Novel Polyethylene Glycol-Conjugated Triazole Derivative with High Thyrointegrin αvβ3 Affinity in Acute Myeloid Leukemia Management
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Tumor Cells and Test Compound
2.2. In Vitro Studies
- (a)
- Evaluation of NF-κB Activity
- (b)
- Cell Vitality Assay (MTT Assay)
2.3. In Vivo Studies
2.4. Statistical Analysis
3. Results
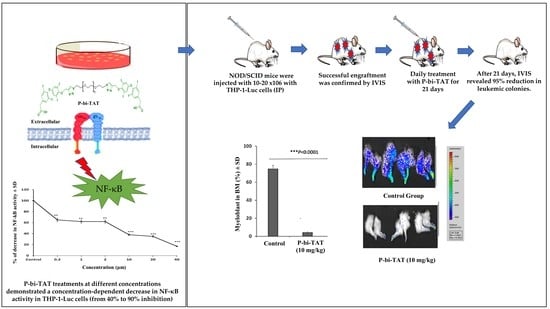
3.1. In Vitro Study of P-bi-TAT Effect on Cell Viability and NF-κB Pathway Activity
3.2. In Vivo Study of P-bi-TAT Therapy Efficacy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cancer Statistics Center. Leukemia Estimates. Available online: https://cancerstatisticscenter.cancer.org/#!/cancer-site/Leukemia (accessed on 11 July 2020).
- Hoffman, R.; Benz, E.J., Jr.; Silberstein, L.E.; Heslop, H.; Anastasi, J.; Weitz, J. Hematology: Basic Principles and Practice; Elsevier Health Sciences: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Kellerman, R.D.; KUSM-W Medical Practice Association. Conn’s Current Therapy 2020, E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Bertoli, S.; Tavitian, S.; Huynh, A.; Borel, C.; Guenounou, S.; Luquet, I.; Delabesse, E.; Sarry, A.; Laurent, G.; Attal, M.; et al. Improved outcome for AML patients over the years 2000–2014. Blood Cancer J. 2017, 7, 635. [Google Scholar] [CrossRef]
- Debreli Coskun, M.; Sudha, T.; Bharali, D.J.; Celikler, S.; Davis, P.J.; Mousa, S.A. αvβ3 Integrin Antagonists Enhance Chemotherapy Response in an Orthotopic Pancreatic Cancer Model. Front. Pharmacol. 2020, 11, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weis, S.M.; Cheresh, D.A. αV integrins in angiogenesis and cancer. Cold Spring Harb. Perspect. Med. 2011, 1, a006478. [Google Scholar] [CrossRef] [Green Version]
- Alday-Parejo, B.; Stupp, R.; Rüegg, C. Are integrins still practicable targets for anti-cancer therapy? Cancers 2019, 11, 978. [Google Scholar] [CrossRef] [Green Version]
- Rajabi, M.; Yalcin, M.; Mousa, S.A. Synthesis of new analogs of tetraiodothyroacetic acid (tetrac) as novel angiogenesis inhibitors for treatment of cancer. Bioorg. Med. Chem. Lett. 2018, 28, 1223–1227. [Google Scholar] [CrossRef]
- Karakus, O.O.; Godugu, K.; Rajabi, M.; Mousa, S.A. Dual Targeting of Norepinephrine Transporter (NET) Function and Thyrointegrin αvβ3 Receptors in the Treatment of Neuroblastoma. J. Med. Chem. 2020, 63, 7653–7662. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Zhao, Y.; Yuan, Z.; Qin, G. Research advances on structure and biological functions of integrins. SpringerPlus 2016, 5, 1094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hudson, S.V.; Dolin, C.E.; Poole, L.G.; Massey, V.L.; Wilkey, D.; Beier, J.I.; Merchant, M.L.; Frieboes, H.B.; Arteel, G.E. Modeling the Kinetics of Integrin Receptor Binding to Hepatic Extracellular Matrix Proteins. Sci. Rep. 2017, 7, 12444. [Google Scholar] [CrossRef] [PubMed]
- Weis, S.M.; Cheresh, D.A. Tumor angiogenesis: Molecular pathways and therapeutic targets. Nat. Med. 2011, 17, 1359–1370. [Google Scholar] [CrossRef]
- Chakraborty, S.; Banerjee, S.; Raina, M.; Haldar, S. Force-Directed “Mechanointeractome” of Talin–Integrin. Biochemistry 2019, 58, 4677–4695. [Google Scholar] [CrossRef]
- Schnittert, J.; Bansal, R.; Storm, G.; Prakash, J. Integrins in wound healing, fibrosis and tumor stroma: High potential targets for therapeutics and drug delivery. Adv. Drug Deliv. Rev. 2018, 129, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, H.; Ivaska, J. Every step of the way: Integrins in cancer progression and metastasis. Nat. Rev. Cancer 2018, 18, 533–548. [Google Scholar] [CrossRef] [Green Version]
- Sökeland, G.; Schumacher, U. The functional role of integrins during intra- and extravasation within the metastatic cascade. Mol. Cancer 2019, 18, 12. [Google Scholar] [CrossRef]
- Yalcin, M.; Lin, H.-Y.; Sudha, T.; Bharali, D.J.; Meng, R.; Tang, H.-Y.; Davis, F.B.; Stain, S.C.; Davis, P.J.; Mousa, S.A. Response of Human Pancreatic Cancer Cell Xenografts to Tetraiodothyroacetic Acid Nanoparticles. Horm. Cancer 2013, 4, 176–185. [Google Scholar] [CrossRef]
- Tai, W.; Mahato, R.; Cheng, K. The role of HER2 in cancer therapy and targeted drug delivery. J. Control Release 2010, 146, 264–275. [Google Scholar] [CrossRef] [Green Version]
- Rajabi, M.; Godugu, K.; Sudha, T.; Bharali, D.J.; Mousa, S.A. Triazole modified tetraiodothyroacetic acid conjugated to polyethylene glycol: High affinity thyrointegrin αvβ3 antagonist with potent anticancer activities in glioblastoma multiforme. Bioconjugate Chem. 2019, 30, 3087–3097. [Google Scholar] [CrossRef]
- Mousa, S.A.; Glinsky, G.V.; Lin, H.-Y.; Ashur-Fabian, O.; Hercbergs, A.; Keating, K.A.; Davis, P.J. Contributions of thyroid hormone to cancer metastasis. Biomedicines 2018, 6, 89. [Google Scholar] [CrossRef] [Green Version]
- Reikvam, H. Inhibition of NF-κB Signaling Alters Acute Myelogenous Leukemia Cell Transcriptomics. Cells 2020, 9, 1677. [Google Scholar] [CrossRef] [PubMed]
- Lira Benício, M.T.; Scheucher, P.S.; Garcia, A.B.; Falcao, R.P.; Rego, E.M. Characterization of leukemic stem cells in AML cell lines using ALDH staining. Blood 2013, 122, 5409. [Google Scholar] [CrossRef]
- Shen, J.; Yang, H.; Ni, W.; Qian, W. Cytotoxicity of homoharringtonine on leukemic stem-like cells in AML cell line KG-1. Zhejiang Da Xue Xue Bao. Yi Xue Ban J. Zhejiang Univ. Med. Sci. 2012, 41, 485–490. [Google Scholar]
- Pabst, T.; Mueller, B.U.; Harakawa, N.; Schoch, C.; Haferlach, T.; Behre, G.; Hiddemann, W.; Zhang, D.E.; Tenen, D.G. AML1-ETO downregulates the granulocytic differentiation factor C/EBPalpha in t(8;21) myeloid leukemia. Nat. Med. 2001, 7, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Trombly, D.J.; Whitfield, T.W.; Padmanabhan, S.; Gordon, J.A.R.; Lian, J.B.; van Wijnen, A.J.; Zaidi, S.K.; Stein, J.L.; Stein, G.S. Genome-wide co-occupancy of AML1-ETO and N-CoR defines the t(8;21) AML signature in leukemic cells. BMC Genom. 2015, 16, 309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleischmann, K.K.; Pagel, P.; Schmid, I.; Roscher, A.A. RNAi-mediated silencing of MLL-AF9 reveals leukemia-associated downstream targets and processes. Mol. Cancer 2014, 13, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shan, W.L.; Ma, X.L. How to establish acute myeloid leukemia xenograft models using immunodeficient mice. Asian Pac. J. Cancer Prev. 2013, 14, 7057–7063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Zhang, Y.; Zhuang, Y.; Wang, J.; Ye, J.; Zhang, S.; Wu, J.; Yu, K.; Han, Y. Matrine induces apoptosis in human acute myeloid leukemia cells via the mitochondrial pathway and Akt inactivation. PLoS ONE 2012, 7, e46853. [Google Scholar] [CrossRef]
- Zhang, Y.; Patel, S.; Abdelouahab, H.; Wittner, M.; Willekens, C.; Shen, S.; Betems, A.; Joulin, V.; Opolon, P.; Bawa, O.; et al. CXCR4 inhibitors selectively eliminate CXCR4-expressing human acute myeloid leukemia cells in NOG mouse model. Cell Death Dis. 2012, 3, e396. [Google Scholar] [CrossRef]
- Lee, M.W.; Kim, H.J.; Yoo, K.H.; Kim, D.S.; Yang, J.M.; Kim, H.R.; Noh, Y.H.; Baek, H.; Kwon, H.; Son, M.H.; et al. Establishment of a bioluminescent imaging-based in vivo leukemia model by intra-bone marrow injection. Int. J. Oncol. 2012, 41, 2047–2056. [Google Scholar] [CrossRef]
- Hodson, D.J.; Screen, M.; Turner, M. RNA-binding proteins in hematopoiesis and hematological malignancy. Blood 2019, 133, 2365–2373. [Google Scholar] [CrossRef] [Green Version]
- Pina-Oviedo, S.; Khogeer, H.A.; Tang, G.; Miranda, R.N. Hematopathology. In Oncological Surgical Pathology; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1729–2141. [Google Scholar]
- Bae, J.; Samur, M.; Richardson, P.; Munshi, N.C.; Anderson, K.C. Selective targeting of multiple myeloma by B cell maturation antigen (BCMA)-specific central memory CD8+ cytotoxic T lymphocytes: Immunotherapeutic application in vaccination and adoptive immunotherapy. Leukemia 2019, 33, 2208–2226. [Google Scholar] [CrossRef]
- Guevara, M.L.; Persano, F.; Persano, S. Nano-immunotherapy: Overcoming tumour immune evasion. Semin. Cancer Biol. 2019, 69, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Olino, K.; Park, T.; Ahuja, N. Exposing Hidden Targets: Combining epigenetic and immunotherapy to overcome cancer resistance. Semin Cancer Biol. 2020, 65, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Bayat Mokhtari, R.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sami, S.A.; Darwish, N.H.E.; Barile, A.N.M.; Mousa, S.A. Current and Future Molecular Targets for Acute Myeloid Leukemia Therapy. Curr. Treat. Options Oncol. 2020, 21, 3. [Google Scholar] [CrossRef] [PubMed]
- Kapp, T.G.; Rechenmacher, F.; Neubauer, S.; Maltsev, O.V.; Cavalcanti-Adam, E.A.; Zarka, R.; Reuning, U.; Notni, J.; Wester, H.-J.; Mas-Moruno, C.; et al. A Comprehensive Evaluation of the Activity and Selectivity Profile of Ligands for RGD-binding Integrins. Sci. Rep. 2017, 7, 39805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theocharis, A.D.; Manou, D.; Karamanos, N.K. The extracellular matrix as a multitasking player in disease. FEBS J. 2019, 286, 2830–2869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darwish, N.H.E.; Sudha, T.; Godugu, K.; Elbaz, O.; Abdelghaffar, H.A.; Hassan, E.E.A.; Mousa, S.A. Acute myeloid leukemia stem cell markers in prognosis and targeted therapy: Potential impact of BMI-1, TIM-3 and CLL-1. Oncotarget 2016, 7, 57811–57820. [Google Scholar] [CrossRef] [Green Version]
- Johansen, S.; Brenner, A.K.; Bartaula-Brevik, S.; Reikvam, H.; Bruserud, Ø. The Possible Importance of β3 Integrins for Leukemogenesis and Chemoresistance in Acute Myeloid Leukemia. Int. J. Mol. Sci. 2018, 19, 251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kechagia, J.Z.; Ivaska, J.; Roca-Cusachs, P. Integrins as biomechanical sensors of the microenvironment. Nat. Rev. Mol. Cell Biol. 2019, 20, 457–473. [Google Scholar] [CrossRef]
- Janiszewska, M.; Primi, M.C.; Izard, T. Cell adhesion in cancer: Beyond the migration of single cells. J. Biol. Chem. 2020, 295, 2495–2505. [Google Scholar] [CrossRef] [Green Version]
- Reichardt, L.F.; Tomaselli, K.J. Extracellular matrix molecules and their receptors: Functions in neural development. Annu. Rev. Neurosci. 1991, 14, 531–570. [Google Scholar] [CrossRef]
- Niu, G.; Chen, X. Why Integrin as a Primary Target for Imaging and Therapy. Theranostics 2011, 1, 30–47. [Google Scholar] [CrossRef] [Green Version]
- Yi, H.; Zeng, D.; Shen, Z.; Liao, J.; Wang, X.; Liu, Y.; Zhang, X.; Kong, P. Integrin alphavbeta3 enhances β-catenin signaling in acute myeloid leukemia harboring Fms-like tyrosine kinase-3 internal tandem duplication mutations: Implications for microenvironment influence on sorafenib sensitivity. Oncotarget 2016, 7, 40387–40397. [Google Scholar] [CrossRef] [Green Version]
- Abbi, S.; Guan, J.L. Focal adhesion kinase: Protein interactions and cellular functions. Histol. Histopathol. 2002, 17, 1163–1171. [Google Scholar] [CrossRef]
- Antenucci, L.; Hytönen, V.P.; Ylänne, J. Phosphorylated immunoreceptor tyrosine-based activation motifs and integrin cytoplasmic domains activate spleen tyrosine kinase via distinct mechanisms. J. Biol. Chem. 2018, 293, 4591–4602. [Google Scholar] [CrossRef] [Green Version]
- Despeaux, M.; Chicanne, G.; Rouer, E.; De Toni-Costes, F.; Bertrand, J.; Mansat-De Mas, V.; Vergnolle, N.; Eaves, C.; Payrastre, B.; Girault, J.A. Focal adhesion kinase splice variants maintain primitive acute myeloid leukemia cells through altered Wnt signaling. Stem Cells 2012, 30, 1597–1610. [Google Scholar] [CrossRef] [PubMed]
- Recher, C.; Ysebaert, L.; Beyne-Rauzy, O.; Mansat-De Mas, V.; Ruidavets, J.-B.; Cariven, P.; Demur, C.; Payrastre, B.; Laurent, G.; Racaud-Sultan, C. Expression of focal adhesion kinase in acute myeloid leukemia is associated with enhanced blast migration, increased cellularity, and poor prognosis. Cancer Res. 2004, 64, 3191–3197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carter, B.Z.; Mak, P.Y.; Wang, X.; Yang, H.; Garcia-Manero, G.; Mak, D.H.; Mu, H.; Ruvolo, V.R.; Qiu, Y.; Coombes, K. Focal adhesion kinase as a potential target in AML and MDS. Mol. Cancer Ther. 2017, 16, 1133–1144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatterjee, A.; Ghosh, J.; Ramdas, B.; Mali, R.S.; Martin, H.; Kobayashi, M.; Vemula, S.; Canela, V.H.; Waskow, E.R.; Visconte, V. Regulation of Stat5 by FAK and PAK1 in oncogenic FLT3-and KIT-driven leukemogenesis. Cell Rep. 2014, 9, 1333–1348. [Google Scholar] [CrossRef] [Green Version]
- Tavernier-Tardy, E.; Cornillon, J.; Campos, L.; Flandrin, P.; Duval, A.; Nadal, N.; Guyotat, D. Prognostic value of CXCR4 and FAK expression in acute myelogenous leukemia. Leuk. Res. 2009, 33, 764–768. [Google Scholar] [CrossRef]
- Tohyama, Y.; Yanagi, S.; Sada, K.; Yamamura, H. Translocation of p72syk to the cytoskeleton in thrombin-stimulated platelets. J. Biol. Chem. 1994, 269, 32796–32799. [Google Scholar] [CrossRef]
- Sudha, T.; Bharali, D.J.; Yalcin, M.; Darwish, N.H.; Coskun, M.D.; Keating, K.A.; Lin, H.-Y.; Davis, P.J.; Mousa, S.A. Targeted delivery of cisplatin to tumor xenografts via the nanoparticle component of nano-diamino-tetrac. Nanomedicine 2017, 12, 195–205. [Google Scholar] [CrossRef] [Green Version]
- Davis, P.J.; Goglia, F.; Leonard, J.L. Nongenomic actions of thyroid hormone. Nat. Rev. Endocrinol. 2016, 12, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Davis, P.J.; Glinsky, G.V.; Lin, H.-Y.; Leith, J.T.; Hercbergs, A.; Tang, H.-Y.; Ashur-Fabian, O.; Incerpi, S.; Mousa, S.A. Cancer cell gene expression modulated from plasma membrane integrin avb3 by thyroid hormone and nanoparticulate tetrac. Front. Endocrinol. 2014, 5, 240. [Google Scholar] [CrossRef] [Green Version]
- Davis, P.J.; Mousa, S.A.; Lin, H.-Y. Nongenomic Actions of Thyroid Hormone: The Integrin Component. Physiol. Rev. 2021, 101, 319–352. [Google Scholar] [CrossRef]
- Ho, Y.; Wu, C.Y.; Chin, Y.T.; Li, Z.L.; Pan, Y.S.; Huang, T.Y.; Su, P.Y.; Lee, S.Y.; Crawford, D.R.; Su, K.W.; et al. NDAT suppresses pro-inflammatory gene expression to enhance resveratrol-induced anti-proliferation in oral cancer cells. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2020, 136, 111092. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.R.; Chen, Y.S.; Chin, Y.T.; Li, Z.L.; Shih, Y.J.; Yang, Y.S.H.; ChangOu, C.A.; Su, P.Y.; Wang, S.H.; Wu, Y.H.; et al. Thyroid hormone-induced expression of inflammatory cytokines interfere with resveratrol-induced anti-proliferation of oral cancer cells. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2019, 132, 110693. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-Y.; Glinsky, G.V.; Mousa, S.A.; Davis, P.J. Thyroid hormone and anti-apoptosis in tumor cells. Oncotarget 2015, 6, 14735. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sudha, T.; Godugu, K.; Darwish, N.H.E.; Nazeer, T.; Mousa, S.A. Novel Polyethylene Glycol-Conjugated Triazole Derivative with High Thyrointegrin αvβ3 Affinity in Acute Myeloid Leukemia Management. Cancers 2021, 13, 4070. https://doi.org/10.3390/cancers13164070
Sudha T, Godugu K, Darwish NHE, Nazeer T, Mousa SA. Novel Polyethylene Glycol-Conjugated Triazole Derivative with High Thyrointegrin αvβ3 Affinity in Acute Myeloid Leukemia Management. Cancers. 2021; 13(16):4070. https://doi.org/10.3390/cancers13164070
Chicago/Turabian StyleSudha, Thangirala, Kavitha Godugu, Noureldien H. E. Darwish, Tipu Nazeer, and Shaker A. Mousa. 2021. "Novel Polyethylene Glycol-Conjugated Triazole Derivative with High Thyrointegrin αvβ3 Affinity in Acute Myeloid Leukemia Management" Cancers 13, no. 16: 4070. https://doi.org/10.3390/cancers13164070
APA StyleSudha, T., Godugu, K., Darwish, N. H. E., Nazeer, T., & Mousa, S. A. (2021). Novel Polyethylene Glycol-Conjugated Triazole Derivative with High Thyrointegrin αvβ3 Affinity in Acute Myeloid Leukemia Management. Cancers, 13(16), 4070. https://doi.org/10.3390/cancers13164070