Suppressive Effect and Molecular Mechanism of Houttuynia cordata Thunb. Extract against Prostate Carcinogenesis and Castration-Resistant Prostate Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Plant Sample Preparation
2.3. Identification of Phytochemicals by HPLC
2.4. Cell Viability Assay
2.5. Cell Cycle Assay
2.6. Apoptosis Assay
2.7. Western Blotting
2.8. Wound-Healing Assay
2.9. TRAP Model Experimental Protocol
2.10. Xenograft Study
2.11. Immunohistochemistry
2.12. Statistical Analysis
3. Results
3.1. Comparison of Phytochemical Compositions in HCT and EA by HPLC
3.2. HCT and EA Inhibit Cell Proliferation and Induce Apoptosis in Prostate Cancer Cell Lines
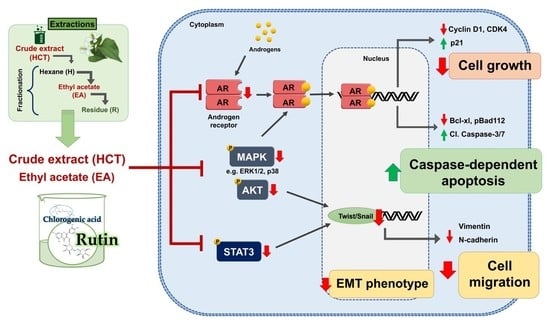
3.3. HCT and EA Induce Apoptosis via Suppressing AR Expression and Inactivating the Phosphorylation of AKT/ERK/p38 MAPK Pathways in Prostate Cancer Cells
3.4. Rutin Is a Responsible Component of HCT to Induce Apoptosis in LNCaP Cell Lines
3.5. HCT and EA Suppress EMT and Prostate Cancer Cell Migration through STAT3/Snail/Twist Pathways
3.6. Suppression of Prostate Carcinogenesis by HCT in the TRAP Model
3.7. HCT Triggers Apoptosis and Suppresses Cell Proliferation via AKT/ERK/p38 MAPK Pathways in the TRAP Model
3.8. HCT Inhibit Tumor Growth and Their Signaling in the PCai1 Xenograft Mice Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADT | Androgen deprivation therapy |
| AKT | Protein kinase B |
| AR | Androgen receptor |
| BPH | Benign prostatic hyperplasia |
| CRPC | Castration-resistant prostate cancer |
| DMSO | Dimethyl sulfoxide |
| EA | Ethyl acetate fraction of H. cordata |
| EMT | Epithelial-mesenchymal transition |
| ERK | Extracellular signal-regulated kinase |
| FBS | Fetal bovine serum |
| H | Hexane fraction of H. cordata |
| HCT | Houttuynia cordata Thunb. |
| HG-PIN | High-grade prostatic intraepithelial neoplasia |
| H&E | Hematoxylin and eosin |
| IHC | Immunohistochemical analysis |
| LG-PIN | Low-grade prostatic intraepithelial neoplasia |
| p38 MAPK | p38 mitogen-activated protein kinase |
| PARP | Poly (ADP-ribose) polymerase |
| R | Residue fraction of H. cordata |
| TRAP | Transgenic rat for adenocarcinoma of prostate |
| TUNEL | Terminal deoxynucleotidyl transferase dUTP nick end labelling |
References
- Culp, M.B.; Soerjomataram, I.; Efstathiou, J.A.; Bray, F.; Jemal, A. Recent global patterns in prostate cancer incidence and mortality rates. Eur. Urol. 2020, 77, 38–52. [Google Scholar] [CrossRef]
- Rawla, P. Epidemiology of prostate cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belev, B.; ŠIPIĆ, T. Pathophysiology of hormone-resistant prostate cancer. Period. Biol. 2014, 116, 387–392. [Google Scholar]
- Shen, M.M.; Abate-Shen, C. Molecular genetics of prostate cancer: New prospects for old challenges. Genes Dev. 2010, 24, 1967–2000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, M.-L.; Kyprianou, N. Androgen receptor and growth factor signaling cross-talk in prostate cancer cells. Endocr. Relat. Cancer 2008, 15, 841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, S.; Niu, Y.; Lee, S.O.; Chang, C. Androgen receptor (AR) positive vs. negative roles in prostate cancer cell deaths including apoptosis, anoikis, entosis, necrosis and autophagic cell death. Cancer Treat. Rev. 2014, 40, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Feldman, B.J.; Feldman, D. The development of androgen-independent prostate cancer. Nat. Rev. Cancer 2001, 1, 34–45. [Google Scholar] [CrossRef]
- Leung, J.K.; Sadar, M.D. Non-genomic actions of the androgen receptor in prostate cancer. Front. Endocrinol. 2017, 8, 2. [Google Scholar] [CrossRef] [Green Version]
- Pelekanou, V.; Notas, G.; Stathopoulos, E.N.; Castanas, E.; Kampa, M. Androgen receptors in early and castration resistant prostate cancer: Friend or foe? Hormones 2013, 12, 224–235. [Google Scholar] [CrossRef] [Green Version]
- Lonergan, P.E.; Tindall, D.J. Androgen receptor signaling in prostate cancer development and progression. J. Carcinog. 2011, 10. [Google Scholar] [CrossRef]
- George, V.C.; Dellaire, G.; Rupasinghe, H.V. Plant flavonoids in cancer chemoprevention: Role in genome stability. J. Nutr. Biochem. 2017, 45, 1–14. [Google Scholar] [CrossRef]
- Cicero, A.F.; Allkanjari, O.; Busetto, G.M.; Cai, T.; Larganà, G.; Magri, V.; Perletti, G.; Della Cuna, F.S.R.; Russo, G.I.; Stamatiou, K. Nutraceutical treatment and prevention of benign prostatic hyperplasia and prostate cancer. Arch. Ital. Urol. Androl. 2019, 91. [Google Scholar] [CrossRef]
- Park, J.-S.; Yeom, M.-H.; Park, W.-S.; Joo, K.-M.; Rho, H.-S.; Kim, D.H.; Chang, I.S. Enzymatic hydrolysis of green tea seed extract and its activity on 5α-reductase inhibition. Biosci. Biotechnol. Biochem. 2006, 70, 387–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiipakka, R.A.; Zhang, H.-Z.; Dai, W.; Dai, Q.; Liao, S. Structure–activity relationships for inhibition of human 5α-reductases by polyphenols. Biochem. Pharmacol. 2002, 63, 1165–1176. [Google Scholar] [CrossRef]
- Noh, S.; Choi, E.; Hwang, C.-H.; Jung, J.H.; Kim, S.-H.; Kim, B. Dietary compounds for targeting prostate cancer. Nutrients 2019, 11, 2401. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Prasad, S.K.; Hemalatha, S. A current update on the phytopharmacological aspects of Houttuynia cordata Thunb. Pharm. Rev. 2014, 8, 22–35. [Google Scholar] [CrossRef] [Green Version]
- Subhawa, S.; Chewonarin, T.; Banjerdpongchai, R. The effects of houttuynia cordata thunb and piper ribesioides wall extracts on breast carcinoma cell proliferation, migration, invasion and apoptosis. Molecules 2020, 25, 1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koyama, T. New botanical materials with anti-androgenic activity. In Prostate Cancer-Original Scientific Reports and Case Studies; Spiess, P.E., Ed.; IntechOpen: London, UK, 2011; pp. 193–206. [Google Scholar]
- Asamoto, M.; Hokaiwado, N.; Cho, Y.-M.; Takahashi, S.; Ikeda, Y.; Imaida, K.; Shirai, T. Prostate carcinomas developing in transgenic rats with SV40 T antigen expression under probasin promoter control are strictly androgen dependent. Cancer Res. 2001, 61, 4693–4700. [Google Scholar]
- Long, N.; Suzuki, S.; Sato, S.; Naiki-Ito, A.; Sakatani, K.; Shirai, T.; Takahashi, S. Purple corn color inhibition of prostate carcinogenesis by targeting cell growth pathways. Cancer Sci. 2013, 104, 298–303. [Google Scholar] [CrossRef]
- Naiki-Ito, A.; Chewonarin, T.; Tang, M.; Pitchakarn, P.; Kuno, T.; Ogawa, K.; Asamoto, M.; Shirai, T.; Takahashi, S. Ellagic acid, a component of pomegranate fruit juice, suppresses androgen-dependent prostate carcinogenesis via induction of apoptosis. Prostate 2015, 75, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Naiki-Ito, A.; Naiki, T.; Kato, H.; Iida, K.; Etani, T.; Nagayasu, Y.; Suzuki, S.; Yamashita, Y.; Inaguma, S.; Onishi, M. Recruitment of miR-8080 by luteolin inhibits androgen receptor splice variant 7 expression in castration-resistant prostate cancer. Carcinogenesis 2020, 41, 1145–1157. [Google Scholar] [CrossRef] [Green Version]
- Tang, M.; Asamoto, M.; Ogawa, K.; Naiki-Ito, A.; Sato, S.; Takahashi, S.; Shirai, T. Induction of apoptosis in the LNCaP human prostate carcinoma cell line and prostate adenocarcinomas of SV40T antigen transgenic rats by the Bowman-Birk inhibitor. Pathol. Int. 2009, 59, 790–796. [Google Scholar] [CrossRef]
- Naiki, T.; Asamoto, M.; Toyoda-Hokaiwado, N.; Naiki-Ito, A.; Tozawa, K.; Kohri, K.; Takahashi, S.; Shirai, T. Organ specific Gst-pi expression of the metastatic androgen independent prostate cancer cells in nude mice. Prostate 2012, 72, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Hokaiwado, N.; Takeshita, F.; Naiki-Ito, A.; Asamoto, M.; Ochiya, T.; Shirai, T. Glutathione S-transferase Pi mediates proliferation of androgen-independent prostate cancer cells. Carcinogenesis 2008, 29, 1134–1138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeewa, R.; Naiki-Ito, A.; Naiki, T.; Kato, H.; Suzuki, S.; Chewonarin, T.; Takahashi, S. Hexane insoluble fraction from purple rice extract retards carcinogenesis and castration-resistant cancer growth of prostate through suppression of androgen receptor mediated cell proliferation and metabolism. Nutrients 2020, 12, 558. [Google Scholar] [CrossRef] [Green Version]
- Naiki, T.; Naiki-Ito, A.; Asamoto, M.; Kawai, N.; Tozawa, K.; Etani, T.; Sato, S.; Suzuki, S.; Shirai, T.; Kohri, K. GPX2 overexpression is involved in cell proliferation and prognosis of castration-resistant prostate cancer. Carcinogenesis 2014, 35, 1962–1967. [Google Scholar] [CrossRef]
- Woranam, K.; Senawong, G.; Utaiwat, S.; Yunchalard, S.; Sattayasai, J.; Senawong, T. Anti-inflammatory activity of the dietary supplement Houttuynia cordata fermentation product in RAW264.7 cells and Wistar rats. PLoS ONE 2020, 15, e0230645. [Google Scholar] [CrossRef] [Green Version]
- Strober, W. Trypan blue exclusion test of cell viability. Curr. Protoc. Immunol. 2015, 111, A3.B.1–A3.B.3. [Google Scholar] [CrossRef]
- Ito, Y.; Naiki-Ito, A.; Kato, H.; Suzuki, S.; Kuno, T.; Ishiguro, Y.; Takahashi, S.; Uemura, H. Chemopreventive effects of angiotensin II receptor type 2 agonist on prostate carcinogenesis by the down-regulation of the androgen receptor. Oncotarget 2018, 9, 13859–13869. [Google Scholar] [CrossRef] [Green Version]
- Sato, S.; Takahashi, S.; Asamoto, M.; Naiki, T.; Naiki-Ito, A.; Asai, K.; Shirai, T. Tranilast suppresses prostate cancer growth and osteoclast differentiation in vivo and in vitro. Prostate 2010, 70, 229–238. [Google Scholar] [CrossRef]
- Yang, H.; Murthy, S.; Sarkar, F.H.; Sheng, S.; Reddy, G.P.V.; Dou, Q.P. Calpain-mediated androgen receptor breakdown in apoptotic prostate cancer cells. J. Cell. Physiol. 2008, 217, 569–576. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Liu, W.-Z.; Liu, T.; Feng, X.; Yang, N.; Zhou, H.-F. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J. Recept. Signal. Transduct. 2015, 35, 600–604. [Google Scholar] [CrossRef]
- Castanares, M.A.; Copeland, B.T.; Chowdhury, W.H.; Liu, M.M.; Rodriguez, R.; Pomper, M.G.; Lupold, S.E.; Foss, C.A. Characterization of a novel metastatic prostate cancer cell line of LNCaP origin. Prostate 2016, 76, 215–225. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Tong, D.; Liu, G.; Xu, J.; Do, K.; Geary, K.; Zhang, D.; Zhang, J.; Zhang, Y.; Li, Y. Metformin reverses prostate cancer resistance to enzalutamide by targeting TGF-β 1/STAT3 axis-regulated EMT. Cell Death Dis. 2017, 8, e3007. [Google Scholar] [CrossRef]
- Chen, C.L.; Mahalingam, D.; Osmulski, P.; Jadhav, R.R.; Wang, C.M.; Leach, R.J.; Chang, T.C.; Weitman, S.D.; Kumar, A.P.; Sun, L. Single-cell analysis of circulating tumor cells identifies cumulative expression patterns of EMT-related genes in metastatic prostate cancer. Prostate 2013, 73, 813–826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odero-Marah, V.; Hawsawi, O.; Henderson, V.; Sweeney, J. Epithelial-Mesenchymal Transition (EMT) and prostate cancer. Adv. Exp. Med. Biol. 2018, 1095, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Madigan, M.; Karhu, E. The role of plant-based nutrition in cancer prevention. J. Unexplored Med. Data 2018, 3, 9. [Google Scholar] [CrossRef]
- Wang, J.-H.; Bose, S.; Shin, N.R.; Chin, Y.-W.; Choi, Y.H.; Kim, H. Pharmaceutical impact of Houttuynia cordata and metformin combination on high-fat-diet-induced metabolic disorders: Link to intestinal microbiota and metabolic endotoxemia. Front. Endocrinol. 2018, 9, 620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Y.; Ren, K.; Dong, H.; Song, F.; Chen, J.; Guo, Y.; Liu, Y.; Tao, W.; Zhang, Y. Flavonoids from persimmon (Diospyros kaki L.) leaves inhibit proliferation and induce apoptosis in PC-3 cells by activation of oxidative stress and mitochondrial apoptosis. Chem. Biol. Interact. 2017, 275, 210–217. [Google Scholar] [CrossRef]
- Iwamoto, H.; Izumi, K.; Natsagdorj, A.; Naito, R.; Makino, T.; Kadomoto, S.; Hiratsuka, K.; Shigehara, K.; Kadono, Y.; Narimoto, K.; et al. Coffee diterpenes kahweol acetate and cafestol synergistically inhibit the proliferation and migration of prostate cancer cells. Prostate 2019, 79, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Gundala, S.R.; Mukkavilli, R.; Vangala, S.; Reid, M.D.; Aneja, R. Synergistic interactions among flavonoids and acetogenins in Graviola (Annona muricata) leaves confer protection against prostate cancer. Carcinogenesis 2015, 36, 656–665. [Google Scholar] [CrossRef] [Green Version]
- Satari, A.; Amini, S.A.; Raeisi, E.; Lemoigne, Y.; Heidarian, E. Synergetic impact of combined 5-fluorouracil and rutin on apoptosis in PC3 cancer cells through the modulation of P53 gene expression. Adv. Pharm. Bull. 2019, 9, 462–469. [Google Scholar] [CrossRef] [Green Version]
- Iriti, M.; Kubina, R.; Cochis, A.; Sorrentino, R.; Varoni, E.M.; Kabała-Dzik, A.; Azzimonti, B.; Dziedzic, A.; Rimondini, L.; Wojtyczka, R.D. Rutin, a quercetin glycoside, restores chemosensitivity in human breast cancer cells. Phytother. Res. 2017, 31, 1529–1538. [Google Scholar] [CrossRef]
- Nafees, S.; Mehdi, S.H.; Zafaryab, M.; Zeya, B.; Sarwar, T.; Rizvi, M.A. Synergistic interaction of rutin and silibinin on human colon cancer cell line. Arch. Med. Res. 2018, 49, 226–234. [Google Scholar] [CrossRef]
- Yamagata, K.; Izawa, Y.; Onodera, D.; Tagami, M. Chlorogenic acid regulates apoptosis and stem cell marker-related gene expression in A549 human lung cancer cells. Mol. Cell. Biochem. 2018, 441, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am. J. Clin. Nutr. 2003, 78, 517S–520S. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H. Potential synergy of phytochemicals in cancer prevention: Mechanism of action. J. Nutr. 2004, 134, 3479S–3485S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- HemaIswarya, S.; Doble, M. Potential synergism of natural products in the treatment of cancer. Phytother. Res. 2006, 20, 239–249. [Google Scholar] [CrossRef]
- Etani, T.; Suzuki, T.; Naiki, T.; Naiki-Ito, A.; Ando, R.; Iida, K.; Kawai, N.; Tozawa, K.; Miyata, N.; Kohri, K.; et al. NCL1, a highly selective lysine-specific demethylase 1 inhibitor, suppresses prostate cancer without adverse effect. Oncotarget 2015, 6, 2865–2878. [Google Scholar] [CrossRef]
- Chen, Y.-F.; Yang, J.-S.; Chang, W.-S.; Tsai, S.-C.; Peng, S.-F.; Zhou, Y.-R. Houttuynia cordata Thunb extract modulates G 0/G 1 arrest and Fas/CD95-mediated death receptor apoptotic cell death in human lung cancer A549 cells. J. Biomed. Sci. 2013, 20, 18. [Google Scholar] [CrossRef] [Green Version]
- Khwaja, A. Akt is more than just a Bad kinase. Nature 1999, 401, 33–34. [Google Scholar] [CrossRef]
- Gao, H.; Ouyang, X.; Banach-Petrosky, W.A.; Gerald, W.L.; Shen, M.M.; Abate-Shen, C. Combinatorial activities of Akt and B-Raf/Erk signaling in a mouse model of androgen-independent prostate cancer. Proc. Natl. Acad. Sci. USA 2006, 103, 14477–14482. [Google Scholar] [CrossRef] [Green Version]
- Nikhil, K.; Sharan, S.; Chakraborty, A.; Roy, P. Pterostilbene-isothiocyanate conjugate suppresses growth of prostate cancer cells irrespective of androgen receptor status. PLoS ONE 2014, 9, e93335. [Google Scholar] [CrossRef]
- Yancey, D.; Nelson, K.C.; Baiz, D.; Hassan, S.; Flores, A.; Pullikuth, A.; Karpova, Y.; Axanova, L.; Moore, V.; Sui, G.; et al. BAD dephosphorylation and decreased expression of MCL-1 induce rapid apoptosis in prostate cancer cells. PLoS ONE 2013, 8, e74561. [Google Scholar] [CrossRef] [PubMed]
- Ballif, B.A.; Blenis, J. Molecular mechanisms mediating mammalian mitogen-activated protein kinase (MAPK) kinase (MEK)-MAPK cell survival signals. Cell Growth Differ. 2001, 12, 397–408. [Google Scholar] [PubMed]
- Wang, Y.; Romigh, T.; He, X.; Orloff, M.S.; Silverman, R.H.; Heston, W.D.; Eng, C. Resveratrol regulates the PTEN/AKT pathway through androgen receptor-dependent and -independent mechanisms in prostate cancer cell lines. Hum. Mol. Genet. 2010, 19, 4319–4329. [Google Scholar] [CrossRef] [PubMed]
- Pastushenko, I.; Blanpain, C. EMT transition states during tumor progression and metastasis. Trends Cell Biol. 2019, 29, 212–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Mai, W.; Chen, M.; Hu, J.; Zhuo, Z.; Lei, X.; Deng, L.; Liu, J.; Yao, N.; Huang, M.; et al. Arenobufagin inhibits prostate cancer epithelial-mesenchymal transition and metastasis by down-regulating β-catenin. Pharmacol. Res. 2017, 123, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Tong, D.; Liu, Q.; Liu, G.; Xu, J.; Lan, W.; Jiang, Y.; Xiao, H.; Zhang, D.; Jiang, J. Metformin inhibits castration-induced EMT in prostate cancer by repressing COX2/PGE2/STAT3 axis. Cancer Lett. 2017, 389, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Zhifang, M.; Liang, W.; Wei, Z.; Bin, H.; Rui, T.; Nan, W.; Shuhai, Z. The androgen receptor plays a suppressive role in epithelial-mesenchymal transition of human prostate cancer stem progenitor cells. BMC Biochem. 2015, 16, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Yang, Z.; Lu, N. A new role for the PI3K/Akt signaling pathway in the epithelial-mesenchymal transition. Cell Adhes. Migr. 2015, 9, 317–324. [Google Scholar] [CrossRef] [Green Version]
- Hasnat, M.; Pervin, M.; Lim, J.H.; Lim, B.O. Apigenin attenuates melanoma cell migration by inducing anoikis through integrin and focal adhesion kinase inhibition. Molecules 2015, 20, 21157–21166. [Google Scholar] [CrossRef]
- Baba, M.; Asano, R.; Takigami, I.; Takahashi, T.; Ohmura, M.; Okada, Y.; Sugimoto, H.; Arika, T.; Nishino, H.; Okuyama, T. Studies on cancer chemoprevention by traditional folk medicines XXV.—Inhibitory effect of isoliquiritigenin on azoxymethane-induced murine colon aberrant crypt focus formation and carcinogenesis. Biol. Pharm. Bull. 2002, 25, 247–250. [Google Scholar] [CrossRef] [Green Version]
- Sharmila, G.; Bhat, F.A.; Arunkumar, R.; Elumalai, P.; Raja Singh, P.; Senthilkumar, K.; Arunakaran, J. Chemopreventive effect of quercetin, a natural dietary flavonoid on prostate cancer in in vivo model. Clin. Nutr. 2014, 33, 718–726. [Google Scholar] [CrossRef]
- Madani, S.; Ameli, S.; Khazaei, S.; Kanani, M.; Izadi, B. Frequency of Ki-67 (MIB-1) and P53 expressions among patients with prostate cancer. Indian J. Pathol. Microbiol. 2011, 54, 688–691. [Google Scholar] [CrossRef]
- Verma, R.; Gupta, V.; Singh, J.; Verma, M.; Gupta, G.; Gupta, S.; Sen, R.; Ralli, M. Significance of p53 and ki-67 expression in prostate cancer. Urol. Ann. 2015, 7, 488–493. [Google Scholar] [CrossRef]
- Ananthanarayanan, V.; Deaton, R.J.; Yang, X.J.; Pins, M.R.; Gann, P.H. Alteration of proliferation and apoptotic markers in normal and premalignant tissue associated with prostate cancer. BMC Cancer 2006, 6, 73. [Google Scholar] [CrossRef] [Green Version]
- Amirghofran, Z.; Monabati, A.; Gholijani, N. Androgen receptor expression in relation to apoptosis and the expression of cell cycle related proteins in prostate cancer. Pathol. Oncol. Res. 2004, 10, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.L.; Su, Y.C.; Tsai, C.C.; Lin, J.S.; Jiang, R.S.; Su, M.C. Postoperative care with Chinese herbal medicine or amoxicillin after functional endoscopic sinus surgery: A randomized, double-blind, placebo-controlled study. Am. J. Rhinol. Allergy 2011, 25, 170–175. [Google Scholar] [CrossRef]
- Liu, Z.; Jin, M.; Li, Y.; Liu, J.; Xiao, X.; Bi, H.; Pan, Z.; Shi, H.; Xie, X.; Zhang, M.; et al. Efficacy and safety of houttuynia eye drops atomization treatment for meibomian gland dysfunction-related dry eye disease: A randomized, double-blinded, placebo-controlled clinical trial. J. Clin. Med. 2020, 9, 4022. [Google Scholar] [CrossRef]
- Yang, J.H.; Hwang, E.J.; Moon, J.; Yoon, J.Y.; Kim, J.W.; Choi, S.; Cho, S.I.; Suh, D.H. Clinical efficacy of herbal extracts in treatment of mild to moderate acne vulgaris: An 8-week, double-blinded, randomized, controlled trial. J. Dermatol. Treat. 2021, 32, 297–301. [Google Scholar] [CrossRef] [PubMed]










| Compounds | H. cordata | Hexane Fraction | Ethyl Acetate Fraction | Residue |
|---|---|---|---|---|
| Gallic acid | 0.44 ± 0.01 | ND | 0.64 ± 0.05 | ND |
| Catechin | 1.64 ± 0.43 | ND | 0.50 ± 0.15 | ND |
| Chlorogenic acid | 25.50 ± 3.41 | ND | 15.15 ± 1.37 | 3.68 ± 0.15 |
| Vanilic acid | 0.84 ± 0.14 | ND | 0.10 ± 0.04 | 0.15 ± 0.02 |
| Ferulic acid | 0.62 ± 0.02 | ND | 1.51 ± 0.18 | ND |
| p-Courmaric acid | 0.12 ± 0.02 | ND | 0.11 ± 0.24 | ND |
| Rutin | 44.00 ± 5.61 | ND | 81.3 ± 5.21 | 2.40 ± 0.27 |
| Rosmarinic acid | 1.49 ± 0.04 | ND | 1.97 ± 0.74 | ND |
| Quercetin | 0.20 ± 0.05 | ND | ND | ND |
| Apigenin | ND | ND | ND | ND |
| Trait | Group | ||
|---|---|---|---|
| Control | 0.2%HCT | 1%HCT | |
| No. of rats | 13 | 12 | 12 |
| Initial body weight (Day 1) (g) | 244.9 ± 6.6 | 248.1 ± 7.3 | 250.2 ± 8.6 |
| Final body weight (Day 70) (g) | 645.0 ± 21.5 | 664.7 ± 15.3 | 656.7 ± 28.9 |
| Average food intake (g/rat/day) | 25.5 ± 1.2 | 25.5 ± 1.1 | 26.0 ± 1.3 |
| Average HCT intake (mg/kg/day) | 0.0 | 115.9 ± 2.2 | 589.2 ± 12.7 |
| Organ weight (g) | |||
| Liver | 23.38 ± 2.61 | 21.31 ± 1.80 | 22.82 ± 2.90 |
| Kidneys | 3.06 ± 0.12 | 3.07 ± 0.11 | 3.12 ± 0.09 |
| Prostate glands | 0.25 ± 0.04 | 0.30 ± 0.05 | 0.29 ± 0.05 |
| Treatments | No. of Rats | Incidence of Adenocarcinoma (%) | Percentage of Lesion in Prostate | ||
|---|---|---|---|---|---|
| LG-PIN | HG-PIN | Adenocarcinoma | |||
| Lateral Lobe | |||||
| Control | 13 | 13 (100%) | 4.72 ± 2.95 | 89.18 ± 3.04 | 6.06 ± 2.69 |
| 0.2%HCT | 12 | 8 (67%) * | 7.60 ± 4.48 | 90.55 ± 5.41 | 2.22 ± 1.96 *** |
| 1%HCT | 12 | 6 (50%) ** | 10.31 ± 4.76 ** | 88.38 ± 4.18 | 1.32 ± 1.65 *** |
| Ventral Lobe | |||||
| Control | 13 | 13 (100%) | 4.61 ± 1.53 | 89.70 ± 2.13 | 5.66 ± 2.42 |
| 0.2%HCT | 12 | 11 (92%) | 6.76 ± 2.89 * | 90.91 ± 2.64 | 2.32 ± 1.51 *** |
| 1%HCT | 12 | 11 (92%) | 8.82 ± 3.80 ** | 87.87 ± 3.55 | 3.33 ± 3.38 |
| Trait | Group | ||
|---|---|---|---|
| Control | 0.2% HCT | 1% HCT | |
| Initial body weight (Day 1) (g) | 25.9 ± 0.7 | 25.8 ± 0.2 | 25.9 ± 0.6 |
| Final body weight (Day 28) (g) | 27.7 ± 0.1 | 27.9 ± 0.6 | 27.8 ± 0.5 |
| Average food consumption (g/mouse/day) | 3.4 ± 0.2 | 3.5 ± 0.1 | 3.5 ± 0.2 |
| Average HCT consumption (mg/kg/day) | 0.0 | 25.2 ± 0.3 | 127.0 ± 1.9 |
| Organ weight (g) | |||
| Liver | 1.51 ± 0.07 | 1.49 ± 0.03 | 1.51 ± 0.04 |
| Kidneys | 0.48 ± 0.01 | 0.49 ± 0.01 | 0.49 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subhawa, S.; Naiki-Ito, A.; Kato, H.; Naiki, T.; Komura, M.; Nagano-Matsuo, A.; Yeewa, R.; Inaguma, S.; Chewonarin, T.; Banjerdpongchai, R.; et al. Suppressive Effect and Molecular Mechanism of Houttuynia cordata Thunb. Extract against Prostate Carcinogenesis and Castration-Resistant Prostate Cancer. Cancers 2021, 13, 3403. https://doi.org/10.3390/cancers13143403
Subhawa S, Naiki-Ito A, Kato H, Naiki T, Komura M, Nagano-Matsuo A, Yeewa R, Inaguma S, Chewonarin T, Banjerdpongchai R, et al. Suppressive Effect and Molecular Mechanism of Houttuynia cordata Thunb. Extract against Prostate Carcinogenesis and Castration-Resistant Prostate Cancer. Cancers. 2021; 13(14):3403. https://doi.org/10.3390/cancers13143403
Chicago/Turabian StyleSubhawa, Subhawat, Aya Naiki-Ito, Hiroyuki Kato, Taku Naiki, Masayuki Komura, Aya Nagano-Matsuo, Ranchana Yeewa, Shingo Inaguma, Teera Chewonarin, Ratana Banjerdpongchai, and et al. 2021. "Suppressive Effect and Molecular Mechanism of Houttuynia cordata Thunb. Extract against Prostate Carcinogenesis and Castration-Resistant Prostate Cancer" Cancers 13, no. 14: 3403. https://doi.org/10.3390/cancers13143403
APA StyleSubhawa, S., Naiki-Ito, A., Kato, H., Naiki, T., Komura, M., Nagano-Matsuo, A., Yeewa, R., Inaguma, S., Chewonarin, T., Banjerdpongchai, R., & Takahashi, S. (2021). Suppressive Effect and Molecular Mechanism of Houttuynia cordata Thunb. Extract against Prostate Carcinogenesis and Castration-Resistant Prostate Cancer. Cancers, 13(14), 3403. https://doi.org/10.3390/cancers13143403