Genomic Characterization and Therapeutic Targeting of HPV Undetected Cervical Carcinomas
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Cohorts
2.2. Defining Tumors with Undetectable HPV (HPVU)
2.3. Genomic DNA Analyses
2.4. RNAseq Analysis
2.5. Cell Line Selection
2.6. HPVU Cell Line Sensitivity to Palbociclib
2.7. Palbociclib Induced G1 Arrest and Proliferation Attenuation
2.8. Statistics
3. Results
3.1. Survival Outcomes Are Poor for Patients with HPVU Cervical Tumors
3.2. Discovery of Significantly Mutated Genes Defines a Distinct Biology in HPVU Tumors
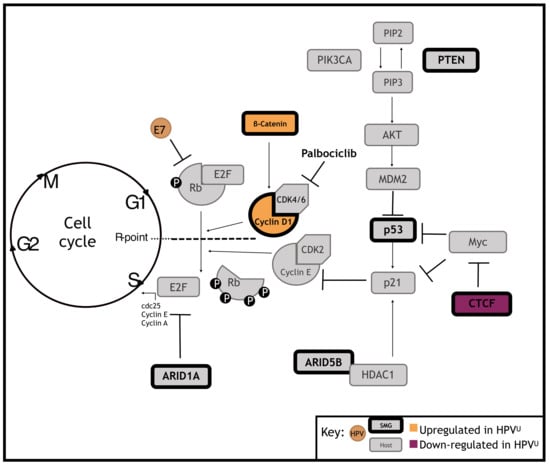
3.3. Significantly Mutated Genes Disrupt Cell Cycle Regulation in HPVU Cervical Cancer
3.4. Identification of a Clinically Actionable Target in HPVU CC
3.5. HPVU Cell Lines with Intact RB1 Are Selectively Sensitivity to CDK4/6 Inhibition
3.6. CDK4/6 Inhibition Induces G1 Arrest and Decreased Proliferation in HPVU Cell Lines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saraiya, M.; Unger, E.R.; Thompson, T.D.; Lynch, C.F.; Hernandez, B.Y.; Lyu, C.W.; Steinau, M.; Watson, M.; Wilkinson, E.J.; Hopenhayn, C.; et al. US Assessment of HPV Types in Cancers: Implications for Current and 9-Valent HPV Vaccines. J. Natl. Cancer Inst. 2015, 107, djv086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reimers, N.; Kasper, H.U.; Weissenborn, S.J.; Stützer, H.; Preuss, S.F.; Hoffmann, T.K.; Speel, E.J.M.; Dienes, H.P.; Pfister, H.J.; Guntinas-Lichius, O.; et al. Combined Analysis of HPV-DNA, P16 and EGFR Expression to Predict Prognosis in Oropharyngeal Cancer. Int. J. Cancer 2007, 120, 1731–1738. [Google Scholar] [CrossRef] [PubMed]
- Fakhry, C.; Lacchetti, C.; Rooper, L.M.; Jordan, R.C.; Rischin, D.; Sturgis, E.M.; Bell, D.; Lingen, M.W.; Harichand-Herdt, S.; Thibo, J.; et al. Human Papillomavirus Testing in Head and Neck Carcinomas: ASCO Clinical Practice Guideline Endorsement of the College of American Pathologists Guideline. J. Clin. Oncol. 2018, 36, 3152–3161. [Google Scholar] [CrossRef]
- Smith, E.M.; Wang, D.; Kim, Y.; Rubenstein, L.M.; Lee, J.H.; Haugen, T.H.; Turek, L.P. P16INK4a Expression, Human Papillomavirus, and Survival in Head and Neck Cancer. Oral Oncol. 2008, 44, 133–142. [Google Scholar] [CrossRef]
- Dayyani, F.; Etzel, C.J.; Liu, M.; Ho, C.-H.; Lippman, S.M.; Tsao, A.S. Meta-Analysis of the Impact of Human Papillomavirus (HPV) on Cancer Risk and Overall Survival in Head and Neck Squamous Cell Carcinomas (HNSCC). Head Neck Oncol. 2010, 2, 15. [Google Scholar] [CrossRef] [Green Version]
- Harima, Y.; Sawada, S.; Nagata, K.; Sougawa, M.; Ohnishi, T. Human Papilloma Virus (HPV) DNA Associated with Prognosis of Cervical Cancer after Radiotherapy. Int. J. Radiat. Oncol. 2002, 52, 1345–1351. [Google Scholar] [CrossRef]
- Lindel, K.; Burri, P.; Studer, H.U.; Altermatt, H.J.; Greiner, R.H.; Gruber, G. Human Papillomavirus Status in Advanced Cervical Cancer: Predictive and Prognostic Significance for Curative Radiation Treatment. Int. J. Gynecol. Cancer 2005, 15, 278–284. [Google Scholar] [CrossRef]
- Rodríguez-Carunchio, L.; Soveral, I.; Steenbergen, R.D.M.; Torné, A.; Martinez, S.; Fusté, P.; Pahisa, J.; Marimon, L.; Ordi, J.; del Pino, M. HPV-Negative Carcinoma of the Uterine Cervix: A Distinct Type of Cervical Cancer with Poor Prognosis. BJOG Int. J. Obstet. Gynaecol. 2015, 122, 119–127. [Google Scholar] [CrossRef]
- Okuma, K.; Yamashita, H.; Yokoyama, T.; Nakagawa, K.; Kawana, K. Undetected Human Papillomavirus DNA and Uterine Cervical Carcinoma. Strahlenther. Onkol. 2016, 192, 55–62. [Google Scholar] [CrossRef]
- Li, P.; Tan, Y.; Zhu, L.-X.; Zhou, L.-N.; Zeng, P.; Liu, Q.; Chen, M.-B.; Tian, Y. Prognostic Value of HPV DNA Status in Cervical Cancer before Treatment: A Systematic Review and Meta-Analysis. Oncotarget 2017, 8, 66352–66359. [Google Scholar] [CrossRef] [Green Version]
- Jeronimo, J.; Castle, P.E.; Temin, S.; Denny, L.; Gupta, V.; Kim, J.J.; Luciani, S.; Murokora, D.; Ngoma, T.; Qiao, Y.; et al. Secondary Prevention of Cervical Cancer: ASCO Resource-Stratified Clinical Practice Guideline. J. Glob. Oncol. 2016, 3, 635–657. [Google Scholar] [CrossRef]
- Uchiyama, M.; Iwasaka, T.; Matsuo, N.; Hachisuga, T.; Mori, M.; Sugimori, H. Correlation between Human Papillomavirus Positivity and P53 Gene Overexpression in Adenocarcinoma of the Uterine Cervix. Gynecol. Oncol. 1997, 65, 23–29. [Google Scholar] [CrossRef]
- Banister, C.E.; Liu, C.; Pirisi, L.; Creek, K.E.; Buckhaults, P.J. Identification and Characterization of HPV-Independent Cervical Cancers. Oncotarget 2017, 8, 13375–13386. [Google Scholar] [CrossRef] [Green Version]
- The Cancer Genome Atlas Research Network. Integrated Genomic and Molecular Characterization of Cervical Cancer. Nature 2017, 543, 378–384. [Google Scholar] [CrossRef]
- Chong, G.O.; Lee, Y.H.; Han, H.S.; Lee, H.J.; Park, J.Y.; Hong, D.G.; Lee, Y.S.; Cho, Y.L. Prognostic Value of Pre-Treatment Human Papilloma Virus DNA Status in Cervical Cancer. Gynecol. Oncol. 2018, 148, 97–102. [Google Scholar] [CrossRef]
- Ojesina, A.I.; Lichtenstein, L.; Freeman, S.S.; Pedamallu, C.S.; Imaz-Rosshandler, I.; Pugh, T.J.; Cherniack, A.D.; Ambrogio, L.; Cibulskis, K.; Bertelsen, B.; et al. Landscape of Genomic Alterations in Cervical Carcinomas. Nature 2014, 506, 371–375. [Google Scholar] [CrossRef]
- Kandoth, C.; McLellan, M.D.; Vandin, F.; Ye, K.; Niu, B.; Lu, C.; Xie, M.; Zhang, Q.; McMichael, J.F.; Wyczalkowski, M.A.; et al. Mutational Landscape and Significance across 12 Major Cancer Types. Nature 2013, 502, 333–339. [Google Scholar] [CrossRef] [Green Version]
- Kostic, A.D.; Ojesina, A.I.; Pedamallu, C.S.; Jung, J.; Verhaak, R.G.W.; Getz, G.; Meyerson, M. PathSeq: Software to Identify or Discover Microbes by Deep Sequencing of Human Tissue. Nat. Biotechnol. 2011, 29, 393–396. [Google Scholar] [CrossRef] [Green Version]
- Cibulskis, K.; Lawrence, M.S.; Carter, S.L.; Sivachenko, A.; Jaffe, D.; Sougnez, C.; Gabriel, S.; Meyerson, M.; Lander, E.S.; Getz, G. Sensitive Detection of Somatic Point Mutations in Impure and Heterogeneous Cancer Samples. Nat. Biotechnol. 2013, 31, 213–219. [Google Scholar] [CrossRef]
- Lawrence, M.S.; Stojanov, P.; Polak, P.; Kryukov, G.V.; Cibulskis, K.; Sivachenko, A.; Carter, S.L.; Stewart, C.; Mermel, C.H.; Roberts, S.A.; et al. Mutational Heterogeneity in Cancer and the Search for New Cancer-Associated Genes. Nature 2013, 499, 214–218. [Google Scholar] [CrossRef]
- Mermel, C.H.; Schumacher, S.E.; Hill, B.; Meyerson, M.L.; Beroukhim, R.; Getz, G. GISTIC2.0 Facilitates Sensitive and Confident Localization of the Targets of Focal Somatic Copy-Number Alteration in Human Cancers. Genome Biol. 2011, 12, R41. [Google Scholar] [CrossRef] [Green Version]
- Fry, D.W.; Harvey, P.J.; Keller, P.R.; Elliott, W.L.; Meade, M.; Trachet, E.; Albassam, M.; Zheng, X.; Leopold, W.R.; Pryer, N.K.; et al. Specific Inhibition of Cyclin-Dependent Kinase 4/6 by PD 0332991 and Associated Antitumor Activity in Human Tumor Xenografts. Mol. Cancer Ther. 2004, 3, 1427–1438. [Google Scholar]
- Cosmic COSMIC—Catalogue of Somatic Mutations in Cancer. Available online: https://cancer.sanger.ac.uk/cell_lines (accessed on 4 November 2019).
- Schlecht, N.F.; Brandwein-Gensler, M.; Nuovo, G.J.; Li, M.; Dunne, A.; Kawachi, N.; Smith, R.V.; Burk, R.D.; Prystowsky, M.B. A Comparison of Clinically Utilized Human Papillomavirus Detection Methods in Head and Neck Cancer. Mod. Pathol. 2011, 24, 1295–1305. [Google Scholar] [CrossRef] [Green Version]
- Götz, C.; Drecoll, E.; Straub, M.; Bissinger, O.; Wolff, K.-D.; Kolk, A. Impact of HPV Infection on Oral Squamous Cell Carcinoma. Oncotarget 2016, 7, 76704–76712. [Google Scholar] [CrossRef] [Green Version]
- The Cancer Genome Atlas Network. Comprehensive Genomic Characterization of Head and Neck Squamous Cell Carcinomas. Nature 2015, 517, 576–582. [Google Scholar] [CrossRef] [Green Version]
- Cancer Genome Atlas Research Network; Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.G.; Pashtan, I.; Shen, R.; et al. Integrated Genomic Characterization of Endometrial Carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Bueno, G.; Rodríguez-Perales, S.; Sánchez-Estévez, C.; Hardisson, D.; Sarrió, D.; Prat, J.; Cigudosa, J.C.; Matias-Guiu, X.; Palacios, J. Cyclin D1 Gene (CCND1) Mutations in Endometrial Cancer. Oncogene 2003, 22, 6115–6118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishii, Y.; Nakahara, T.; Kataoka, M.; Kusumoto-Matsuo, R.; Mori, S.; Takeuchi, T.; Kukimoto, I. Identification of TRAPPC8 as a Host Factor Required for Human Papillomavirus Cell Entry. PLoS ONE 2013, 8, e80297. [Google Scholar] [CrossRef]
- Paris, C.; Pentland, I.; Groves, I.; Roberts, D.C.; Powis, S.J.; Coleman, N.; Roberts, S.; Parish, J.L. CCCTC-Binding Factor Recruitment to the Early Region of the Human Papillomavirus 18 Genome Regulates Viral Oncogene Expression. J. Virol. 2015, 89, 4770–4785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uesugi, A.; Kozaki, K.-I.; Tsuruta, T.; Furuta, M.; Morita, K.-I.; Imoto, I.; Omura, K.; Inazawa, J. The Tumor Suppressive MicroRNA MiR-218 Targets the MTOR Component Rictor and Inhibits AKT Phosphorylation in Oral Cancer. Cancer Res. 2011, 71, 5765–5778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, I.; Gardiner, A.S.; Board, K.F.; Monzon, F.A.; Edwards, R.P.; Khan, S.A. Human Papillomavirus Type 16 Reduces the Expression of MicroRNA-218 in Cervical Carcinoma Cells. Oncogene 2008, 27, 2575–2582. [Google Scholar] [CrossRef] [Green Version]
- Akgül, S.; Li, Y.; Zheng, S.; Kool, M.; Treisman, D.M.; Li, C.; Wang, Y.; Gröbner, S.; Ikenoue, T.; Shen, Y.; et al. Opposing Tumor-Promoting and -Suppressive Functions of Rictor/MTORC2 Signaling in Adult Glioma and Pediatric SHH Medulloblastoma. Cell Rep. 2018, 24, 463–478.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizutani, A.; Koinuma, D.; Seimiya, H.; Miyazono, K. The Arkadia-ESRP2 Axis Suppresses Tumor Progression: Analyses in Clear-Cell Renal Cell Carcinoma. Oncogene 2016, 35, 3514–3523. [Google Scholar] [CrossRef] [Green Version]
- Sharma, V.; Antonacopoulou, A.G.; Tanaka, S.; Panoutsopoulos, A.A.; Bravou, V.; Kalofonos, H.P.; Episkopou, V. Enhancement of TGF-β Signaling Responses by the E3 Ubiquitin Ligase Arkadia Provides Tumor Suppression in Colorectal Cancer. Cancer Res. 2011, 71, 6438–6449. [Google Scholar] [CrossRef] [Green Version]
- Briones-Orta, M.A.; Levy, L.; Madsen, C.D.; Das, D.; Erker, Y.; Sahai, E.; Hill, C.S. Arkadia Regulates Tumor Metastasis by Modulation of the TGF-β Pathway. Cancer Res. 2013, 73, 1800–1810. [Google Scholar] [CrossRef] [Green Version]
- Davis, T.L.; Walker, J.R.; Campagna-Slater, V.; Finerty, P.J.; Paramanathan, R.; Bernstein, G.; MacKenzie, F.; Tempel, W.; Ouyang, H.; Lee, W.H.; et al. Structural and Biochemical Characterization of the Human Cyclophilin Family of Peptidyl-Prolyl Isomerases. PLoS Biol. 2010, 8, e1000439. [Google Scholar] [CrossRef] [Green Version]
- Jurica, M.S.; Licklider, L.J.; Gygi, S.R.; Grigorieff, N.; Moore, M.J. Purification and Characterization of Native Spliceosomes Suitable for Three-Dimensional Structural Analysis. RNA 2002, 8, 426–439. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Eom, K.; Kim, J.; Bang, H.; Wang, H.; Ahn, S.; Kim, G.; Jang, H.; Kim, S.; Lee, D.; et al. MiR-9, MiR-21, and MiR-155 as Potential Biomarkers for HPV Positive and Negative Cervical Cancer. BMC Cancer 2017, 17, 658. [Google Scholar] [CrossRef]
- Kennedy, A.L.; Rai, R.; Isingizwe, Z.R.; Zhao, Y.D.; Lightfoot, S.A.; Benbrook, D.M. Complementary Targeting of Rb Phosphorylation and Growth in Cervical Cancer Cell Cultures and a Xenograft Mouse Model by SHetA2 and Palbociclib. Cancers 2020, 12, 1269. [Google Scholar] [CrossRef]
- Xiong, Y.; Li, T.; Assani, G.; Ling, H.; Zhou, Q.; Zeng, Y.; Zhou, F.; Zhou, Y. Ribociclib, a Selective Cyclin D Kinase 4/6 Inhibitor, Inhibits Proliferation and Induces Apoptosis of Human Cervical Cancer in Vitro and in Vivo. Biomed. Pharmacother. Biomed. Pharmacother. 2019, 112, 108602. [Google Scholar] [CrossRef]
- Göttgens, E.-L.; Bussink, J.; Leszczynska, K.B.; Peters, H.; Span, P.N.; Hammond, E.M. Inhibition of CDK4/CDK6 Enhances Radiosensitivity of HPV Negative Head and Neck Squamous Cell Carcinomas. Int. J. Radiat. Oncol. 2019, 105, 548–558. [Google Scholar] [CrossRef]




| Whole-Exome Analysis: 1569 Genes | ||||
| NOR-TCGA | Gene | Relative Frequency of HPV Undetected Tumors with Mutation (%) (n = 24) | Relative Frequency of HPV Positive Tumors with Mutation (%) (n = 355) | FDR p-Value |
| TP53 | 50.0 | 3.1 | 2.65 × 10−7 | |
| ARID1A | 33.3 | 5.4 | 2.05 × 10−2 | |
| RICTOR | 20.8 | 0.6 | 1.01 × 10−2 | |
| ARHGEF2 | 20.8 | 1.4 | 3.05 × 10−2 | |
| ZNF331 | 16.7 | 0.3 | 3.14 × 10−2 | |
| CTNNB1 | 16.7 | 0.3 | 3.14 × 10−2 | |
| KIAA1012 | 16.7 | 0.3 | 3.14 × 10−2 | |
| MC5R | 12.5 | 0 | 4.41 × 10−2 | |
| Combined Targeted Gene Panel and Whole-Exome Analysis: 211 Genes | ||||
| NOR-TCGA + WUSM | Gene | Relative Frequency of HPV Undetected Tumors with Mutation (%) (n = 33) | Relative Frequency of HPV Positive Tumors with Mutation (%) (n = 418) | FDR p-Value |
| TP53 | 45.45 | 3.83 | 8.82 × 10−9 | |
| ARID1A | 33.33 | 6.22 | 9.61 × 10−4 | |
| PTEN | 30.3 | 5.74 | 1.75 × 10−3 | |
| ARID5B | 21.21 | 0.72 | 6.43 × 10−5 | |
| CTNNB1 | 15.15 | 0.96 | 6.69 × 10−3 | |
| CTCF | 15.15 | 1.44 | 1.84 × 10−2 | |
| CCND1 | 9.09 | 0.24 | 4.11 × 10−2 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz, F.J.; Sundaresan, A.; Zhang, J.; Pedamallu, C.S.; Halle, M.K.; Srinivasasainagendra, V.; Zhang, J.; Muhammad, N.; Stanley, J.; Markovina, S.; et al. Genomic Characterization and Therapeutic Targeting of HPV Undetected Cervical Carcinomas. Cancers 2021, 13, 4551. https://doi.org/10.3390/cancers13184551
Ruiz FJ, Sundaresan A, Zhang J, Pedamallu CS, Halle MK, Srinivasasainagendra V, Zhang J, Muhammad N, Stanley J, Markovina S, et al. Genomic Characterization and Therapeutic Targeting of HPV Undetected Cervical Carcinomas. Cancers. 2021; 13(18):4551. https://doi.org/10.3390/cancers13184551
Chicago/Turabian StyleRuiz, Fiona J., Aishwarya Sundaresan, Jin Zhang, Chandra S. Pedamallu, Mari K. Halle, Vinodh Srinivasasainagendra, Jianqing Zhang, Naoshad Muhammad, Jennifer Stanley, Stephanie Markovina, and et al. 2021. "Genomic Characterization and Therapeutic Targeting of HPV Undetected Cervical Carcinomas" Cancers 13, no. 18: 4551. https://doi.org/10.3390/cancers13184551
APA StyleRuiz, F. J., Sundaresan, A., Zhang, J., Pedamallu, C. S., Halle, M. K., Srinivasasainagendra, V., Zhang, J., Muhammad, N., Stanley, J., Markovina, S., Tiwari, H. K., Grigsby, P. W., Krakstad, C., Schwarz, J. K., & Ojesina, A. I. (2021). Genomic Characterization and Therapeutic Targeting of HPV Undetected Cervical Carcinomas. Cancers, 13(18), 4551. https://doi.org/10.3390/cancers13184551