An Overview on the Histogenesis and Morphogenesis of Salivary Gland Neoplasms and Evolving Diagnostic Approaches
Abstract
:Simple Summary
Abstract
1. Introduction
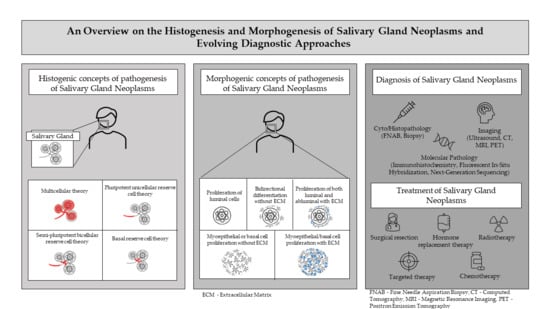
1.1. Pathogenesis of Salivary Gland Neoplasms (SGN)
1.1.1. Histogenic Concepts
- Semi-pluripotent bicellular reserve cell theory: A more plausible interpretation of the reserve cell theory suggested that the basal cells of the excretory duct (excretory duct reserve cells) produced squamous or mucin-producing columnar cells, and those from the intercalated ducts (intercalated duct reserve cells) were responsible for development of intercalated, striated, and acinar elements [1,2,10];
- Multicellular theory: Further investigation provided evidence that all mature cell types, including acinar and basal cells in salivary gland tissue were capable of proliferation. This theory presumes that SGN originated from the differentiated or adult cell counterpart from within the functional salivary ducto-acinar complex [1,2].
1.1.2. Morphogenic Concepts
2. Classification of SGN
3. Diagnostic Workup and Recent Advances in Diagnosis
3.1. Clinical History
3.2. Fine-Needle Aspiration Biopsy (FNAB)
3.3. Ultrasound (US)
3.4. Computerized Tomography (CT)
3.5. Magnetic Resonance Imaging (MRI)
3.6. Positron Emission Tomography (PET)
| Imaging Technique | Principle | Interpretation Guidelines (Parameters Studied) | Sensitivity | Specificity |
|---|---|---|---|---|
| Ultrasound (US) [31,32] | Use of high-frequency sound waves to generate images of internal tissues and organs for diagnosis | Tumor: location, dimensions, shape, structure, margins, vascularization | 63% | 92% |
| Computerized tomography (CT) [31,32] | Using a series of X-ray images to produce a cross-sectional image of tissues for diagnosis | Tumor boundary, enhancement pattern, calcification | 83% | 85% |
| Magnetic resonance imaging (MRI) [31,32] | Use of magnetic field and radio waves to produce images for diagnosis | T1-, T2-weighted images for tumor localization, extent, perineural infiltration and relation to adjacent structures. Other parameters: apparent diffusion coefficient, time–intensity curve, amide proton transfer-telated signal intensity | 81% | 89% |
| Positron emission tomography (PET) [43] | Use of radioactive tracers to visualize and evaluate tissues and organs for diagnosis | Tumor maximum standardized uptake value, clinicopathlogic parameters (local tumor invasion, T and N categories, TNM stage, loco-regional and distant lymph node metastasis) | 80.5% (cervical lymphnode levels with metastases) | 89.5% (cervical lymphnode levels with metastases) |
3.7. Biopsy and Histopathological Diagnosis
4. Oncogenes as a Novel Diagnostic Tool
4.1. Pleomorphic Adenoma
4.2. Mucoepidermoid Carcinoma
4.3. Adenoid Cystic Carcinoma
4.4. Acinic Cell Carcinoma
4.5. Polymorphous Adenocarcinoma
4.6. Clear Cell Carcinoma
4.7. Salivary Duct Carcinoma
4.8. Myoepithelial Carcinoma
4.9. Epithelial–Myoepithelial Carcinoma
5. The Management of SGN
5.1. Surgery
5.2. Radiotherapy
5.3. Chemotherapy
5.4. Other Therapeutic Interventions
5.5. Relative Problems of SGN Therapy
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dardick, I.; Burford-Mason, A.P. Current Status of Histogenetic and Morphogenetic Concepts of Salivary Gland Tumorigenesis. Crit. Rev. Oral Biol. Med. 1993, 4, 639–677. [Google Scholar] [CrossRef] [Green Version]
- Sreeja, C.; Shahela, T.; Aesha, S.; Satish, M.K. Taxonomy of salivary gland neoplasm. J. Clin. Diagn. Res. 2014, 8, 291–293. [Google Scholar] [PubMed]
- Harunaga, J.; Hsu, J.; Yamada, K. Dynamics of Salivary Gland Morphogenesis. J. Dent. Res. 2011, 90, 1070–1077. [Google Scholar] [CrossRef] [Green Version]
- Denny, P.; Ball, W.; Redman, R. Salivary Glands: A Paradigm for Diversity of Gland Development. Crit. Rev. Oral Biol. Med. 1997, 8, 51–75. [Google Scholar] [CrossRef] [Green Version]
- Tran, O.N.; Wang, H.; Dean, D.D.; Chen, X.D.; Yeh, C.K. Chapter 14—Stem Cell–Based Restoration of Salivary Gland Function. In A Roadmap to Non-Hematopoietic Stem Cell-Based Therapeutics, 1st ed.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2018; p. 544. [Google Scholar]
- Proctor, G.B.; Shaalan, A.K. Chapter 37—Salivary Gland Secretion. In Physiology of the Gastrointestinal Tract, 6th ed.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Gontarz, M.; Bargiel, J.; Gąsiorowski, K.; Marecik, T.; Szczurowski, P.; Zapała, J.; Wyszyńska-Pawelec, G. Epidemiology of Primary Epithelial Salivary Gland Tumors in Southern Poland—A 26-Year, Clinicopathologic, Retrospective Analysis. J. Clin. Med. 2021, 10, 1663. [Google Scholar] [CrossRef]
- Ostović, K.T.; Luksić, I.; Virag, M.; Macan, D.; Müllers, D.; Manojlović, S. The importance of team work of cytologist and surgeon in preoperative diagnosis of intraoral minor salivary gland tumours. Coll. Antropol. 2012, 36 (Suppl. 2), 151–157. [Google Scholar] [PubMed]
- Porcheri, C.; Meisel, C.T.; Mitsiadis, T.A. Molecular and Cellular Modelling of Salivary Gland Tumors Open New Landscapes in Diagnosis and Treatment. Cancers 2020, 12, 3107. [Google Scholar] [CrossRef] [PubMed]
- Eversole, L.R. Histogenic classification of salivary tumors. Arch. Pathol. 1971, 92, 433–443. [Google Scholar] [PubMed]
- Batsakis, J.G.; Ordonez, N.G.; Ro, J.; Meis, J.M.; Bruner, J.M. S-100 protein and myoepithelial neoplasms. J. Laryngol. Otol. 1986, 100, 687–698. [Google Scholar] [CrossRef]
- Dardick, I.; Van Nostrand, A.W. Morphogenesis of salivary gland tumors. A prerequisite to improving classification. Pathol. Annu. 1987, 22, 1–53. [Google Scholar] [PubMed]
- Jagdish, A.K.; Janarthanam, J.; Parthasarathy, S.; Santosham, K. Histogenetic and Morphogenetic Concepts of Salivary Gland Neoplasms. Int. J. Sci. Res. 2014, 3, 575–581. [Google Scholar]
- Dardick, I.; van Nostrand, A.P.; Phillips, M.J. Histogenesis of salivary gland pleomorphic adenoma (mixed tumor) with an evaluation of the role of the myoepithelial cell. Hum. Pathol. 1982, 13, 62–75. [Google Scholar] [CrossRef]
- El-Naggar, A.K.; Chan, J.K.C.; Grandis, J.R.; Takata, T.; Slootweg, P.J. (Eds.) World Health Organization Classification of Tumours: Pathology and Genetics of Head and Neck Tumours, 4th ed.; International Agency for Research on Cancer (IARC): Lyon, France, 2017. [Google Scholar]
- Seethala, R.R.; Stenman, G. Update from the 4th Edition of the World Health Organization Classification of Head and Neck Tumours: Tumors of the Salivary Gland. Head Neck Pathol. 2017, 11, 55–67. [Google Scholar] [CrossRef] [Green Version]
- Speight, P.M.; Barrett, A.W. Salivary gland tumours: Diagnostic challenges and an update on the latest WHO classification. Diagn. Histopathol. 2020, 26, 147–158. [Google Scholar] [CrossRef]
- Jung, Y.J.; Han, M.; Ha, E.J.; Choi, J.W. Differentiation of salivary gland tumors through tumor heterogeneity: A comparison between pleomorphic adenoma and Warthin tumor using CT texture analysis. Neuroradiology 2020, 62, 1451–1458. [Google Scholar] [CrossRef] [PubMed]
- Rudack, C.; Jörg, S.; Kloska, S.; Stoll, W.; Thiede, O. Neither MRI, CT nor US is superior to diagnose tumors in the salivary glands--an extended case study. Head Face Med. 2007, 3, 19. [Google Scholar] [CrossRef] [Green Version]
- Buchbender, C.; Heusner, T.A.; Lauenstein, T.C.; Bockisch, A.; Antoch, G. Oncologic PET/MRI, part 1: Tumors of the brain, head and neck, chest, abdomen, and pelvis. J. Nucl. Med. 2012, 53, 928–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mouminah, A.; Borja, A.J.; Hancin, E.C.; Chang, Y.C.; Werner, T.J.; Swisher-McClure, S.; Korostoff, J.; Alavi, A.; Revheim, M.-E. 18F-FDG-PET/CT in radiation therapy-induced parotid gland inflammation. Eur. J. Hybrid Imaging 2020, 4, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, M.H.; Bagherihagh, A.; Saeedi, M.; Dabirmoghaddam, P.; Kouhi, A.; Amirzade-Iranaq, M.H. Chapter 3—Salivary Gland Cancers: A Survey through History, Classifications and Managements. In Diagnosis and Management of Head and Neck Cancer; IntechOpen: London, UK, 2017. [Google Scholar]
- Stodulski, D.; Mikaszewski, B.; Stankiewicz, C. Signs and symptoms of parotid gland carcinoma and their prognostic value. Int. J. Oral Maxillofac. Surg. 2012, 41, 801–806. [Google Scholar] [CrossRef]
- Son, E.; Panwar, A.; Mosher, C.H.; Lydiatt, D. Cancers of the Major Salivary Gland. J. Oncol. Pract. 2018, 14, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Sarmento, D.J.; Morais, M.L.; Costa, A.L.; Silveira, É.J. Minor intraoral salivary gland tumors: A clinical-pathological study. Einstein 2016, 14, 508–512. [Google Scholar] [CrossRef] [Green Version]
- To, V.S.H.; Chan, J.Y.W.; Tsang, R.K.Y.; Wei, W.I. Review of Salivary Gland Neoplasms. ISRN Otolaryngol. 2012, 2012, 1–6. [Google Scholar] [CrossRef]
- Edizer, D.T.; Server, E.A.; Yigit, O.; Yıldız, M. Role of Fine-Needle Aspiration Biopsy in the Management of Salivary Gland Masses. Turk. Arch. Otorhinolaryngol. 2016, 54, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Kala, C.; Kala, S.; Khan, L. Milan system for reporting salivary gland cytopathology: An experience with the implication for risk of malignancy. J. Cytol. 2019, 36, 160–164. [Google Scholar] [CrossRef]
- Białek, E.J.; Jakubowski, W.; Karpińska, G. Role of ultrasonography in diagnosis and differentiation of pleomorphic adenomas: Work in progress. Arch. Otolaryngol. Head Neck Surg. 2003, 129, 929–933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith-Francis, M.; Orr, P. Ultrasound studies. Crit. Care Nurs. Clin. N. Am. 2010, 22, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Thoeny, H.C. Imaging of salivary gland tumours. Cancer Imaging 2007, 7, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, J.; Tan, Y.-R.; Xiong, P.; Zhong, L.-P. Accuracy of diagnosis of salivary gland tumors with the use of ultrasonography, computed tomography, and magnetic resonance imaging: A meta-analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2015, 119, 238–245.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burke, C.; Thomas, R.; Howlett, D. Imaging the major salivary glands. Br. J. Oral Maxillofac. Surg. 2011, 49, 261–269. [Google Scholar] [CrossRef]
- Kim, T.-Y.; Lee, Y. Contrast-enhanced Multi-detector CT Examination of Parotid Gland Tumors: Determination of the Most Helpful Scanning Delay for Predicting Histologic Subtypes. J. Belg. Soc. Radiol. 2019, 103. [Google Scholar] [CrossRef]
- Abdel-Wahed, N.; Amer, M.E.; Abo-Taleb, N.S.M. Assessment of the role of cone beam computed sialography in diagnosing salivary gland lesions. Imaging Sci. Dent. 2013, 43, 17–23. [Google Scholar] [CrossRef] [Green Version]
- Takumi, K.; Nagano, H.; Kikuno, H.; Kumagae, Y.; Fukukura, Y.; Yoshiura, T. Differentiating malignant from benign salivary gland lesions: A multiparametric non-contrast MR imaging approach. Sci. Rep. 2021, 11, 1–9. [Google Scholar] [CrossRef]
- Tartaglione, T.; Botto, A.; Sciandra, M.; Gaudino, S.; Danieli, L.; Parrilla, C.; Paludetti, G.; Colosimo, C. Differential diagnosis of parotid gland tumours: Which magnetic resonance findings should be taken in account? Acta Otorhinolaryngol. Ital. 2015, 35, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Davachi, B.; Imanimoghaddam, M.; Majidi, M.R.; Sahebalam, A.; Johari, M.; Langaroodi, A.J.; Shakeri, M.T. The Efficacy of Magnetic Resonance Imaging and Color Doppler Ultrasonography in Diagnosis of Salivary Gland Tumors. J. Dent. Res. Dent. Clin. Dent. Prospect. 2014, 8, 246–251. [Google Scholar] [CrossRef]
- Bae, Y.J.; Choi, B.S.; Jeong, W.-J.; Jung, Y.H.; Park, J.H.; Sunwoo, L.; Jung, C.; Kim, J.H. Amide Proton Transfer-weighted MRI in the Diagnosis of Major Salivary Gland Tumors. Sci. Rep. 2019, 9, 8349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freling, N.; Crippa, F.; Maroldi, R. Staging and follow-up of high-grade malignant salivary gland tumours: The role of traditional versus functional imaging approaches—A review. Oral Oncol. 2016, 60, 157–166. [Google Scholar] [CrossRef] [Green Version]
- Kato, H.; Kanematsu, M.; Watanabe, H.; Mizuta, K.; Aoki, M. Salivary gland tumors of the parotid gland: CT and MR imaging findings with emphasis on intratumoral cystic components. Neuroradiology 2014, 56, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.F.; Piccini, P.; Chinnery, P.F. Applications of Positron Emission Tomography (PET) in Neurology. J. Neurol. Neurosurg. Psychiatry 2006, 377–399. [Google Scholar] [CrossRef] [Green Version]
- Roh, J.-L.; Ryu, C.H.; Choi, S.-H.; Kim, J.S.; Lee, J.H.; Cho, K.-J.; Nam, S.Y.; Kim, S.Y. Clinical utility of 18F-FDG PET for patients with salivary gland malignancies. J. Nucl. Med. 2007, 48, 240–246. [Google Scholar]
- Moutasim, K.A.; Thomas, G.J. Salivary gland tumours: Update on molecular diagnostics. Diagn. Histopathol. 2020, 26, 159–164. [Google Scholar] [CrossRef]
- Bobati, S.S.; Patil, B.V.; Dombale, V.D. Histopathological study of salivary gland tumors. J. Oral Maxillofac. Pathol. 2017, 21, 46–50. [Google Scholar] [CrossRef]
- Thompson, L.D.; Lewis, J.S.; Skálová, A.; Bishop, J.A. Don’t stop the champions of research now: A brief history of head and neck pathology developments. Hum. Pathol. 2020, 95, 1–23. [Google Scholar] [CrossRef]
- Toper, M.H.; Sarioglu, S. Molecular Pathology of Salivary Gland Neoplasms: Diagnostic, Prognostic, and Predictive Perspective. Adv. Anat. Pathol. 2021, 28, 81–93. [Google Scholar] [CrossRef]
- Nagao, T.; Sato, E.; Inoue, R.; Oshiro, H.; Takahashi, R.H.; Nagai, T.; Yoshida, M.; Suzuki, F.; Obikane, H.; Yamashina, M.; et al. Immunohistochemical Analysis of Salivary Gland Tumors: Application for Surgical Pathology Practice. Acta Histochem. Cytochem. 2012, 45, 269–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bokhari, M.R.; Greene, J. Pleomorphic Adenoma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Asahina, M.; Saito, T.; Hayashi, T.; Fukumura, Y.; Mitani, K.; Yao, T. Clinicopathological effect of PLAG1 fusion genes in pleomorphic adenoma and carcinoma ex pleomorphic adenoma with special emphasis on histological features. Histopathology 2018, 74, 514–525. [Google Scholar] [CrossRef]
- Katabi, N.; Xu, B.; Jungbluth, A.A.; Zhang, L.; Shao, S.Y.; Lane, J.; Ghossein, R.; Antonescu, C.R. PLAG1 immunohistochemistry is a sensitive marker for pleomorphic adenoma: A comparative study with PLAG1 genetic abnormalities. Histopathology 2017, 72, 285–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffith, C.C.; Schmitt, A.C.; Little, J.L.; Magliocca, K.R. New Developments in Salivary Gland Pathology: Clinically Useful Ancillary Testing and New Potentially Targetable Molecular Alterations. Arch. Pathol. Lab. Med. 2017, 141, 381–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mito, J.K.; Jo, V.Y.; Chiosea, S.; Cin, P.D.; Krane, J.F. HMGA2 is a specific immunohistochemical marker for pleomorphic adenoma and carcinoma ex-pleomorphic adenoma. Histopathology 2017, 71, 511–521. [Google Scholar] [CrossRef]
- Darras, N.; Mooney, K.L.; Long, S.R. Diagnostic utility of fluorescence in situ hybridization testing on cytology cell blocks for the definitive classification of salivary gland neoplasms. J. Am. Soc. Cytopathol. 2019, 8, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Evrard, S.M.; Meilleroux, J.; Daniel, G.; Basset, C.; Lacoste-Collin, L.; Vergez, S.; Uro-Coste, E.; Courtade-Saidi, M. Use of fluorescent in-situ hybridisation in salivary gland cytology: A powerful diagnostic tool. Cytopathology 2017, 28, 312–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wasserman, J.K.; Dickson, B.C.; Smith, A.; Swanson, D.; Purgina, B.M.; Weinreb, I. Metastasizing Pleomorphic Adenoma: Recurrent PLAG1/HMGA2 Rearrangements and Identification of a Novel HMGA2-TMTC2 Fusion. Am. J. Surg. Pathol. 2019, 43, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Kaye, F.J. Mutation-associated fusion cancer genes in solid tumors. Mol. Cancer Ther. 2009, 8, 1399–1408. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Ni, W.; Li, J.-L.; Lin, S.; Zhou, X.; Sun, Y.; Li, J.W.; Leon, M.E.; Hurtado, M.D.; Zolotukhin, S.; et al. The CRTC1-MAML2 fusion is the major oncogenic driver in mucoepidermoid carcinoma. JCI Insight 2021, 6. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, N.A.; Lusardi, J.J.; McElherne, J.; Pearson, A.T.; Olivas, A.D.; Fitzpatrick, C.; Lingen, M.W.; Blair, E.A. Mucoepidermoid Carcinoma: A Comparison of Histologic Grading Systems and Relationship to MAML2 Rearrangement and Prognosis. Am. J. Surg. Pathol. 2019, 43, 885–897. [Google Scholar] [CrossRef] [PubMed]
- Okumura, Y.; Nakano, S.; Murase, T.; Ueda, K.; Kawakita, D.; Nagao, T.; Kusafuka, K.; Urano, M.; Yamamoto, H.; Kano, S.; et al. Prognostic impact of CRTC1/3-MAML2 fusions in salivary gland mucoepidermoid carcinoma: A multiinstitutional retrospective study. Cancer Sci. 2020, 111, 4195–4204. [Google Scholar] [CrossRef] [PubMed]
- Todorovic, E.; Dickson, B.C.; Weinreb, I. Salivary Gland Cancer in the Era of Routine Next-Generation Sequencing. Head Neck Pathol. 2020, 14, 311–320. [Google Scholar] [CrossRef]
- Shinomiya, H.; Ito, Y.; Kubo, M.; Yonezawa, K.; Otsuki, N.; Iwae, S.; Inagaki, H.; Nibu, K.-I. Expression of amphiregulin in mucoepidermoid carcinoma of the major salivary glands: A molecular and clinicopathological study. Hum. Pathol. 2016, 57, 37–44. [Google Scholar] [CrossRef]
- Ishida, E.; Ogawa, T.; Rokugo, M.; Ishikawa, T.; Wakamori, S.; Ohkoshi, A.; Usubuchi, H.; Higashi, K.; Ishii, R.; Nakanome, A.; et al. Management of adenoid cystic carcinoma of the head and neck: A single-institute study with over 25-year follow-up. Head Face Med. 2020, 16, 1–9. [Google Scholar] [CrossRef]
- Dhouib, F.; Siala, W.; Hassine, S.B.; Fourati, N.; Mnejja, W.; Hammami, B.; Daoud, J. Adenoid cystic carcinoma of head and neck. PAMJ Clin. Med. 2020, 3, 1–9. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, Z.-Q.; Chen, F.-P.; Yan, J.-Y.; Huang, X.-D.; Li, F.; Sun, Y.; Zhou, G.-Q. Role of Postoperative Radiotherapy in Nonmetastatic Head and Neck Adenoid Cystic Carcinoma. J. Natl. Compr. Cancer Netw. 2020, 18, 1476–1484. [Google Scholar] [CrossRef]
- Ho, A.S.; Ochoa, A.; Jayakumaran, G.; Zehir, A.; Mayor, C.V.; Tepe, J.; Makarov, V.; Dalin, M.G.; He, J.; Bailey, M.; et al. Genetic hallmarks of recurrent/metastatic adenoid cystic carcinoma. J. Clin. Investig. 2019, 129, 4276–4289. [Google Scholar] [CrossRef]
- Ferrarotto, R.; Mitani, Y.; Diao, L.; Guijarro, I.; Wang, J.; Zweidler-McKay, P.; Bell, D.; William, W.N., Jr.; Glisson, B.S.; Wick, M.J.; et al. Activating NOTCH1 Mutations Define a Distinct Subgroup of Patients With Adenoid Cystic Carcinoma Who Have Poor Prognosis, Propensity to Bone and Liver Metastasis, and Potential Responsiveness to Notch1 Inhibitors. J. Clin. Oncol. 2017, 35, 352–360. [Google Scholar] [CrossRef]
- Rettig, E.M.; Talbot, C.C.; Sausen, M.; Jones, S.; Bishop, J.A.; Wood, L.D.; Tokheim, C.; Niknafs, N.; Karchin, R.; Fertig, E.; et al. Whole-Genome Sequencing of Salivary Gland Adenoid Cystic Carcinoma. Cancer Prev. Res. 2016, 9, 265–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drier, Y.; Cotton, M.J.; Williamson, K.E.; Gillespie, S.; Ryan, R.; Kluk, M.J.; Carey, C.D.; Rodig, S.J.; Sholl, L.M.; Afrogheh, A.H.; et al. An oncogenic MYB feedback loop drives alternate cell fates in adenoid cystic carcinoma. Nat. Genet. 2016, 48, 265–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.; Zhao, F.; Yang, W.; Chen, C.; Du, Z.; Fu, M.; Ge, X.; Li, S. MYB promotes the growth and metastasis of salivary adenoid cystic carcinoma. Int. J. Oncol. 2019, 54, 1579–1590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haller, F.; Skálová, A.; Ihrler, S.; Märkl, B.; Bieg, M.; Moskalev, E.A.; Erber, R.; Blank, S.; Winkelmann, C.; Hebele, S.; et al. Nuclear NR4A3 Immunostaining Is a Specific and Sensitive Novel Marker for Acinic Cell Carcinoma of the Salivary Glands. Am. J. Surg. Pathol. 2019, 43, 1264–1272. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, S.; Varma, S.; Barasch, N.; Thompson, L.D.; Miettinen, M.; Rooper, L.; Stelow, E.B.; Agander, T.K.; Seethala, R.R.; Chiosea, S.I.; et al. The HTN3-MSANTD3 Fusion Gene Defines a Subset of Acinic Cell Carcinoma of the Salivary Gland. Am. J. Surg. Pathol. 2019, 43, 489–496. [Google Scholar] [CrossRef]
- Xu, B.; Barbieri, A.L.; Bishop, J.A.; Chiosea, S.I.; Dogan, S.; di Palma, S.; Faquin, W.C.; Ghossein, R.; Hyrcza, M.; Jo, V.Y.; et al. Histologic Classification and Molecular Signature of Polymorphous Adenocarcinoma (PAC) and Cribriform Adenocarcinoma of Salivary Gland (CASG): An International Interobserver Study. Am. J. Surg. Pathol. 2020, 44, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Sebastiao, A.P.M.; Xu, B.; Lozada, J.; Pareja, F.; Geyer, F.C.; Paula, A.D.C.; Da Silva, E.M.; Ghossein, R.A.; Weinreb, I.; De Noronha, L.; et al. Histologic spectrum of polymorphous adenocarcinoma of the salivary gland harbor genetic alterations affecting PRKD genes. Mod. Pathol. 2019, 33, 65–73. [Google Scholar] [CrossRef]
- Fisher, C. The diversity of soft tissue tumours withEWSR1gene rearrangements: A review. Histopathology 2013, 64, 134–150. [Google Scholar] [CrossRef]
- Shah, A.A.; LeGallo, R.D.; van Zante, A.; Frierson, H.F., Jr.; Mills, S.E.; Berean, K.W.; Mentrikoski, M.J.; Stelow, E.B. EWSR1 genetic rearrangements in salivary gland tumors: A specific and very common feature of hyalinizing clear cell carcinoma. Am. J. Surg. Pathol. 2013, 37, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Udager, A.M.; Chiosea, S.I. Salivary Duct Carcinoma: An Update on Morphologic Mimics and Diagnostic Use of Androgen Receptor Immunohistochemistry. Head Neck Pathol. 2017, 11, 288–294. [Google Scholar] [CrossRef] [Green Version]
- Shimura, T.; Tada, Y.; Hirai, H.; Kawakita, D.; Kano, S.; Tsukahara, K.; Shimizu, A.; Takase, S.; Imanishi, Y.; Ozawa, H.; et al. Prognostic and histogenetic roles of gene alteration and the expression of key potentially actionable targets in salivary duct carcinomas. Oncotarget 2017, 9, 1852–1867. [Google Scholar] [CrossRef] [Green Version]
- Khoo, T.K.; Yu, B.; Smith, J.A.; Clarke, A.J.; Luk, P.P.; Selinger, C.I.; Mahon, K.L.; Kraitsek, S.; Palme, C.; Boyer, M.J.; et al. Somatic mutations in salivary duct carcinoma and potential therapeutic targets. Oncotarget 2017, 8, 75893–75903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shenoy, N. Aggressive myoepithelial carcinoma with EWSR1-POU5F1 fusion highly responsive to Ewing sarcoma combination chemotherapy. Cancer 2020, 126, 5198–5201. [Google Scholar] [CrossRef]
- Urano, M.; Nakaguro, M.; Yamamoto, Y.; Hirai, H.; Tanigawa, M.; Saigusa, N.; Shimizu, A.; Tsukahara, K.; Tada, Y.; Sakurai, K.; et al. Diagnostic Significance of HRAS Mutations in Epithelial-Myoepithelial Carcinomas Exhibiting a Broad Histopathologic Spectrum. Am. J. Surg. Pathol. 2019, 43, 984–994. [Google Scholar] [CrossRef] [PubMed]
- Sood, S.; McGurk, M.; Vaz, F. Management of Salivary Gland Tumours: United Kingdom National Multidisciplinary Guidelines. J. Laryngol. Otol. 2016, 130, S142–S149. [Google Scholar] [CrossRef] [PubMed]
- Adelstein, D.J.; Koyfman, S.A.; El-Naggar, A.K.; Hanna, E. Biology and Management of Salivary Gland Cancers. Semin. Radiat. Oncol. 2012, 22, 245–253. [Google Scholar] [CrossRef]
- Mantravadi, A.V.; Moore, M.G.; Rassekh, C.H. AHNS series: Do you know your guidelines? Diagnosis and management of salivary gland tumors. Head Neck 2018, 41, 269–280. [Google Scholar] [CrossRef]
- Young, A.; Okuyemi, O.T. Benign Salivary Gland Tumors; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Lewis, A.G.; Tong, T.; Maghami, E. Diagnosis and Management of Malignant Salivary Gland Tumors of the Parotid Gland. Otolaryngol. Clin. North Am. 2016, 49, 343–380. [Google Scholar] [CrossRef] [PubMed]
- Mifsud, M.J.; Burton, J.N.; Trotti, A.M.; Padhya, T.A. Multidisciplinary Management of Salivary Gland Cancers. Cancer Control 2016, 23, 242–248. [Google Scholar] [CrossRef] [Green Version]
- Thielker, J.; Grosheva, M.; Ihrler, S.; Wittig, A.; Guntinas-Lichius, O. Contemporary Management of Benign and Malignant Parotid Tumors. Front. Surg. 2018, 5, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panwar, A.; Kozel, J.A.; Lydiatt, W.M. Cancers of Major Salivary Glands. Surg. Oncol. Clin. North Am. 2015, 24, 615–633. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.M.; Lau, V.H.; Farwell, D.G.; Luu, Q.; Donald, P.J. Mucoepidermoid carcinoma of the parotid gland treated by surgery and postoperative radiation therapy: Clinicopathologic correlates of outcome. Laryngoscope 2013, 123, 3049–3055. [Google Scholar] [CrossRef] [PubMed]
- Holtzman, A.; Morris, C.G.; Amdur, R.J.; Dziegielewski, P.T.; Boyce, B.; Mendenhall, W.M. Outcomes after primary or adjuvant radiotherapy for salivary gland carcinoma. Acta Oncol. 2016, 56, 484–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spratt, D.E.; LSalgado, R.; Riaz, N.; Doran, M.G.; Tam, M.; Wolden, S.; Katsoulakis, E.; Rao, S.; Ho, A.; Wong, R.; et al. Results of photon radiotherapy for unresectable salivary gland tumors: Is neutron radiotherapy’s local control superior? Radiol. Oncol. 2014, 48, 56–61. [Google Scholar] [CrossRef] [Green Version]
- Lagha, A.; Chraiet, N.; Ayadi, M.; Krimi, S.; Allani, B.; Rifi, H.; Raies, H.; Mezlini, A. Systemic therapy in the management of metastatic or advanced salivary gland cancers. Head Neck Oncol. 2012, 4, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, C.-E.; Lin, C.-Y.; Lee, L.-Y.; Yang, L.-Y.; Wang, C.-C.; Wang, H.-M.; Chang, J.T.-C.; Fan, K.-H.; Liao, C.-T.; Yen, T.-C.; et al. Adding concurrent chemotherapy to postoperative radiotherapy improves locoregional control but Not overall survival in patients with salivary gland adenoid cystic carcinoma-a propensity score matched study. Radiat. Oncol. 2016, 11, 47. [Google Scholar] [CrossRef] [Green Version]
- Gebhardt, B.J.; Ohr, J.P.; Ferris, R.L.; Duvvuri, U.; Kim, S.; Johnson, J.T.; Heron, D.E.; Clump, D.A. Concurrent Chemoradiotherapy in the Adjuvant Treatment of High-risk Primary Salivary Gland Malignancies. Am. J. Clin. Oncol. 2018, 41, 888–893. [Google Scholar] [CrossRef]
- Mifsud, M.J.; Tanvetyanon, T.; McCaffrey, J.C.; Otto, K.J.; Padhya, T.A.; Kish, J.; Trotti, A.M.; Harrison, L.B.; Caudell, J.J. Adjuvant radiotherapy versus concurrent chemoradiotherapy for the management of high-risk salivary gland carcinomas. Head Neck 2016, 38, 1628–1633. [Google Scholar] [CrossRef] [PubMed]
- Locati, L.D.; Quattrone, P.; Bossi, P.; Marchianò, A.V.; Cantù, G.; Licitra, L. A complete remission with androgen-deprivation therapy in a recurrent androgen receptor-expressing adenocarcinoma of the parotid gland. Ann. Oncol. 2003, 14, 1327–1328. [Google Scholar] [CrossRef]
- Elkin, A.D.; Jacobs, C.D. Tamoxifen for salivary gland adenoid cystic carcinoma: Report of two cases. J. Cancer Res. Clin. Oncol. 2008, 134, 1151–1153. [Google Scholar] [CrossRef]
- Nahlieli, O. Complications of traditional and modern therapeutic salivary approaches. Acta Otorhinolaryngol. Ital. 2017, 37, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.B.; Vissink, A.; Limesand, K.H.; Reyland, M.E. Salivary Gland Hypofunction and Xerostomia in Head and Neck Radiation Patients. J. Natl. Cancer Inst. Monogr. 2019, 2019. [Google Scholar] [CrossRef]
- Aarup-Kristensen, S.; Hansen, C.R.; Forner, L.; Brink, C.; Eriksen, J.G.; Johansen, J. Osteoradionecrosis of the mandible after radiotherapy for head and neck cancer: Risk factors and dose-volume correlations. Acta Oncol. 2019, 58, 1373–1377. [Google Scholar] [CrossRef] [PubMed]
- Strojan, P.; Hutcheson, K.; Eisbruch, A.; Beitler, J.J.; Langendijk, J.A.; Lee, A.W.; Corry, J.; Mendenhall, W.M.; Smee, R.; Rinaldo, A.; et al. Treatment of late sequelae after radiotherapy for head and neck cancer. Cancer Treat. Rev. 2017, 59, 79–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben-David, M.A.; Diamante, M.; Radawski, J.D.; Vineberg, K.A.; Stroup, C.; Murdoch-Kinch, C.-A.; Zwetchkenbaum, S.R.; Eisbruch, A. Lack of Osteoradionecrosis of the Mandible After Intensity-Modulated Radiotherapy for Head and Neck Cancer: Likely Contributions of Both Dental Care and Improved Dose Distributions. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 396–402. [Google Scholar] [CrossRef] [Green Version]
- Hamakawa, H.; Nakashiro, K.-I.; Sumida, T.; Shintani, S.; Myers, J.N.; Takes, R.P.; Rinaldo, A.; Ferlito, A. Basic evidence of molecular targeted therapy for oral cancer and salivary gland cancer. Head Neck 2008, 30, 800–809. [Google Scholar] [CrossRef]
- Lagha, A.; Chraiet, N.; Ayadi, M.; Krimi, S.; Allani, B.; Rifi, H.; Raies, H.; Mezlini, A. RETRACTED: Systemic therapy in the management of metastatic or advanced salivary gland cancers. Oral Oncol. 2012, 48, 948–957. [Google Scholar] [CrossRef] [PubMed]
- Can, N.T.; Lingen, M.W.; Mashek, H.; McElherne, J.; Briese, R.; Fitzpatrick, C.; Van Zante, A.; Cipriani, N.A. Expression of Hormone Receptors and HER-2 in Benign and Malignant Salivary Gland Tumors. Head Neck Pathol. 2017, 12, 95–104. [Google Scholar] [CrossRef]



| Histopathological Variant | ICD-O Code | Histopathological Variant | ICD-O Code |
|---|---|---|---|
| Malignant epithelial tumors | Benign tumors | ||
| Acinic cell carcinoma | 8550/3 | Pleomorphic adenoma | 8940/0 |
| Secretory carcinoma | 8502/3 | Myoepithelioma | 8982/0 |
| Mucoepidermoid carcinoma | 8430/3 | Basal cell adenoma | 8147/0 |
| Adenoid cystic carcinoma | 8200/3 | Warthin tumor | 8561/0 |
| Polymorphous adenocarcinoma | 8525/3 | Oncocytoma | 8290/0 |
| Epithelial–myoepithelial carcinoma | 8562/3 | Lymphoadenoma | 8563/0 |
| Clear cell carcinoma | 8310/3 | Cystadenoma | 8440/0 |
| Basal cell adenocarcinoma | 8147/3 | Sialadenoma papilliferum | 8406/0 |
| Sebaceous adenocarcinoma | 8410/3 | Ductal papillomas | 8503/0 |
| Intraductal carcinoma | 8500/2 | Sebaceous adenoma | 8410/0 |
| Cystadenocarcinoma | 8440/3 | Canalicular adenoma and other ductal adenomas | 8149/0 |
| Adenocarcinoma, NOS | 8140/3 | ||
| Salivary duct carcinoma | 8500/3 | Other epithelial lesions | |
| Myoepithelial carcinoma | 8982/3 | Sclerosing polycystic adenosis | |
| Carcinoma ex pleomorphic adenoma | 8941/3 | Nodular oncocytic hyperplasia | |
| Carcinosarcoma | 8980/3 | Lymphoepithelial lesions | |
| Poorly differentiated carcinoma: | Intercalated duct hyperplasia | ||
| Neuroendocrine and non-endocrine | |||
| Undifferentiated carcinoma | 8020/3 | Soft tissue tumors | |
| Large cell neuroendocrine carcinoma | 8013/3 | Hemangioma | 9120/0 |
| Small cell neuroendocrine carcinoma | 8041/3 | Lipoma/sialolipoma | 8850/0 |
| Lymphoepithelial carcinoma | 8082/3 | Nodular fasciitis | 8828/0 |
| Squamous cell carcinoma | 8070/3 | ||
| Oncocytic carcinoma | 8290/3 | Hematolymphoid tumors | |
| Borderline tumour | Extranodal marginal zone lymphoma of MALT | 9699/3 | |
| Sialoblastoma | 8974/1 | ||
| Diagnostic Category | Risk of Malignancy % | Management |
|---|---|---|
| Non-diagnostic | 25 | Clinical and radiologic correlation/repeat FNAC |
| Non-neoplastic | 10 | Clinical follow-up and radiological correlation |
| Atypia of undetermined significance (AUS) | 20 | Repeat FNAC or surgery |
| Neoplasm: benign | <5 | Surgery or clinical follow-up |
| Neoplasm: salivary gland neoplasm of uncertain malignant potential (SUMP) | 35 | Surgery |
| Suspicious for malignancy (SM) | 60 | Surgery |
| Malignant | 90 | Surgery |
| Tumor Subtype | Genetic/Molecular Alterations | Role of Alteration |
|---|---|---|
| Pleomorphic adenoma | PLAG1 alterations | Diagnostic |
| HMGA2 alterations | Diagnostic | |
| HER2 overexpression AR overexpression | Predictive for therapeutic response Predictive for therapeutic response | |
| Mucoepidermoid carcinoma | CRTC1–MAML2 fusion | Diagnostic/prognostic |
| CRTC3–MAML2 fusion | Diagnostic/prognostic | |
| Adenoid cystic carcinoma | MYB/MYBL1 rearrangements | Diagnostic/predictive (MYB overexpression for therapeutic response) |
| MYB–NFIB fusion NOTCH1 mutations | Diagnostic Prognostic | |
| Acinic cell carcinoma | NR4A3 rearrangements | Diagnostic |
| Polymorphous low-grade adenocarcinoma | PRKD1/2/3 rearrangements PRKD1 E710D hot spot mutations | Diagnostic Diagnostic/prognostic |
| Clear cell carcinoma | EWSR1–ATR fusion | Diagnostic |
| Salivary duct carcinoma | AR gene alterations | Diagnostic/predictive for androgen–deprivation therapy response |
| ERBB2 amplifications | Diagnostic/prognostic | |
| TP53, PIK3CA, H-RAS mutations KIT, EGFR, BRAF, AKT1, N-RAS, FBXW7, ATM, NFI mutations | Diagnostic/prognostic (only TP53) | |
| Loss of heterozygosity of CDKN2A, p16, PTEN | Diagnostic | |
| Myoepithelial carcinoma | EWSR1 rearrangements | No confirmatory role |
| Epithelial–myoepithelial carcinoma | HRAS mutations | No confirmatory role |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Submitted for possible open access publication under the terms and conditions of the Creative Com-mons Attribution (CC BY-NC-ND 4.0) license (https://creativecommons.org/licenses/by-nc-nd/4.0/).
Share and Cite
Iyer, J.; Hariharan, A.; Cao, U.M.N.; Mai, C.T.T.; Wang, A.; Khayambashi, P.; Nguyen, B.H.; Safi, L.; Tran, S.D. An Overview on the Histogenesis and Morphogenesis of Salivary Gland Neoplasms and Evolving Diagnostic Approaches. Cancers 2021, 13, 3910. https://doi.org/10.3390/cancers13153910
Iyer J, Hariharan A, Cao UMN, Mai CTT, Wang A, Khayambashi P, Nguyen BH, Safi L, Tran SD. An Overview on the Histogenesis and Morphogenesis of Salivary Gland Neoplasms and Evolving Diagnostic Approaches. Cancers. 2021; 13(15):3910. https://doi.org/10.3390/cancers13153910
Chicago/Turabian StyleIyer, Janaki, Arvind Hariharan, Uyen Minh Nha Cao, Crystal To Tam Mai, Athena Wang, Parisa Khayambashi, Bich Hong Nguyen, Lydia Safi, and Simon D. Tran. 2021. "An Overview on the Histogenesis and Morphogenesis of Salivary Gland Neoplasms and Evolving Diagnostic Approaches" Cancers 13, no. 15: 3910. https://doi.org/10.3390/cancers13153910
APA StyleIyer, J., Hariharan, A., Cao, U. M. N., Mai, C. T. T., Wang, A., Khayambashi, P., Nguyen, B. H., Safi, L., & Tran, S. D. (2021). An Overview on the Histogenesis and Morphogenesis of Salivary Gland Neoplasms and Evolving Diagnostic Approaches. Cancers, 13(15), 3910. https://doi.org/10.3390/cancers13153910