proteins activate NOTCH3-signaling in leukemic T-cells. BMC Cancer 2009, 9, 371. [Google Scholar] [CrossRef] [Green Version]
- Cohen, S.M.; Bronner, G.; Kuttner, F.; Jurgens, G.; Jackle, H. Distal-less encodes a homoeodomain protein required for limb development in Drosophila. Nature 1989, 338, 432–434. [Google Scholar] [CrossRef]
- Depew, M.J.; Liu, J.K.; Long, J.E.; Presley, R.; Meneses, J.J.; Pedersen, R.A.; Rubenstein, J.L. Dlx5 regulates regional development of the branchial arches and sensory capsules. Development 1999, 126, 3831–3846. [Google Scholar] [CrossRef] [PubMed]
- Sumiyama, K.; Irvine, S.Q.; Stock, D.W.; Weiss, K.M.; Kawasaki, K.; Shimizu, N.; Shashikant, C.S.; Miller, W.; Ruddle, F.H. Genomic structure and functional control of the Dlx3-7 bigene cluster. Proc. Natl. Acad. Sci. USA 2002, 99, 780–785. [Google Scholar] [CrossRef] [Green Version]
- Poitras, L.; Ghanem, N.; Hatch, G.; Ekker, M. The proneural determinant MASH1 regulates forebrain Dlx1/2 expression through the I12b intergenic enhancer. Development 2007, 134, 1755–1765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghanem, N.; Yu, M.; Long, J.; Hatch, G.; Rubenstein, J.L.; Ekker, M. Distinct cis-regulatory elements from the Dlx1/Dlx2 locus mark different progenitor cell populations in the ganglionic eminences and different subtypes of adult cortical interneurons. J. Neurosci. 2007, 27, 5012–5022. [Google Scholar] [CrossRef]
- Ghanem, N.; Jarinova, O.; Amores, A.; Long, Q.; Hatch, G.; Park, B.K.; Rubenstein, J.L.; Ekker, M. Regulatory roles of conserved intergenic domains in vertebrate Dlx bigene clusters. Genome Res. 2003, 13, 533–543. [Google Scholar] [CrossRef] [Green Version]
- Sumiyama, K.; Irvine, S.Q.; Ruddle, F.H.. The role of gene duplication in the evolution and function of the vertebrate Dlx/distal-less bigene clusters. J. Struct. Funct. Genomics 2003, 3, 151–159. [Google Scholar] [CrossRef]
- Sumiyama, K.; Ruddle, F.H. Regulation of Dlx3 gene expression in visceral arches by evolutionarily conserved enhancer elements. Proc. Natl. Acad. Sci. USA 2003, 100, 4030–4034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stock, D.W.; Ellies, D.L.; Zhao, Z.; Ekker, M.; Ruddle, F.H.; Weiss, K.M. The evolution of the vertebrate Dlx gene family. Proc. Natl. Acad. Sci. USA 1996, 93, 10858–10863. [Google Scholar] [CrossRef] [Green Version]
- Pollard, S.L.; Holland, P.W. Evidence for 14 homeobox gene clusters in human genome ancestry. Curr. Biol. 2000, 10, 1059–1062. [Google Scholar] [CrossRef] [Green Version]
- Park, B.K.; Sperber, S.M.; Choudhury, A.; Ghanem, N.; Hatch, G.T.; Sharpe, P.T.; Thomas, B.L.; Ekker, M. Intergenic enhancers with distinct activities regulate Dlx gene expression in the mesenchyme of the branchial arches. Dev. Biol. 2004, 268, 532–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruest, L.B.; Hammer, R.E.; Yanagisawa, M.; Clouthier, D.E. Dlx5/6-enhancer directed expression of Cre recombinase in the pharyngeal arches and brain. Genesis 2003, 37, 188–194. [Google Scholar] [CrossRef] [Green Version]
- Ellies, D.L.; Stock, D.W.; Hatch, G.; Giroux, G.; Weiss, K.M.; Ekker, M. Relationship between the genomic organization and the overlapping embryonic expression patterns of the zebrafish dlx genes. Genomics 1997, 45, 580–590. [Google Scholar] [CrossRef]
- Zerucha, T.; Ekker, M. Distal-less-related homeobox genes of vertebrates: Evolution, function, and regulation. Biochem. Cell Biol. 2000, 78, 593–601. [Google Scholar] [CrossRef]
- Qiu, M.; Bulfone, A.; Ghattas, I.; Meneses, J.J.; Christensen, L.; Sharpe, P.T.; Presley, R.; Pedersen, R.A.; Rubenstein, J.L. Role of the Dlx homeobox genes in proximodistal patterning of the branchial arches: Mutations of Dlx-1, Dlx-2, and Dlx-1 and -2 alter morphogenesis of proximal skeletal and soft tissue structures derived from the first and second arches. Dev. Biol. 1997, 185, 165–184. [Google Scholar] [CrossRef] [Green Version]
- Qiu, M.; Bulfone, A.; Martinez, S.; Meneses, J.J.; Shimamura, K.; Pedersen, R.A.; Rubenstein, J.L. Null mutation of Dlx-2 results in abnormal morphogenesis of proximal first and second branchial arch derivatives and abnormal differentiation in the forebrain. Genes. Dev. 1995, 9, 2523–2538. [Google Scholar] [CrossRef] [Green Version]
- Anderson, S.A.; Qiu, M.; Bulfone, A.; Eisenstat, D.D.; Meneses, J.; Pedersen, R.; Rubenstein, J.L. Mutations of the homeobox genes Dlx-1 and Dlx-2 disrupt the striatal subventricular zone and differentiation of late born striatal neurons. Neuron 1997, 19, 27–37. [Google Scholar] [CrossRef] [Green Version]
- De Melo, J.; Du, G.; Fonseca, M.; Gillespie, L.A.; Turk, W.J.; Rubenstein, J.L.; Eisenstat, D.D. Dlx1 and Dlx2 function is necessary for terminal differentiation and survival of late-born retinal ganglion cells in the developing mouse retina. Development 2005, 132, 311–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Q.P.; Le, T.N.; Qiu, X.; Spencer, V.; de Melo, J.; Du, G.; Plews, M.; Fonseca, M.; Sun, J.M.; Davie, J.R.; et al. Identification of a direct Dlx homeodomain target in the developing mouse forebrain and retina by optimization of chromatin immunoprecipitation. Nucleic. Acids Res. 2004, 32, 884–892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acampora, D.; Merlo, G.R.; Paleari, L.; Zerega, B.; Postiglione, M.P.; Mantero, S.; Bober, E.; Barbieri, O.; Simeone, A.; Levi, G. Craniofacial, vestibular and bone defects in mice lacking the Distal-less-related gene Dlx5. Development 1999, 126, 3795–3809. [Google Scholar] [CrossRef] [PubMed]
- Robledo, R.F.; Rajan, L.; Li, X.; Lufkin, T. The Dlx5 and Dlx6 homeobox genes are essential for craniofacial, axial, and appendicular skeletal development. Genes. Dev. 2002, 16, 1089–1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Marijanovic, I.; Kronenberg, M.S.; Erceg, I.; Stover, M.L.; Velonis, D.; Mina, M.; Heinrich, J.G.; Harris, S.E.; Upholt, W.B.; et al. Expression and function of Dlx genes in the osteoblast lineage. Dev. Biol. 2008, 316, 458–470. [Google Scholar] [CrossRef] [Green Version]
- Robledo, R.F.; Lufkin, T. Dlx5 and Dlx6 homeobox genes are required for specification of the mammalian vestibular apparatus. Genesis 2006, 44, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Lezot, F.; Thomas, B.; Greene, S.R.; Hotton, D.; Yuan, Z.A.; Castaneda, B.; Bolanos, A.; Depew, M.; Sharpe, P.; Gibson, C.W.; et al. Physiological implications of DLX homeoproteins in enamel formation. J. Cell Physiol. 2008, 216, 688–697. [Google Scholar] [CrossRef]
- Nishida, H.; Miyagawa, S.; Vieux-Rochas, M.; Morini, M.; Ogino, Y.; Suzuki, K.; Nakagata, N.; Choi, H.S.; Levi, G.; Yamada, G. Positive regulation of steroidogenic acute regulatory protein gene expression through the interaction between Dlx and GATA-4 for testicular steroidogenesis. Endocrinology 2008, 149, 2090–2097. [Google Scholar] [CrossRef] [Green Version]
- Verzi, M.P.; Agarwal, P.; Brown, C.; McCulley, D.J.; Schwarz, J.J.; Black, B.L. The transcription factor MEF2C is required for craniofacial development. Dev. Cell 2007, 12, 645–652. [Google Scholar] [CrossRef] [Green Version]
- Lo Iacono, N.; Mantero, S.; Chiarelli, A.; Garcia, E.; Mills, A.A.; Morasso, M.I.; Costanzo, A.; Levi, G.; Guerrini, L.; Merlo, G.R. Regulation of Dlx5 and Dlx6 gene expression by p63 is involved in EEC and SHFM congenital limb defects. Development 2008, 135, 1377–1388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, K.K.; Reiss, J.A.; Crow, K.; Ferguson, H.L.; Kelly, C.; Fritzsch, B.; Morton, C.C. Deletion of an enhancer near DLX5 and DLX6 in a family with hearing loss, craniofacial defects, and an inv(7)(q21.3q35). Hum. Genet. 2010, 127, 19–31. [Google Scholar] [CrossRef] [Green Version]
- Radoja, N.; Guerrini, L.; Lo Iacono, N.; Merlo, G.R.; Costanzo, A.; Weinberg, W.C.; La Mantia, G.; Calabrò, V.; Morasso, M.I. Homeobox gene Dlx3 is regulated by p63 during ectoderm development: Relevance in the pathogenesis of ectodermal dysplasias. Development 2007, 134, 13–18. [Google Scholar] [CrossRef] [Green Version]
- Isaac, J.; Erthal, J.; Gordon, J.; Duverger, O.; Sun, H.W.; Lichtler, A.C.; Stein, G.S.; Lian, J.B.; Morasso, M.I. DLX3 regulates bone mass by targeting genes supporting osteoblast differentiation and mineral homeostasis in vivo. Cell Death. Differ. 2014, 21, 1365–1376. [Google Scholar] [CrossRef] [Green Version]
- Artigas, N.; Gamez, B.; Cubillos-Rojas, M.; Sanchez-de Diego, C.; Valer, J.A.; Pons, G.; Rosa, J.L.; Ventura, F. p53 inhibits SP7/Osterix activity in the transcriptional program of osteoblast differentiation. Cell Death. Differ. 2017, 24, 2022–2031. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Roodman, G.D.; Feng, J.Q.; Song, I.S.; Amin, K.; Hart, P.S.; Wright, J.T.; Haruyama, N.; Hart, T.C. In vivo impact of a 4 bp deletion mutation in the DLX3 gene on bone development. Dev. Biol. 2009, 325, 129–137. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Mandal, S.; Choi, A.; Anderson, A.; Prochazkova, M.; Perry, H.; Gil-Da-Silva-Lopes, V.L.; Lao, R.; Wan, E.; Tang, P.L.; et al. DLX4 is associated with orofacial clefting and abnormal jaw development. Hum. Mol. Genet. 2015, 24, 4340–4352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, H.; Han, G.; Sun, B.; Fang, X.; Dai, X.; Zhou, S.; Mao, H.; Wang, B. Activating PIK3CA mutation promotes osteogenesis of bone marrow mesenchymal stem cells in macrodactyly. Cell Death. Dis. 2020, 11, 505. [Google Scholar] [CrossRef] [PubMed]
- Twomey-Kozak, J.; Desai, S.; Liu, W.; Li, N.Y.; Lemme, N.; Chen, Q.; Owens, B.D.; Jayasuriya, C.T. Distal-less homeobox 5 is a therapeutic target for attenuating hypertrophy and apoptosis of mesenchymal progenitor cells. Int. J. Mol. Sci. 2020, 21, 4823. [Google Scholar] [CrossRef]
- Tamaoki, N.; Takahashi, K.; Aoki, H.; Iida, K.; Kawaguchi, T.; Hatakeyama, D.; Inden, M.; Chosa, N.; Ishisaki, A.; Kunisada, T.; et al. The homeobox gene DLX4 promotes generation of human induced pluripotent stem cells. Sci. Rep. 2014, 4, 7283. [Google Scholar] [CrossRef] [Green Version]
- Nardelli, J.; Thiesson, D.; Fujiwara, Y.; Tsai, F.Y.; Orkin, S.H. Expression and genetic interaction of transcription factors GATA-2 and GATA-3 during development of the mouse central nervous system. Dev. Biol. 1999, 210, 305–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huber, T.L.; Zhou, Y.; Mead, P.E.; Zon, L.I. Cooperative effects of growth factors involved in the induction of hematopoietic mesoderm. Blood 1998, 92, 4128–4137. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Lu, S.; Vida, L.; Thomson, J.A.; Honig, G.R. Bone morphogenetic protein 4 induces efficient hematopoietic differentiation of rhesus monkey embryonic stem cells in vitro. Blood 2001, 98, 335–342. [Google Scholar] [CrossRef] [Green Version]
- Chiba, S.; Takeshita, K.; Imai, Y.; Kumano, K.; Kurokawa, M.; Masuda, S.; Shimizu, K.; Nakamura, S.; Ruddle, F.H.; Hirai, H. Homeoprotein DLX-1 interacts with Smad4 and blocks a signaling pathway from activin A in hematopoietic cells. Proc. Natl. Acad. Sci. USA 2003, 100, 15577–15582. [Google Scholar] [CrossRef] [Green Version]
- Woodside, K.J.; Shen, H.; Muntzel, C.; Daller, J.A.; Sommers, C.L.; Love, P.E. Expression of Dlx and Lhx family homeobox genes in fetal thymus and thymocytes. Gene Expr. Patterns 2004, 4, 315–320. [Google Scholar] [CrossRef]
- Sunwoo, J.B.; Kim, S.; Yang, L.; Naik, T.; Higuchi, D.A.; Rubenstein, J.L.; Yokoyama, W.M. Distal-less homeobox transcription factors regulate development and maturation of natural killer cells. Proc. Natl. Acad. Sci. USA 2008, 105, 10877–10882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, J.H.; Haggadone, M.D.; Sunwoo, J.B. Transcription factor Dlx3 induces aryl hydrocarbon receptor promoter activity. Biochem. Biophys. Rep. 2016, 7, 353–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimamoto, T.; Nakamura, S.; Bollekens, J.; Ruddle, F.H.; Takeshita, K. Inhibition of DLX-7 homeobox gene causes decreased expression of GATA-1 and c-myc genes and apoptosis. Proc. Natl. Acad. Sci. USA 1997, 94, 3245–3249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trinh, B.Q.; Barengo, N.; Kim, S.B.; Lee, J.S.; Zweidler-McKay, P.A.; Naora, H. The homeobox gene DLX4 regulates erythro-megakaryocytic differentiation by stimulating IL-1beta and NF-kappaB signaling. J. Cell Sci. 2015, 128, 3055–3067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagel, S.; Pommerenke, C.; Meyer, C.; Kaufmann, M.; MacLeod, R.A.F.; Drexler, H.G. NKL homeobox gene MSX1 acts like a tumor suppressor in NK-cell leukemia. Oncotarget 2017, 8, 66815–66832. [Google Scholar] [CrossRef] [Green Version]
- Nagel, S.; Pommerenke, C.; Scherr, M.; Meyer, C.; Kaufmann, M.; Battmer, K.; MacLeod, R.A.; Drexler, H.G. NKL homeobox gene activities in hematopoietic stem cells, T-cell development and T-cell leukemia. PLoS ONE 2017, 12, e0171164. [Google Scholar] [CrossRef] [PubMed]
- Starkova, J.; Gadgil, S.; Qiu, Y.H.; Zhang, N.; Hermanova, I.; Kornblau, S.M.; Drabkin, H.A. Up-regulation of homeodomain genes, DLX1 and DLX2, by FLT3 signaling. Haematologica 2011, 96, 820–828. [Google Scholar] [CrossRef]
- Ferrari, N.; Palmisano, G.L.; Paleari, L.; Basso, G.; Mangioni, M.; Fidanza, V.; Albini, A.; Croce, C.M.; Levi, G.; Brigati, C. DLX genes as targets of ALL-1: DLX 2,3,4 down-regulation in t(4;11) acute lymphoblastic leukemias. J. Leukoc. Biol. 2003, 74, 302–305. [Google Scholar] [CrossRef]
- Campo Dell’Orto, M.; Banelli, B.; Giarin, E.; Accordi, B.; Trentin, L.; Romani, M.; te Kronnie, G.; Basso, G. Down-regulation of DLX3 expression in MLL-AF4 childhood lymphoblastic leukemias is mediated by promoter region hypermethylation. Oncol. Rep. 2007, 18, 417–423. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.J.; Xu, Z.J.; Gu, Y.; Wen, X.M.; Ma, J.C.; Zhang, W.; Deng, Z.Q.; Leng, J.Y.; Qian, J.; Lin, J.; et al. Identification and validation of prognosis-related DLX5 methylation as an epigenetic driver in myeloid neoplasms. Clin. Transl. Med. 2020, 10, e29. [Google Scholar] [CrossRef]
- Tan, Y.; Timakhov, R.A.; Rao, M.; Altomare, D.A.; Xu, J.; Liu, Z.; Gao, Q.; Jhanwar, S.C.; Di Cristofano, A.; Wiest, D.L.; et al. A novel recurrent chromosomal inversion implicates the homeobox gene Dlx5 in T-cell lymphomas from Lck-Akt2 transgenic mice. Cancer Res. 2008, 68, 1296–1302. [Google Scholar] [CrossRef] [Green Version]
- Tan, Y.; Sementino, E.; Xu, J.; Pei, J.; Liu, Z.; Ito, T.K.; Cai, K.Q.; Peri, S.; Klein-Szanto, A.J.; Wiest, D.L.; et al. The homeoprotein Dlx5 drives murine T-cell lymphomagenesis by directly transactivating Notch and upregulating Akt signaling. Oncotarget 2017, 8, 14941–14956. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Sementino, E.; Pei, J.; Kadariya, Y.; Ito, T.K.; Testa, J.R. Co-targeting of Akt and Myc inhibits viability of lymphoma cells from Lck-Dlx5 mice. Cancer Biol. Ther. 2015, 16, 580–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, O.H.; Erbilgin, Y.; Firtina, S.; Celkan, T.; Karakas, Z.; Aydogan, G.; Turkkan, E.; Yildirmak, Y.; Timur, C.; Zengin, E.; et al. Deregulated WNT signaling in childhood T-cell acute lymphoblastic leukemia. Blood Cancer J. 2014, 4, e192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Z.; Dose, M.; Kovalovsky, D.; Chang, R.; O’Neil, J.; Look, A.T.; von Boehmer, H.; Khazaie, K.; Gounari, F. Beta-catenin stabilization stalls the transition from double-positive to single-positive stage and predisposes thymocytes to malignant transformation. Blood 2007, 109, 5463–5472. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Sementino, E.; Liu, Z.; Cai, K.Q.; Testa, J.R. Wnt signaling mediates oncogenic synergy between Akt and Dlx5 in T-cell lymphomagenesis by enhancing cholesterol synthesis. Sci. Rep. 2020, 10, 15837. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Q.; Sack, L.; Kang, C.; Elledge, S.J. A gain-of-function senescence bypass screen identifies the homeobox transcription factor DLX2 as a regulator of ATM-p53 signaling. Genes Dev. 2016, 30, 293–306. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, M.; Maass, D.; Tiwari, N.; Waldmeier, L.; Schmidt, P.; Lehembre, F.; Christofori, G. Transcription factor Dlx2 protects from TGFbeta-induced cell-cycle arrest and apoptosis. EMBO J. 2011, 30, 4489–4499. [Google Scholar] [CrossRef] [Green Version]
- Nieto, M.A. The snail superfamily of zinc-finger transcription factors. Nat. Rev. Mol. Cell Biol. 2002, 3, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Jeon, H.M.; Ju, M.K.; Jeong, E.K.; Kim, C.H.; Yoo, M.A.; Park, H.G.; Han, S.I.; Kang, H.S. Dlx-2 is implicated in TGF-beta- and Wnt-induced epithelial-mesenchymal, glycolytic switch, and mitochondrial repression by Snail activation. Int. J. Oncol. 2015, 46, 1768–1780. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Jeon, H.M.; Ju, M.K.; Jeong, E.K.; Kim, C.H.; Park, H.G.; Han, S.I.; Kang, H.S. Dlx-2 and glutaminase upregulate epithelial-mesenchymal transition and glycolytic switch. Oncotarget 2016, 7, 7925–7939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, Z.H.; Bao, Z.S.; Yan, W.; Liu, Y.W.; Zhang, C.B.; Wang, H.J.; Feng, Y.; Wang, Y.Z.; Zhang, W.; You, G.; et al. Upregulation of DLX2 confers a poor prognosis in glioblastoma patients by inducing a proliferative phenotype. Curr. Mol. Med. 2013, 13, 438–445. [Google Scholar]
- Lee, S.Y.; Jeon, H.M.; Kim, C.H.; Ju, M.K.; Bae, H.S.; Park, H.G.; Lim, S.C.; Han, S.I.; Kang, H.S. Homeobox gene Dlx-2 is implicated in metabolic stress-induced necrosis. Mol. Cancer 2011, 10, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morini, M.; Astigiano, S.; Gitton, Y.; Emionite, L.; Mirisola, V.; Levi, G.; Barbieri, O. Mutually exclusive expression of DLX2 and DLX5/6 is associated with the metastatic potential of the human breast cancer cell line MDA-MB-231. BMC Cancer 2010, 10, 649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trinh, B.Q.; Barengo, N.; Naora, H. Homeodomain protein DLX4 counteracts key transcriptional control mechanisms of the TGF-beta cytostatic program and blocks the antiproliferative effect of TGF-beta. Oncogene 2011, 30, 2718–2729. [Google Scholar] [CrossRef] [Green Version]
- Trinh, B.Q.; Ko, S.Y.; Barengo, N.; Lin, S.Y.; Naora, H. Dual functions of the homeoprotein DLX4 in modulating responsiveness of tumor cells to topoisomerase II-targeting drugs. Cancer Res. 2013, 73, 1000–1010. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Yang, M.; Gan, L.; He, T.; Xiao, X.; Stewart, M.D.; Liu, X.; Yang, L.; Zhang, T.; Zhao, Y.; et al. DLX4 upregulates TWIST and enhances tumor migration, invasion and metastasis. Int. J. Biol. Sci. 2012, 8, 1178–1187. [Google Scholar] [CrossRef]
- Torresan, C.; Oliveira, M.M.; Pereira, S.R.; Ribeiro, E.M.; Marian, C.; Gusev, Y.; Lima, R.S.; Urban, C.A.; Berg, P.E.; Haddad, B.R.; et al. Increased copy number of the DLX4 homeobox gene in breast axillary lymph node metastasis. Cancer Genet. 2014, 207, 177–187. [Google Scholar] [CrossRef]
- Trinh, B.; Ko, S.Y.; Haria, D.; Barengo, N.; Naora, H. The homeoprotein DLX4 controls inducible nitric oxide synthase-mediated angiogenesis in ovarian cancer. Mol. Cancer. 2015, 14, 97. [Google Scholar] [CrossRef] [Green Version]
- Haria, D.; Trinh, B.Q.; Ko, S.Y.; Barengo, N.; Liu, J.; Naora, H. The homeoprotein DLX4 stimulates NF-kappaB activation and CD44-mediated tumor-mesothelial cell interactions in ovarian cancer. Am. J. Pathol. 2015, 185, 2298–2308. [Google Scholar] [CrossRef]
- Xu, J.; Testa, J.R. DLX5 (distal-less homeobox 5) promotes tumor cell proliferation by transcriptionally regulating MYC. J. Biol. Chem. 2009, 284, 20593–20601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, Y.; Cheung, M.; Pei, J.; Menges, C.W.; Godwin, A.K.; Testa, J.R. Upregulation of DLX5 promotes ovarian cancer cell proliferation by enhancing IRS-2-AKT signaling. Cancer Res. 2010, 70, 9197–9206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rauch, T.; Wang, Z.; Zhang, X.; Zhong, X.; Wu, X.; Lau, S.K.; Kernstine, K.H.; Riggs, A.D.; Pfeifer, G.P. Homeobox gene methylation in lung cancer studied by genome-wide analysis with a microarray-based methylated CpG island recovery assay. Proc. Natl. Acad. Sci. USA 2007, 104, 5527–5532. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.; Yang, F.; Zhu, Y.; Zhang, S. KDM4A promotes the growth of non-small cell lung cancer by mediating the expression of Myc via DLX5 through the Wnt/beta-catenin signaling pathway. Life Sci. 2020, 262, 118508. [Google Scholar] [CrossRef]
- Kato, T.; Sato, N.; Takano, A.; Miyamoto, M.; Nishimura, H.; Tsuchiya, E.; Kondo, S.; Nakamura, Y.; Daigo, Y. Activation of placenta-specific transcription factor distal-less homeobox 5 predicts clinical outcome in primary lung cancer patients. Clin. Cancer Res. 2008, 14, 2363–2370. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, N.; Mortensen, S.; Sorensen, S.B.; Pedersen, M.W.; Rieneck, K.; Bovin, L.F.; Poulsen, H.S. Transcriptional gene expression profiling of small cell lung cancer cells. Cancer Res. 2003, 63, 1943–1953. [Google Scholar] [PubMed]
- Maxwell, G.L.; Chandramouli, G.V.; Dainty, L.; Litzi, T.J.; Berchuck, A.; Barrett, J.C.; Risinger, J.I. Microarray analysis of endometrial carcinomas and mixed mullerian tumors reveals distinct gene expression profiles associated with different histologic types of uterine cancer. Clin. Cancer Res. 2005, 11, 4056–4066. [Google Scholar] [CrossRef] [Green Version]
- Bai, P.; Li, W.; Wan, Z.; Xiao, Y.; Xiao, W.; Wang, X.; Wu, Z.; Zhang, K.; Wang, Y.; Chen, B.; et al. miR-489-3p inhibits prostate cancer progression by targeting DLX1. Cancer Manag. Res. 2020, 12, 2719–2729. [Google Scholar] [CrossRef] [Green Version]
- Xie, X.H.; Xu, X.P.; Sun, C.Y.; Yu, Z.J. Regulation of the oncogenic function of distal-less 4 by microRNA-122 in hepatocellular carcinoma. Mol. Med. Rep. 2015, 12, 1375–1380. [Google Scholar] [CrossRef] [PubMed]
- Larroux, C.; Fahey, B.; Degnan, S.M.; Adamski, M.; Rokhsar, D.S.; Degnan, B.M. The NK homeobox gene cluster predates the origin of Hox genes. Curr. Biol. 2007, 17, 706–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, Y.; Testa, J.R. DLX Genes: Roles in Development and Cancer. Cancers 2021, 13, 3005. https://doi.org/10.3390/cancers13123005
Tan Y, Testa JR. DLX Genes: Roles in Development and Cancer. Cancers. 2021; 13(12):3005. https://doi.org/10.3390/cancers13123005
Chicago/Turabian StyleTan, Yinfei, and Joseph R. Testa. 2021. "DLX Genes: Roles in Development and Cancer" Cancers 13, no. 12: 3005. https://doi.org/10.3390/cancers13123005
APA StyleTan, Y., & Testa, J. R. (2021). DLX Genes: Roles in Development and Cancer. Cancers, 13(12), 3005. https://doi.org/10.3390/cancers13123005