Anosmia but Not Ageusia as a COVID-19-Related Symptom among Cancer Patients—First Results from the PAPESCO-19 Cohort Study
Abstract
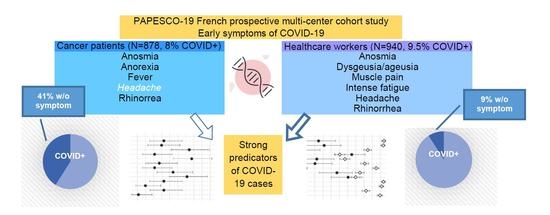
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Participants
2.3. Data Collection
2.3.1. Study Questionnaires
2.3.2. Blood Samples, Serological Tests and Routine RT-PCR Reported
2.3.3. Reported Symptoms
2.4. COVID-19 Test Outcomes and Symptoms
2.5. Statistical Analysis
3. Results
3.1. Population Characteristics
3.2. COVID-19 Outcomes
3.3. Single Symptoms
3.4. Combined Symptoms Predicting COVID-19
3.5. Model Validation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bakouny, Z.; Hawley, J.E.; Choueiri, T.K.; Peters, S.; Rini, B.I.; Warner, J.L.; Painter, C.A. COVID-19 and Cancer: Current Challenges and Perspectives. Cancer Cell 2020, 38, 629–646. [Google Scholar] [CrossRef]
- Lee, L.Y.W.; Cazier, J.-B.; Starkey, T.; Briggs, S.E.W.; Arnold, R.; Bisht, V.; Booth, S.; Campton, N.A.; Cheng, V.W.T.; Collins, G.; et al. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: A prospective cohort study. Lancet Oncol. 2020, 21, 1309–1316. [Google Scholar] [CrossRef]
- Dai, M.; Liu, D.; Liu, M.; Zhou, F.; Li, G.; Chen, Z.; Zhang, Z.; You, H.; Wu, M.; Zheng, Q.; et al. Patients with Cancer Appear more vulnerable to SARS-CoV-2: A Multicenter study during the COVID-19 outbreak. Cancer Discov. 2020, 10, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Berghoff, A.S.; Gansterer, M.; Bathke, A.C.; Trutschnig, W.; Hungerländer, P.; Berger, J.M.; Kreminger, J.; Starzer, A.M.; Strassl, R.; Schmidt, R.; et al. SARS-CoV-2 testing in patients with cancer treated at a tertiary care hospital during the COVID-19 pandemic. J. Clin. Oncol. 2020, 38, 3547–3554. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, H.; Wang, W.; Liu, Q.; Zhu, C. Clinical risk factors for mortality in patients with cancer and COVID-19: A systematic review and meta-analysis of recent observational studies. Null 2020, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.Y.; Cazier, J.-B.; Angelis, V.; Arnold, R.; Bisht, V.; Campton, N.A.; Chackathayil, J.; Cheng, V.W.; Curley, H.M.; Fittall, M.W.; et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: A prospective cohort study. Lancet 2020, 395, 1919–1926. [Google Scholar] [CrossRef]
- Albiges, L.; Foulon, S.; Bayle, A.; Gachot, B.; Pommeret, F.; Willekens, C.; Stoclin, A.; Merad, M.; Griscelli, F.; Lacroix, L. Determinants of the outcomes of patients with cancer infected with SARS-CoV-2: Results from the gustave roussy cohort. Nat. Cancer 2020, 1, 965–975. [Google Scholar] [CrossRef]
- Lièvre, A.; Turpin, A.; Ray-Coquard, I.; Le Malicot, K.; Thariat, J.; Ahle, G.; Neuzillet, C.; Paoletti, X.; Bouché, O.; Aldabbagh, K.; et al. Risk factors for coronavirus disease 2019 (COVID-19) severity and mortality among solid cancer patients and impact of the disease on anticancer treatment: A French nationwide cohort study (GCO-002 CACOVID-19). Eur. J. Cancer 2020, 141, 62–81. [Google Scholar] [CrossRef]
- Bénézit, F.; Le Turnier, P.; Declerck, C.; Paillé, C.; Revest, M.; Dubée, V.; Tattevin, P.; Arvieux, C.; Baldeyrou, M.; Chapplain, J.-M. Utility of hyposmia and hypogeusia for the diagnosis of COVID-19. Lancet Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Iravani, B.; Arshamian, A.; Ravia, A.; Mishor, E.; Snitz, K.; Shushan, S.; Roth, Y.; Perl, O.; Honigstein, D.; Weissgross, R. Relationship between odor intensity estimates and COVID-19 prevalence prediction in a Swedish population. Chem. Senses 2020. [Google Scholar] [CrossRef]
- Menni, C.; Valdes, A.M.; Freidin, M.B.; Sudre, C.H.; Nguyen, L.H.; Drew, D.A.; Ganesh, S.; Varsavsky, T.; Cardoso, M.J.; El-Sayed Moustafa, J.S.; et al. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat. Med. 2020, 26, 1037–1040. [Google Scholar] [CrossRef]
- Lechien, J.R.; Chiesa-Estomba, C.M.; De Siati, D.R.; Horoi, M.; Le Bon, S.D.; Rodriguez, A.; Dequanter, D.; Blecic, S.; El Afia, F.; Distinguin, L. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the Coronavirus Disease (COVID-19): A multicenter european study. Eur. Arch. Oto Rhino Laryngol. 2020, 1–11. [Google Scholar] [CrossRef]
- Petersen, I.; Phillips, A. Three quarters of people with SARS-CoV-2 infection are asymptomatic: Analysis of English household survey data. Clin. Epidemiol. 2020, 12, 1039. [Google Scholar] [CrossRef] [PubMed]
- Pierron, D.; Pereda-Loth, V.; Mantel, M.; Moranges, M.; Bignon, E.; Alva, O.; Kabous, J.; Heiske, M.; Pacalon, J.; David, R.; et al. Smell and taste changes are early indicators of the COVID-19 pandemic and political decision effectiveness. Nat. Commun. 2020, 11, 5152. [Google Scholar] [CrossRef] [PubMed]
- Roland, L.T.; Gurrola, J.G.; Loftus, P.A.; Cheung, S.W.; Chang, J.L. Smell and taste symptom-based predictive model for COVID-19 diagnosis. Int. Forum Allergy Rhinol. 2020, 10, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.; Pottinger, G.; Scott, A.; Hopkins, C. Anosmia and loss of smell in the era of Covid-19. BMJ 2020, 370. [Google Scholar] [CrossRef]
- Carrillo-Larco, R.M.; Altez-Fernandez, C. Anosmia and Dysgeusia in COVID-19: A systematic review. Wellcome Open Res. 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Steinbach, S.; Hummel, T.; Böhner, C.; Berktold, S.; Hundt, W.; Kriner, M.; Heinrich, P.; Sommer, H.; Hanusch, C.; Prechtl, A.; et al. Qualitative and quantitative assessment of taste and smell changes in patients undergoing chemotherapy for breast cancer or gynecologic malignancies. JCO 2009, 27, 1899–1905. [Google Scholar] [CrossRef] [PubMed]
- De Vries, Y.C.; Boesveldt, S.; Kelfkens, C.S.; Posthuma, E.E.; van den Berg, M.M.G.A.; de Kruif, J.T.C.M.; Haringhuizen, A.; Sommeijer, D.W.; Buist, N.; Grosfeld, S.; et al. Taste and smell perception and quality of life during and after systemic therapy for breast cancer. Breast Cancer Res. Treat. 2018, 170, 27–34. [Google Scholar] [CrossRef] [Green Version]
- McGreevy, J.; Orrevall, Y.; Belqaid, K.; Wismer, W.; Tishelman, C.; Bernhardson, B.-M. Characteristics of taste and smell alterations reported by patients after starting treatment for lung cancer. Support. Care Cancer 2014, 22, 2635–2644. [Google Scholar] [CrossRef] [Green Version]
- Hong, J.; Omur-Ozbek, P.; Stanek, B.; Dietrich, A.; Duncan, S.; Lee, Y.; Lesser, G. Taste and odor abnormalities in cancer patients. J. Support Oncol. 2009, 7, 58–65. [Google Scholar] [PubMed]
- Gamper, E.-M.; Zabernigg, A.; Wintner, L.M.; Giesinger, J.M.; Oberguggenberger, A.; Kemmler, G.; Sperner-Unterweger, B.; Holzner, B. Coming to your senses: Detecting taste and smell alterations in chemotherapy patients. A systematic review. J. Pain Symptom. Manag. 2012, 44, 880–895. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Camacho, M.; Gonella, S.; Campbell, S.; Scrimger, R.A.; Wismer, W.V. A systematic review of smell alterations after radiotherapy for head and neck cancer. Cancer Treat. Rev. 2017, 54, 110–121. [Google Scholar] [CrossRef]
- Russell, B.; Moss, C.; Rigg, A.; Hopkins, C.; Papa, S.; Van Hemelrijck, M. Anosmia and Ageusia are emerging as symptoms in patients with COVID-19: What does the current evidence say? Ecancermedicalscience 2020, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, L.H.; Drew, D.A.; Graham, M.S.; Joshi, A.D.; Guo, C.-G.; Ma, W.; Mehta, R.S.; Warner, E.T.; Sikavi, D.R.; Lo, C.-H.; et al. Risk of COVID-19 among front-line health-care workers and the general community: A prospective cohort study. Lancet Publ. Health 2020, 5, e475–e483. [Google Scholar] [CrossRef]
- Rudberg, A.-S.; Havervall, S.; Månberg, A.; Falk, A.J.; Aguilera, K.; Ng, H.; Gabrielsson, L.; Salomonsson, A.-C.; Hanke, L.; Murrell, B. SARS-CoV-2 Exposure, Symptoms and Seroprevalence in Healthcare Workers in Sweden. Nat. Commun. 2020, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ladoire, S.; Goussot, V.; Redersdorff, E.; Cueff, A.; Ballot, E.; Truntzer, C.; Ayati, S.; Bengrine-Lefevre, L.; Bremaud, N.; Coudert, B.; et al. Seroprevalence of SARS-CoV-2 among the staff and patients of a french cancer centre after first lockdown: The CanSEROcov Study. Eur. J. Cancer 2021, 148, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Favara, D.M.; McAdam, K.; Cooke, A.; Bordessa-Kelly, A.; Budriunaite, I.; Bossingham, S.; Houghton, S.; Doffinger, R.; Ainsworth, N.; Corrie, P.G. SARS-CoV-2 infection and antibody seroprevalence among UK Healthcare professionals working with cancer patients during the first wave of the COVID-19 pandemic. Clin. Oncol. R Coll. Radiol. 2021. [Google Scholar] [CrossRef]
- Institut Cancerologie de l’Ouest. Patients and Health Staff of Cancer Centres During the Covid-19 Pandemic: Constitution of a Biological Collection Linked to a Prospective, Multicenter Cohort Study; US National Library of Medicine: Bethsedan, MD, USA, 2020.
- Cauchemez, S.; Kiem, C.T.; Paireau, J.; Rolland, P.; Fontanet, A. lockdown impact on COVID-19 epidemics in regions across metropolitan France. Lancet 2020, 396, 1068–1069. [Google Scholar] [CrossRef]
- Nicol, T.; Lefeuvre, C.; Serri, O.; Pivert, A.; Joubaud, F.; Dubée, V.; Kouatchet, A.; Ducancelle, A.; Lunel-Fabiani, F.; Le Guillou-Guillemette, H. Assessment of SARS-CoV-2 serological tests for the diagnosis of COVID-19 through the evaluation of three immunoassays: Two Automated immunoassays (euroimmun and abbott) and one rapid lateral flow immunoassay (NG Biotech). J. Clin. Virol. 2020, 129, 104511. [Google Scholar] [CrossRef]
- Sweeney, N.; Merrick, B.; Galão, R.P.; Pickering, S.; Botgros, A.; Wilson, H.D.; Signell, A.W.; Betancor, G.; Tan, M.K.I.; Ramble, J.; et al. Clinical utility of targeted SARS-CoV-2 serology testing to aid the diagnosis and management of suspected missed, late or post-COVID-19 infection syndromes: Results from a pilot service implemented during the first pandemic wave. PLoS ONE 2021, 16, e0249791. [Google Scholar] [CrossRef]
- Ghaffari, A.; Meurant, R.; Ardakani, A. COVID-19 serological tests: How well do they actually perform? Diagnostics 2020, 10, 453. [Google Scholar] [CrossRef] [PubMed]
- Pickering, S.; Betancor, G.; Galão, R.P.; Merrick, B.; Signell, A.W.; Wilson, H.D.; Ik, M.T.K.; Seow, J.; Graham, C.; Acors, S.; et al. Comparative assessment of multiple COVID-19 serological technologies supports continued evaluation of point-of-care lateral flow assays in hospital and community healthcare settings. PLoS Pathog. 2020, 16, e1008817. [Google Scholar] [CrossRef]
- Marklund, E.; Leach, S.; Axelsson, H.; Nyström, K.; Norder, H.; Bemark, M.; Angeletti, D.; Lundgren, A.; Nilsson, S.; Andersson, L.-M.; et al. Serum-IgG Responses to SARS-CoV-2 after mild and severe COVID-19 infection and analysis of IgG non-responders. PLoS ONE 2020, 15. [Google Scholar] [CrossRef] [PubMed]
- WHO. Coronavirus-Symptoms. Available online: https://www.who.int/westernpacific/health-topics/coronavirus (accessed on 15 January 2021).
- French Ministry of Health (Ministère des Solidarités et de la Santé). Les Réponses à Vos Questions Sur la COVID-19. Available online: https://solidarites-sante.gouv.fr/soins-et-maladies/maladies/maladies-infectieuses/coronavirus/tout-savoir-sur-la-covid-19/article/les-reponses-a-vos-questions-sur-la-covid-19 (accessed on 15 January 2021).
- Hanson, K.E.; Caliendo, A.M.; Arias, C.A.; Hayden, M.K.; Englund, J.A.; Lee, M.J.; Loeb, M.; Patel, R.; El Alayli, A.; Altayar, O.; et al. The infectious diseases society of america guidelines on the diagnosis of COVID-19: Molecular diagnostic testing. Clin. Infect. Dis. 2021. [Google Scholar] [CrossRef]
- Hosmer, D.W., Jr.; Lemeshow, S.; Sturdivant, R.X. Applied Logistic Regression; John Wiley & Sons: Hoboken, NJ, USA, 2013; Volume 398, ISBN 0-470-58247-2. [Google Scholar]
- Louviere, J.J.; Hensher, D.A.; Swait, J.D. Stated Choice Methods: Analysis and Applications; Cambridge University Press: Cambridge, UK, 2000; ISBN 0-521-78830-7. [Google Scholar]
- Basse, C.; Diakite, S.; Servois, V.; Frelaut, M.; Noret, A.; Bellesoeur, A.; Moreau, P.; Massiani, M.-A.; Bouyer, A.-S.; Vuagnat, P.; et al. Characteristics and outcome of SARS-CoV-2 infection in cancer patients. JNCI Cancer Spectr. 2021, 5. [Google Scholar] [CrossRef]
- Pullano, G.; Di Domenico, L.; Sabbatini, C.E.; Valdano, E.; Turbelin, C.; Debin, M.; Guerrisi, C.; Kengne-Kuetche, C.; Souty, C.; Hanslik, T.; et al. Underdetection of COVID-19 cases in france threatens epidemic control. Nature 2020, 1–9. [Google Scholar] [CrossRef]
- Carrat, F.; Touvier, M.; Severi, G.; Meyer, L.; Jusot, F.; Lapidus, N.; Rahib, D.; Lydié, N.; Charles, M.-A.; Ancel, P.-Y.; et al. Incidence and risk factors of COVID-19-like symptoms in the french general population during the lockdown period: A multi-cohort study. BMC Infect. Dis. 2021, 21, 169. [Google Scholar] [CrossRef]
- Kluytmans-van den Bergh, M.F.Q.; Buiting, A.G.M.; Pas, S.D.; Bentvelsen, R.G.; van den Bijllaardt, W.; van Oudheusden, A.J.G.; van Rijen, M.M.L.; Verweij, J.J.; Koopmans, M.P.G.; Kluytmans, J.A.J.W. Prevalence and clinical presentation of health care workers with symptoms of Coronavirus disease 2019 in 2 dutch hospitals during an early phase of the pandemic. JAMA Netw. Open 2020, 3, e209673. [Google Scholar] [CrossRef]
- Delfraissy, J.F.; Atlani Duault, L.; Benamouzig, D.; Bouadma, L.; Cauchemez, S.; Chauvin, F.; Druais, P.L.; Fontanet, A.; Grard, M.A.; Hoang, A.; et al. Avis Du Conseil Scientifique COVID-19; 2020. Available online: https://www.vie-publique.fr/sites/default/files/rapport/pdf/avis_conseil_scientifique_12_mars_2020.pdf (accessed on 15 June 2021).
- Oved, K.; Olmer, L.; Shemer-Avni, Y.; Wolf, T.; Supino-Rosin, L.; Prajgrod, G.; Shenhar, Y.; Payorsky, I.; Cohen, Y.; Kohn, Y.; et al. Multi-center nationwide comparison of seven serology assays reveals a SARS-CoV-2 Non-responding seronegative subpopulation. EClinicalMedicine 2020, 29–30, 100651. [Google Scholar] [CrossRef]
- Ong, D.S.Y.; Fragkou, P.C.; Schweitzer, V.A.; Chemaly, R.F.; Moschopoulos, C.D.; Skevaki, C. How to interpret and use COVID-19 serology and immunology tests. Clin. Microbiol. Infect. 2021. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, Y.; Hu, M.; Wen, L.; Wen, C.; Wang, Y.; Zhu, W.; Tai, S.; Jiang, Z.; Xiao, K.; et al. Antibody seroconversion in asymptomatic and symptomatic patients infected with severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2). Clin. Translat. Immunol. 2020, 9, e1182. [Google Scholar] [CrossRef]
- Long, Q.-X.; Tang, X.-J.; Shi, Q.-L.; Li, Q.; Deng, H.-J.; Yuan, J.; Hu, J.-L.; Xu, W.; Zhang, Y.; Lv, F.-J.; et al. Clinical and Immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 2020, 26, 1200–1204. [Google Scholar] [CrossRef]
- Bastos, M.L.; Tavaziva, G.; Abidi, S.K.; Campbell, J.R.; Haraoui, L.-P.; Johnston, J.C.; Lan, Z.; Law, S.; MacLean, E.; Trajman, A.; et al. Diagnostic Accuracy of serological tests for Covid-19: Systematic review and meta-analysis. BMJ 2020, 370, m2516. [Google Scholar] [CrossRef]
- Mekonnen, D.; Mengist, H.M.; Derbie, A.; Nibret, E.; Munshea, A.; He, H.; Li, B.; Jin, T. Diagnostic accuracy of serological tests and kinetics of severe acute respiratory syndrome Coronavirus 2 antibody: A systematic review and meta-analysis. Rev. Med. Virol. 2021, 31, e2181. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.-P.; Lin, R.T.P.; Renia, L.; Ng, L.F.P. Serological approaches for COVID-19: Epidemiologic perspective on surveillance and control. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef]
- Doust, J.A.; Bell, K.J.L.; Leeflang, M.M.G.; Dinnes, J.; Lord, S.J.; Mallett, S.; van de Wijgert, J.H.H.M.; Sandberg, S.; Adeli, K.; Deeks, J.J.; et al. Guidance for the design and reporting of studies evaluating the clinical performance of tests for present or past SARS-CoV-2 infection. BMJ 2021, 372, n568. [Google Scholar] [CrossRef]


| Characteristics | Cancer Patients N (%) |
|---|---|
| N = 878 | |
| Sex | |
| Male | 275 (31.3) |
| Female | 603 (68.7) |
| Age | |
| Median (Range) | 62 (18–91) |
| 18–49 | 171 (19.5) |
| 50–64 | 334 (38.0) |
| 65–74 | 264 (30.1) |
| ≥75 | 109 (12.4) |
| BMI | |
| Median (Range) | 25 (17–43) |
| Obesity (BMI ≥ 30) | 141 (19.9) |
| missing data | 170 |
| Tobacco smoking status | |
| Non-smoker | 299 (47.8) |
| Former smoker | 228 (36.4) |
| Current smoker | 99 (15.8) |
| missing data | 252 |
| Public-facing role 1 | |
| No | 283 (58.1) |
| Yes | 204 (41.9) |
| missing data | 391 |
| No. of Comorbidities 2 | |
| ≥1 | 330 (40.7) |
| missing data | 67 |
| No. of Comedications 3 | |
| ≥1 | 235 (29.0) |
| missing data | 68 |
| Centers of inclusion | |
| Nantes | 201 (22.9) |
| Angers | 238 (27.1) |
| Clermont-Ferrand | 159 (18.1) |
| Nancy | 280 (31.9) |
| Symptoms 4 | |
| Symptomatic | 282 (32.1) |
| Asymptomatic | 596 (67.9) |
| COVID-19 tests 5 | |
| Any positive test | 70 (8.0) |
| Positive serological test | 59 (6.7) |
| Positive RT-PCR test | 26 (3.0) |
| Cancer Features | COVID− N (%) | COVID+ N (%) | Total N (%) |
|---|---|---|---|
| N = 808 | N = 70 | N = 878 | |
| Location | |||
| Breast | 335 (45) | 36 (54.5) | 371 (45.7) |
| Uterine. Endometrial. Cervical | 81 (10.9) | 5 (7.6) | 86 (10.6) |
| Colorectal | 32 (4.3) | 3 (4.5) | 35 (4.3) |
| Gastrointestinal | 22 (3) | 1 (1.5) | 23 (2.8) |
| Prostate | 59 (7.9) | 0 (0) | 59 (7.3) |
| Urological | 62 (8.3) | 6 (9.1) | 68 (8.4) |
| Lung | 65 (8.7) | 8 (12.1) | 73 (9) |
| Miscellaneous 1 | 89 (11.9) | 7 (10.6) | 96 (11.8) |
| missing data | 63 | 4 | 67 |
| Treatment status | |||
| Undergoing treatment | 782 (96.8) | 63 (90.0) | 845 (96.2) |
| Being monitored | 26 (3.2) | 7 (10.0) | 33 (3.8) |
| Stage | |||
| Localized | 196 (27.3) | 19 (31.7) | 215 (27.6) |
| Locally advanced | 122 (17.0) | 9 (15.0) | 131 (16.8) |
| Metastatic | 401 (55.8) | 32 (53.3) | 433 (55.6) |
| missing data | 89 | 10 | 99 |
| ECOG PS | |||
| 0 | 263 (41.9) | 21 (38.2) | 284 (41.6) |
| 1 | 333 (53.1) | 31 (56.4) | 364 (53.4) |
| ≥2 | 31 (4.9) | 3 (5.5) | 34 (5) |
| missing data | 181 | 15 | 196 |
| Years since the first cancer diagnosis | |||
| ≥1 year | 467 (62.9) | 44 (66.7) | 511 (63.2) |
| missing data | 65 | 4 | 69 |
| Last treatment before inclusion | |||
| Chemotherapy | 425 (58.7) | 37 (57.8) | 462 (58.6) |
| Immunotherapy | 113 (15.6) | 10 (15.6) | 123 (15.6) |
| Targeted therapy | 139 (19.2) | 16 (25.0) | 155 (19.7) |
| Hormone therapy | 88 (12.2) | 7 (10.9) | 95 (12.1) |
| Radiotherapy | 41 (5.7) | 2 (3.1) | 43 (5.5) |
| Surgery | 22 (3.0) | 4 (6.3) | 26 (3.3) |
| Miscellaneous | 23 (3.2) | 0 (0) | 23 (2.9) |
| missing data | 67 | 6 | 73 |
| Predictors | OR (95% CI) | Wald | p-Value 1 |
|---|---|---|---|
| Cancer Patients’ Full Model (N = 878) | |||
| Anosmia | 9.71 (2.99–31.57) | 14.3 | < 0.001 |
| Dysgeusia/Ageusia | 0.77 (0.26–2.35) | 0.21 | 0.651 |
| Fever | 3.23 (1.54–6.78) | 9.57 | 0.002 |
| Headache | 0.33 (0.12–0.90) | 4.75 | 0.029 |
| Rhinorrhea | 1.98 (0.95–4.10) | 3.36 | 0.067 |
| Cough | 0.61 (0.23–1.59) | 1.02 | 0.313 |
| Shortness of breath | 1.34 (0.48–3.72) | 0.32 | 0.575 |
| Muscle pain | 1.15 (0.50–2.67) | 0.11 | 0.738 |
| Intense fatigue | 1.17 (0.46–2.99) | 0.11 | 0.738 |
| Anorexia | 4.52 (1.69–12.09) | 9.03 | 0.003 |
| Red eyes | 1.16 (0.35–3.91) | 0.06 | 0.809 |
| Digestive disorders | 0.65 (0.25–1.66) | 0.82 | 0.366 |
| Chest pain | 0.42 (0.11–1.67) | 1.50 | 0.221 |
| Cancer Patients’ Final Model (N = 878) | |||
| Anosmia | 7.48 (2.96–18.89) | 18.12 | < 0.001 |
| Anorexia | 3.82 (1.66–8.76) | 9.99 | 0.002 |
| Fever | 3.07 (1.53–6.17) | 9.90 | 0.002 |
| Headache | 0.30 (0.12–0.76) | 6.49 | 0.011 |
| Rhinorrhea | 1.81 (0.93–3.51) | 3.08 | 0.079 |
| Healthcare Workers’ Full Model (N = 940) | |||
| Anosmia | 6.11 (2.26–16.49) | 12.76 | < 0.001 |
| Dysgeusia/Ageusia | 5.30 (1.96–14.34) | 10.78 | 0.001 |
| Fever | 0.65 (0.30–1.41) | 1.18 | 0.276 |
| Headache | 2.08 (0.96–4.48) | 3.47 | 0.062 |
| Rhinorrhea | 0.67 (0.33–1.34) | 1.29 | 0.256 |
| Cough | 1.26 (0.57–2.79) | 0.32 | 0.570 |
| Shortness of breath | 0.90 (0.37–2.20) | 0.05 | 0.825 |
| Muscle pain | 2.01 (0.91–4.41) | 3.01 | 0.083 |
| Intense fatigue | 2.05 (0.89–4.73) | 2.87 | 0.090 |
| Anorexia | 1.25 (0.47–3.30) | 0.20 | 0.654 |
| Red eyes | 0.91 (0.31–2.65) | 0.03 | 0.866 |
| Digestive disorders | 0.78 (0.38–1.60) | 0.47 | 0.494 |
| Chest pain | 2.61 (1.07–6.35) | 4.49 | 0.034 |
| Healthcare Workers’ Final Model (N = 940) | |||
| Anosmia | 5.71 (2.21–14.75) | 12.93 | < 0.001 |
| Dysgeusia/Ageusia | 5.14 (2.01–13.14) | 11.68 | 0.001 |
| Muscle pain | 1.75 (0.82–3.75) | 2.08 | 0.149 |
| Intense fatigue | 1.78 (0.85–3.72) | 2.34 | 0.126 |
| Headache | 1.88 (0.86–4.11) | 2.53 | 0.111 |
| Chest pain | 2.42 (1.11–5.27) | 4.95 | 0.026 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, K.; Blanc-Lapierre, A.; Seegers, V.; Boisdron-Celle, M.; Bigot, F.; Bourdon, M.; Mahammedi, H.; Lambert, A.; Campone, M.; Conroy, T.; et al. Anosmia but Not Ageusia as a COVID-19-Related Symptom among Cancer Patients—First Results from the PAPESCO-19 Cohort Study. Cancers 2021, 13, 3389. https://doi.org/10.3390/cancers13143389
Zhou K, Blanc-Lapierre A, Seegers V, Boisdron-Celle M, Bigot F, Bourdon M, Mahammedi H, Lambert A, Campone M, Conroy T, et al. Anosmia but Not Ageusia as a COVID-19-Related Symptom among Cancer Patients—First Results from the PAPESCO-19 Cohort Study. Cancers. 2021; 13(14):3389. https://doi.org/10.3390/cancers13143389
Chicago/Turabian StyleZhou, Ke, Audrey Blanc-Lapierre, Valérie Seegers, Michèle Boisdron-Celle, Frédéric Bigot, Marianne Bourdon, Hakim Mahammedi, Aurélien Lambert, Mario Campone, Thierry Conroy, and et al. 2021. "Anosmia but Not Ageusia as a COVID-19-Related Symptom among Cancer Patients—First Results from the PAPESCO-19 Cohort Study" Cancers 13, no. 14: 3389. https://doi.org/10.3390/cancers13143389
APA StyleZhou, K., Blanc-Lapierre, A., Seegers, V., Boisdron-Celle, M., Bigot, F., Bourdon, M., Mahammedi, H., Lambert, A., Campone, M., Conroy, T., Penault-Llorca, F., Bellanger, M. M., & Raoul, J. -L. (2021). Anosmia but Not Ageusia as a COVID-19-Related Symptom among Cancer Patients—First Results from the PAPESCO-19 Cohort Study. Cancers, 13(14), 3389. https://doi.org/10.3390/cancers13143389