1. Introduction
Non-melanoma skin cancer (NMSC) represents the most common cancer in fair-skin people. Basal cell carcinoma (BCC) accounts for approximately 75% of cases, the remaining mainly being squamous cell carcinomas (SCC) [
1,
2]. While BCC rarely metastasizes, metastases from high-risk SCCs are not uncommon and may be fatal [
3,
4,
5,
6,
7].
Management of NMSC is based on early diagnosis and treatment, as well as monitoring of recurrences [
8,
9,
10,
11].
Several techniques, based on optical properties of skin, and including dermoscopy, reflectance confocal microscopy (RCM), multiphoton microscopy, fluorescence evaluation, diffuse reflectance and Raman spectroscopy, have been variously used as tools for non-invasive diagnosis of NMSCs [
12,
13,
14]. Among these, dermoscopy and RCM are reliable techniques for melanoma diagnosis even if their efficacy for preoperative delineation of SCC surgical margins is questionable [
15,
16,
17,
18].
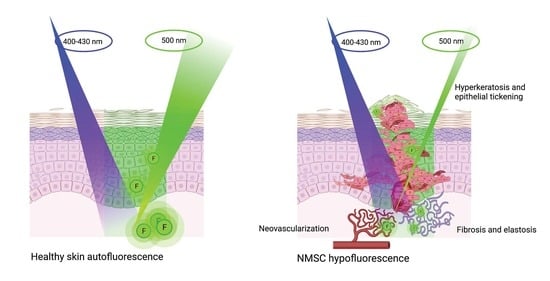
Autofluorescence (AF) is a type of luminescence depending on the optical properties of specific molecules (fluorophores) able to absorb and re-emit light of specific wavelengths.
Alteration of fluorescence (hyper and/or hypofluorescence) has been documented in neoplastic tissues, presumptively resulting from changes in the amount and distribution of fluorophores and in the chemico-physical properties of their environment [
19]. In particular, modifications of collagen, elastin and NADH seem to account for the alteration of fluorescence observed in malignant tumors. Therefore, evaluation and measurement of AF could be a promising technique for non-invasive diagnosis and management of NMSCs [
20].
Gross architectural alterations (e.g., hyperkeratosis, neoangiogenesis) associated with hyper- and hypofluorescence in pathologic tissues from the oral mucosa have been recently described [
21]. Only a few other papers have been published in this field, and, to our knowledge, this is the first work on NMSC autofluorescence and its histopathological correlation [
22]
The aims of the present study are 1. to investigate the differences in AF intensity between NMSC and healthy skin; 2. to analyze the histopathological determinants possibly accounting for AF alterations in pathologic skin.
2. Materials and Methods
2.1. Study Setting and Design
The study was conducted at the Policlinico of Modena, University of Modena and Reggio Emilia (Modena, Italy) from October 2019 to March 2020.
The study was a prospective, mono-centric, pre-clinical ex vivo study approved by the local Ethical Committee (n°1167.2018 Area Vasta Emilia Nord Ethical Committee, Policlinico di Modena, Via Largo del Pozzo 71, 41124). The research was conducted according to the Helsinki Declaration. All patients signed informed consent to the use of their data for research purposes.
2.2. AF Detection System
The AF detection system, (
Figure 1) included:
- (a)
A LED source (LLS, Ocean Optics®, Dunedin, FL, USA), emitting light in the violet spectrum (400–430 nm), used to elicit AF in healthy and pathological skin;
- (b)
Dichroic filters (Thorlabs®, Newton, NJ, USA), used to select wavelengths either from the LED source and from captured skin AF;
- (c)
aA bifurcated optical fiber (Ocean Optics®) to illuminate tissues and capture the AF;
- (d)
A spectrophotometer (Flame, Ocean Optics®), used to measure the spectrum of the captured AF emission light in the range;
- (e)
A software application (SW) was also developed (in C language) and integrated into the AF detection system. The SW acquired and processed the captured AF spectra, which were then organized within a database (
Figure 1).
2.3. Study Population and AF Evaluation
Seventy-five (46 males (61%); 29 females (39%); mean age: 79 years (ranging from 42 to 93) consecutive subjects underwent preemptive routine skin surgery for lesions suggestive for NMSC. For every patient, clinical history was considered, in particular, smoking, diabetes, hypertension, autoimmune diseases and anticoagulant therapy.
Skin phototype was as follows: type II in 71 cases (95%) and type III in 4 cases (5%). Ten (13%) patients were smokers. Overall, 98 lesions were excised from the population study.
Forty-three (43.9%) lesions were located in the H-zone (high-risk zone: eyelids, eyebrow, periorbital region, nose, lip, chin, mandibular region, preauricular and retroauricular region, ear, temple, genitalia, hands and feet), 30 (30.6%) in the I-zone (intermediate-riskzone: cheeks, forehead, scalp, neck and pretibial region) (n = 30) and 25 (25.5%) in L-zone (low-risk zone: trunk and extremities).
One-hundred and fifteen lesional points were identified on the 98 excised specimens (ex vivo), and for each of these, a control point on clinically perilesional healthy skin was evaluated.
Lesional and control points were irradiated with a 400–430 nm excitation wavelength, and the emitted light was filtered in order to remove the reflected excitation light and to selectively isolate the AF emission. AF emission spectrum was measured by a spectrophotometer and then recorded.
The ratio between the number of photons emitted at 500 nm in the healthy skin and that emitted in the pathological tissue was utilized as a measure of AF (autofluorescence intensity ratio AFIR).
AFIR > 1 indicates a decrease in AF, while AFIR < 1 indicates an increase in AF.
After evaluation, all points were marked through suture stiches as reference for histologic analysis (
Figure 2).
Histologic analysis of the marked points evaluated was performed and on the basis of histopathological diagnosis. BCCs were subclassified into: superficial, nodular, morpheiform and pigmented. Lesions in the SCC group were subclassified into in situ SCC, Bowen disease, infiltrative SCC and keratoacanthoma. Actinic keratoses were included in the SCC group.
2.4. Histological Variables
The histopathological determinants evaluated for each diagnosis of NMSC and their degrees were the following:
Hyperkeratosis (absent (0), mild (1), moderate (2) and severe (3));
epithelial thickening (defined as an increased distance between the corneum and the basal layer) (absent (0), mild (1), moderate (2), severe (3) and with presence of ulcer (4));
Fibrosis (absent (0) and present (1));
Elastosis (absent (0) and present (1));
Neovascularization (absent (0), mild (1), moderate (2) and severe (3));
Cellular atypia (absent (0), present with grade 1, 2 or 3).
Each histopathological variable was correlated to AFIR.
2.5. Statistical Analysis
Data analysis was performed using the commercial package IBM SPSS Statistics for Windows (version 22, IBM Corp., Armonk, NY) and the open-source statistical system Jamovi v.1.8.4 (The jamovi project (2021). jamovi. (Version 1.8) [Computer Software]. Retrieved from
https://www.jamovi.org, accessed on 30 June 2021, based on the R system. Measures of central tendency, dispersion and shape were calculated for all the variables in the data set. Summaries included arithmetic mean, median, standard deviation, interquartile range, minimum, maximum, asymmetry, kurtosis and the relevant standard errors and 95% confidence intervals. Normality of the data was tested by the Shapiro–Wilk test. Categorical data were reported in frequency tables and expressed as absolute, relative and cumulated frequencies and percentages.
Univariate comparisons between continuous variables were performed using both parametric tests (Student’s t-test, ANOVA) and non-parametric test (Mann–Whitney’s U-test, Kruskal–Wallis test). Comparisons between categorical variables in contingency tables were performed using the chi-square test and Fisher’s exact test.
The combined effects of different predictors on outcome variables were tested by multiple linear regression. The results were considered statistically significant for a p-value less than 5% (p < 0.05).
4. Discussion
Despite the development of several adjunctive diagnostic aids which can possibly help the clinician in the pre-operative differentiation between benign and malignant lesions, the definitive diagnosis of the vast majority of skin conditions requires histopathological analysis. In case of doubts, the collection of one or more specimens through biopsies is the gold-standard procedure that allows the planning of an appropriate treatment [
23].
As some malignant tumors (e.g., NMSCs in the early phases of development) can mimic benign lesions, the availability of a reliable, non-invasive, highly specific and sensible tool able to identify peculiar features suggestive of malignancy would provide strong input to the management of skin diseases.
Within this context, and following previous analyses performed on other anatomical sites (e.g., oral cavity) [
21,
24,
25], we designed and conducted the present study, which attempts to evaluate the usefulness of AF for the diagnosis and the management of a heterogeneous group of NMSCs.
To date, different excitation wavelengths have been applied to fluorescence spectroscopy of the skin and in particular high-energy wavelengths in the UV region. It is known that wavelengths in the UV region, in addition to AF generation, induce oxidative stress in cells, resulting in modifications in the cellular redox state, damage to the cell structures and cell death [
19]. Based on recent observation, for the present study, we selected a 400–430 excitation wavelength in the blue region of visible light [
26,
27].
The results of the present analysis demonstrate that all ex vivo specimens harboring neoplastic alterations at the histological level show a marked alteration of AF compared to perilesional, histologically healthy skin.
Such alterations can take the form of AF enhancement or AF reduction and are invariably present, conferring a specific optical appearance of NMSCs. In the present analysis, the vast majority of cases displayed a marked decrease in AF, which was on average 4.4–4.5 times lower than that of clinically healthy skin.
The decrease in AF in NMSCs after 400–430 excitation are in line with data already present in the literature [
13,
28,
29,
30]. In 2002, Panjehour et al. highlighted that healthy skin exhibited a stronger fluorescence emission than BCC and SCC when excited at 410 nm [
31].
Assessment of AFIR in different BCCs and SCCs histological subtypes showed a trend to decline not statistically significantly but directly proportional to tumor thickness and infiltration. This suggests a different fluorophore distribution between healthy and lesional skin, in line with previous findings [
20,
29,
30,
32].
The histological analysis showed a correlation between histological variables and AF alteration. Our data show that fibrosis and elastosis (present vs. absent), neoangiogenesis (absent vs. present with grades 1, 2 and 3), hyperkeratosis (absent versus present with grade 3) and epithelial thickening (absent vs. present with grades 1, 2, 3 and 4) were significantly associated with alteration of AF. Cellular atypia was not significantly associated with alteration of AF.
As previously demonstrated by clinical and histological studies conducted on the oral mucosa the main fluorophores within the range of 400–460 nm are NADH and FAD cellular coenzymes, collagen and elastin [
33,
34]. These data may support the hypothesis that the positive association between the presence of fibrosis and elastosis with the decrease in AF can be due to alteration in the distribution and concentration of these fluorophores [
32,
33].
The presence of epithelial thickening, conditions characterized by an increased distance between the corneum and the basal layer, is associated with a higher decrease in AF when compared to the normal epidermis. A thickened epithelium can probably obstruct the penetration of specific wavelengths in deep dermal layers, where the main known fluorophores are located.
Last, the presence of neovascularization was also associated with a decrease in AF. In this case, the decrease of AF can be influenced by hemoglobin reabsorption of the fluorescence from deeper dermal layer [
35].
Previous studies demonstrated the correlation between AF intensity and skin phototype. Fluorescence-based methods have higher specificity and sensibility when applied to fair-skinned people [
31]. Our study population is rather homogeneous with a phototype II in almost all cases evaluated (95%), thus allowing an unbiased interpretation of the results.
As extensively described in the literature, other “optical biopsy” techniques such as dermoscopy and confocal microscopy have been demonstrated to improve diagnostic accuracy in NMSC [
17,
36], However, dermoscopy and/or confocal imaging are used for the assessment of surgical margins either for BCC and lentigo maligna, while no evidence still exists about potential benefits of these techniques for SCCs [
15,
16].