The Cancer-Associated Antigens Sialyl Lewisa/x and Sda: Two Opposite Faces of Terminal Glycosylation
Abstract
:Simple Summary
Abstract
1. Introduction
2. The Sda Antigen: Structure and Biosynthesis
3. Sda/B4GALNT2 in Development, Differentiation, and Cancer
3.1. Development and Differentiation
3.2. Cancer
3.2.1. Clinical Studies
Colon Cancer
Gastric Cancer
Breast Cancer
4. Sialyl Lewis Antigens
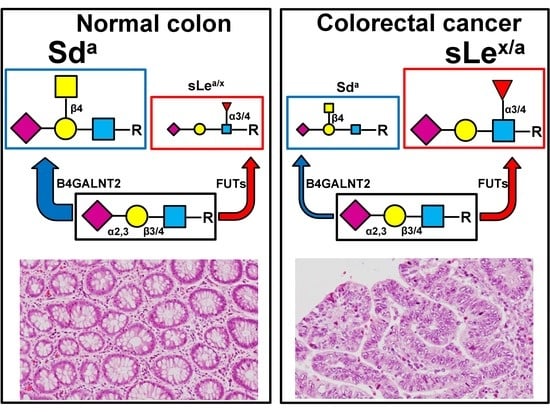
4.1. Structure and Biosynthesis in Normal and Cancer Colon
4.2. Role in Malignancy
5. Phenotypic Effects of B4GALNT2 on Cancer Phenotype
6. Discussion
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BRCA | breast cancer |
| COADREAD | colon adenocarcinoma rectal adenocarcinoma |
| CRC | colorectal cancer |
| DTDST | diastrophic dysplasia sulfate transporter |
| HBE | high-B4GALNT2 expressers |
| LBE | low-B4GALNT2 expressers |
| NS | northern shore |
| OS | open sea |
| RT-PCR | reverse transcriptase-polymerase chain reaction |
| sLea | sialyl Lewisa |
| sLex | sialyl Lewisx |
| SCLC | small cell lung cancer |
| SS | southern shore |
| STAD | stomach adenocarcinoma |
| TCGA | The Cancer Genome Atlas |
| TLR4 | Toll-like receptor 4 |
References
- Dall’Olio, F.; Malagolini, N.; Trinchera, M.; Chiricolo, M. Mechanisms of cancer-associated glycosylation changes. Front. Biosci. 2012, 17, 670–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dall’Olio, F.; Malagolini, N.; Trinchera, M.; Chiricolo, M. Sialosignaling: Sialyltransferases as engines of self-fueling loops in cancer progression. Biochim. Biophys. Acta 2014, 1840, 2752–2764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinho, S.S.; Reis, C.A. Glycosylation in cancer: Mechanisms and clinical implications. Nat. Rev. Cancer 2015, 15, 540–555. [Google Scholar] [CrossRef]
- Morton, J.A.; Pickles, M.M.; Terry, A.M. The Sda blood group antigen in tissues and body fluids. Vox Sang. 1970, 19, 472–482. [Google Scholar]
- Morton, J.A.; Pickles, M.M.; Vanhegan, R.I. The Sda antigen in the human kidney and colon. Immunol. Investig. 1988, 17, 217–224. [Google Scholar] [CrossRef]
- Renton, P.H.; Howell, P.; Ikin, E.W.; Giles, C.M.; Goldsmith, K.L. Anti Sda: A new blood group antibody. Vox Sang. 1967, 13, 493–501. [Google Scholar] [CrossRef]
- Macvie, S.I.; Morton, J.A.; Pickles, M.M. The reactions and inheritance of a new blood group antigen. Vox Sang. 1967, 13, 485–492. [Google Scholar]
- Cramer, M.L.; Xu, R.; Martin, P.T. Soluble Heparin Binding EGF-like Growth Factor (HB-EGF) is a regulator of GALGT2 expression and GALGT2-dependent muscle and neuromuscular phenotypes. Mol. Cell Biol. 2019, 39, e00140-19. [Google Scholar] [CrossRef] [Green Version]
- Xu, R.; Singhal, N.; Serinagaoglu, Y.; Chandrasekharan, K.; Joshi, M.; Bauer, J.A.; Janssen, P.M.; Martin, P.T. Deletion of Galgt2 (B4Galnt2) Reduces Muscle Growth in Response to Acute Injury and Increases Muscle Inflammation and Pathology in Dystrophin-Deficient Mice. Am. J. Pathol. 2015, 185, 2668–2684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallier, M.; Abou, C.M.; Hindersin, L.; Linnenbrink, M.; Traulsen, A.; Baines, J.F. Evaluating the maintenance of disease-associated variation at the blood group-related gene B4galnt2 in house mice. BMC Evol. Biol. 2017, 17, 187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linnenbrink, M.; Johnsen, J.M.; Montero, I.; Brzezinski, C.R.; Harr, B.; Baines, J.F. Long-term balancing selection at the blood group-related gene B4galnt2 in the genus Mus (Rodentia; Muridae). Mol. Biol. Evol. 2011, 28, 2999–3003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staubach, F.; Kunzel, S.; Baines, A.C.; Yee, A.; McGee, B.M.; Backhed, F.; Baines, J.F.; Johnsen, J.M. Expression of the blood-group-related glycosyltransferase B4galnt2 influences the intestinal microbiota in mice. ISME J. 2012, 6, 1345–1355. [Google Scholar] [CrossRef] [Green Version]
- Galeev, A.; Suwandi, A.; Cepic, A.; Basu, M.; Baines, J.F.; Grassl, G.A. The role of the blood group-related glycosyltransferases FUT2 and B4GALNT2 in susceptibility to infectious disease. Int. J. Med. Microbiol. 2021, 311, 151487. [Google Scholar] [CrossRef] [PubMed]
- Ben, J.S.; Ruesche, J.; Sarry, J.; Woloszyn, F.; Lassoued, N.; Fabre, S. The high prolificacy of D’man sheep is associated with the segregation of the FecL(L) mutation in the B4GALNT2 gene. Reprod. Domest. Anim. 2018, 54, 531–537. [Google Scholar] [CrossRef]
- Byrne, G.; Ahmad-Villiers, S.; Du, Z.; McGregor, C. B4GALNT2 and xenotransplantation: A newly appreciated xenogeneic antigen. Xenotransplantation 2018, 25, e12394. [Google Scholar] [CrossRef] [PubMed]
- Heaton, B.E.; Kennedy, E.M.; Dumm, R.E.; Harding, A.T.; Sacco, M.T.; Sachs, D.; Heaton, N.S. A CRISPR Activation Screen Identifies a Pan-avian Influenza Virus Inhibitory Host Factor. Cell Rep. 2017, 20, 1503–1512. [Google Scholar] [CrossRef] [Green Version]
- Wong, H.H.; Fung, K.; Nicholls, J.M. MDCK-B4GalNT2 cells disclose a a2,3-sialic acid requirement for the 2009 pandemic H1N1 A/California/04/2009 and NA aid entry of A/WSN/33. Emerg. Microbes. Infect. 2019, 8, 1428–1437. [Google Scholar] [CrossRef] [Green Version]
- Donald, A.S.; Yates, A.D.; Soh, C.P.; Morgan, W.T.; Watkins, W.M. A blood group Sda-active pentasaccharide isolated from Tamm-Horsfall urinary glycoprotein. Biochem. Biophys. Res. Commun. 1983, 115, 625–631. [Google Scholar] [CrossRef]
- Dall’Olio, F.; Malagolini, N.; Chiricolo, M.; Trinchera, M.; Harduin-Lepers, A. The expanding roles of the Sda/Cad carbohydrate antigen and its cognate glycosyltransferase B4GALNT2. Biochim. Biophys. Acta 2014, 1840, 443–453. [Google Scholar] [CrossRef] [Green Version]
- Blanchard, D.; Piller, F.; Gillard, B.; Marcus, D.; Cartron, J.P. Identification of a novel ganglioside on erythrocytes with blood group Cad specificity. J. Biol. Chem. 1985, 260, 7813–7816. [Google Scholar] [CrossRef]
- Serafini-Cessi, F.; Dall’Olio, F. Guinea-pig kidney b-N-acetylgalactosaminyltransferase towards Tamm- Horsfall glycoprotein. Requirement of sialic acid in the acceptor for transferase activity. Biochem. J. 1983, 215, 483–489. [Google Scholar] [CrossRef]
- Smith, P.L.; Lowe, J.B. Molecular cloning of a murine N-acetylgalactosamine transferase cDNA that determines expression of the T lymphocyte-specific CT oligosaccharide differentiation antigen. J. Biol. Chem. 1994, 269, 15162–15171. [Google Scholar] [CrossRef]
- Lo Presti, L.; Cabuy, E.; Chiricolo, M.; Dall’Olio, F. Molecular Cloning of the Human b1,4 N-Acetylgalactosaminyltransferase Responsible for the Biosynthesis of the Sda Histo-Blood Group Antigen: The Sequence Predicts a Very Long Cytoplasmic Domain. J. Biochem. 2003, 134, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Montiel, M.D.; Krzewinski-Recchi, M.A.; Delannoy, P.; Harduin-Lepers, A. Molecular cloning, gene organization and expression of the human UDP-GalNAc:Neu5Aca2-3Galb-R b1,4-N-acetylgalactosaminyltransferase responsible for the biosynthesis of the blood group Sda/Cad antigen: Evidence for an unusual extended cytoplasmic domain. Biochem. J. 2003, 373, 369–379. [Google Scholar] [CrossRef] [Green Version]
- Groux-Degroote, S.; Schulz, C.; Cogez, V.; Noel, M.; Portier, L.; Vicogne, D.; Solorzano, C.; Dall’Olio, F.; Steenackers, A.; Mortuaire, M.; et al. The extended cytoplasmic tail of the human B4GALNT2 is critical for its Golgi targeting and post-Golgi sorting. FEBS J. 2018, 285, 3442–3463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malagolini, N.; Santini, D.; Chiricolo, M.; Dall’Olio, F. Biosynthesis and expression of the Sda and sialyl Lewis x antigens in normal and cancer colon. Glycobiology 2007, 17, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Groux-Degroote, S.; Wavelet, C.; Krzewinski-Recchi, M.A.; Portier, L.; Mortuaire, M.; Mihalache, A.; Trinchera, M.; Delannoy, P.; Malagolini, N.; Chiricolo, M.; et al. B4GALNT2 gene expression controls the biosynthesis of Sda and sialyl Lewis X antigens in healthy and cancer human gastrointestinal tract. Int. J. Biochem. Cell Biol. 2014, 53, 442–449. [Google Scholar] [CrossRef]
- Stenfelt, L.; Hellberg, A.; Moller, M.; Thornton, N.; Larson, G.; Olsson, M.L. Missense mutations in the C-terminal portion of the B4GALNT2-encoded glycosyltransferase underlying the Sda phenotype. Biochem. Biophys. Rep. 2019, 19, 100659. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.R.; Hsieh, C.Y.; Twu, Y.C.; Yu, L.C. Expression of the human Sda b-1,4-N-acetylgalactosaminyltransferase II gene is dependent on the promoter methylation status. Glycobiology 2008, 18, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, Y.I.; Toyota, M.; Kawashima, R.; Hagiwara, T.; Suzuki, H.; Imai, K.; Shinomura, Y.; Tokino, T.; Kannagi, R.; Dohi, T. DNA hypermethylation contributes to incomplete synthesis of carbohydrate determinants in gastrointestinal cancer. Gastroenterology 2008, 135, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Wavelet-Vermuse, C.; Groux-Degroote, S.; Vicogne, D.; Cogez, V.; Venturi, G.; Trinchera, M.; Brysbaert, G.; Krzewinski-Recchi, M.A.; Bachir, E.H.; Schulz, C.; et al. Analysis of the proximal promoter of the human colon-specific B4GALNT2 (Sda synthase) gene: B4GALNT2 is transcriptionally regulated by ETS1. Biochim. Biophys. Acta Gene Regul. Mech. 2021, 1864, 194747. [Google Scholar] [CrossRef]
- Dall’Olio, F.; Malagolini, N.; Serafini-Cessi, F. Tissue distribution and age-dependent expression of b-4-N- acetylgalactosaminyl-transferase in guinea-pig. Biosci. Rep. 1987, 7, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Dall’Olio, F.; Malagolini, N.; Di Stefano, G.; Ciambella, M.; Serafini-Cessi, F. Postnatal development of rat colon epithelial cells is associated with changes in the expression of the b 1,4-N-acetylgalactosaminyltransferase involved in the synthesis of Sda antigen and of a 2,6-sialyltransferase activity towards N-acetyllactosamine. Biochem. J. 1990, 270, 519–524. [Google Scholar] [CrossRef]
- Robbe-Masselot, C.; Maes, E.; Rousset, M.; Michalski, J.C.; Capon, C. Glycosylation of human fetal mucins: A similar repertoire of O-glycans along the intestinal tract. Glycoconj. J. 2009, 26, 397–413. [Google Scholar] [CrossRef]
- Lefrancois, L. Carbohydrate differentiation antigens of murine T cells: Expression on intestinal lymphocytes and intestinal epithelium. J. Immunol. 1987, 138, 3375–3384. [Google Scholar] [PubMed]
- Malagolini, N.; Dall’Olio, F.; Serafini-Cessi, F. UDP-GalNAc:NeuAc a 2,3Gal b-R (GalNAc to Gal) b 1,4-N- acetylgalactosaminyltransferase responsible for the Sda specificity in human colon carcinoma CaCo-2 cell line. Biochem. Biophys. Res. Commun. 1991, 180, 681–686. [Google Scholar] [CrossRef]
- Malagolini, N.; Dall’Olio, F.; Di Stefano, G.; Minni, F.; Marrano, D.; Serafini-Cessi, F. Expression of UDP-GalNAc:NeuAc a2,3Gal b-R beta 1,4(GalNAc to Gal) N-acetylgalactosaminyltransferase involved in the synthesis of Sda antigen in human large intestine and colorectal carcinomas. Cancer Res. 1989, 49, 6466–6470. [Google Scholar] [PubMed]
- Dohi, T.; Yuyama, Y.; Natori, Y.; Smith, P.L.; Lowe, J.B.; Oshima, M. Detection of N-acetylgalactosaminyltransferase mRNA which determines expression of Sda blood group carbohydrate structure in human gastrointestinal mucosa and cancer. Int. J. Cancer 1996, 67, 626–631. [Google Scholar] [CrossRef]
- Robbe-Masselot, C.; Herrmann, A.; Maes, E.; Carlstedt, I.; Michalski, J.C.; Capon, C. Expression of a core 3 disialyl-Lex hexasaccharide in human colorectal cancers: A potential marker of malignant transformation in colon. J. Proteome. Res. 2009, 8, 702–711. [Google Scholar] [CrossRef] [PubMed]
- Low, E.N.D.; Mokhtar, N.M.; Wong, Z.; Raja Ali, R.A. Colonic Mucosal Transcriptomic Changes in Patients with Long-Duration Ulcerative Colitis Revealed Colitis-Associated Cancer Pathways. J. Crohns. Colitis. 2019, 13, 755–763. [Google Scholar] [CrossRef]
- Pucci, M.; Gomes Ferreira, I.; Orlandani, M.; Malagolini, N.; Ferracin, M.; Dall’Olio, F. High Expression of the Sda Synthase B4GALNT2 Associates with Good Prognosis and Attenuates Stemness in Colon Cancer. Cells 2020, 9, 948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pucci, M.; Malagolini, N.; Dall’Olio, F. Glycosyltransferase B4GALNT2 as a Predictor of Good Prognosis in Colon Cancer: Lessons from Databases. Int. J. Mol. Sci. 2021, 22, 4331. [Google Scholar] [CrossRef] [PubMed]
- Dohi, T.; Ohta, S.; Hanai, N.; Yamaguchi, K.; Oshima, M. Sialylpentaosylceramide detected with anti-GM2 monoclonal antibody. Structural characterization and complementary expression with GM2 in gastric cancer and normal gastric mucosa. J. Biol. Chem. 1990, 265, 7880–7885. [Google Scholar] [CrossRef]
- Tanaka-Okamoto, M.; Hanzawa, K.; Mukai, M.; Takahashi, H.; Ohue, M.; Miyamoto, Y. Identification of internally sialylated carbohydrate tumor marker candidates, including Sda/CAD antigens, by focused glycomic analyses utilizing the substrate specificity of neuraminidase. Glycobiology 2018, 28, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Qusa, M.H.; Abdelwahed, K.S.; Siddique, A.B.; El Sayed, K.A. Comparative Gene Signature of (-)-Oleocanthal Formulation Treatments in Heterogeneous Triple Negative Breast Tumor Models: Oncological Therapeutic Target Insights. Nutrients 2021, 13, 1706. [Google Scholar] [CrossRef]
- Yu, P.; Zhu, L.; Cui, K.; Du, Y.; Zhang, C.; Ma, W.; Guo, J. B4GALNT2 Gene Promotes Proliferation, and Invasiveness and Migration Abilities of Model Triple Negative Breast Cancer (TNBC) Cells by Interacting With HLA-B Protein. Front Oncol. 2021, 11, 722828. [Google Scholar] [CrossRef] [PubMed]
- Holst, S.; Wuhrer, M.; Rombouts, Y. Glycosylation characteristics of colorectal cancer. Adv. Cancer Res. 2015, 126, 203–256. [Google Scholar] [PubMed]
- Blanas, A.; Sahasrabudhe, N.M.; Rodriguez, E.; van Kooyk, Y.; van Vliet, S.J. Fucosylated Antigens in Cancer: An Alliance toward Tumor Progression, Metastasis, and Resistance to Chemotherapy. Front Oncol. 2018, 8, 39. [Google Scholar] [CrossRef]
- Aronica, A.; Avagliano, L.; Caretti, A.; Tosi, D.; Bulfamante, G.P.; Trinchera, M. Unexpected distribution of CA19.9 and other type 1 chain Lewis antigens in normal and cancer tissues of colon and pancreas: Importance of the detection method and role of glycosyltransferase regulation. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 3210–3220. [Google Scholar] [CrossRef] [PubMed]
- Mare, L.; Caretti, A.; Albertini, R.; Trinchera, M. CA19.9 antigen circulating in the serum of colon cancer patients: Where is it from? Int. J. Biochem. Cell Biol. 2013, 45, 792–797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trinchera, M.; Aronica, A.; Dall’Olio, F. Selectin Ligands Sialyl-Lewis a and Sialyl-Lewis x in Gastrointestinal Cancers. Biology 2017, 6, 16. [Google Scholar] [CrossRef]
- Ferreira, J.A.; Magalhaes, A.; Gomes, J.; Peixoto, A.; Gaiteiro, C.; Fernandes, E.; Santos, L.L.; Reis, C.A. Protein glycosylation in gastric and colorectal cancers: Toward cancer detection and targeted therapeutics. Cancer Lett. 2017, 387, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Trinchera, M.; Malagolini, N.; Chiricolo, M.; Santini, D.; Minni, F.; Caretti, A.; Dall’Olio, F. The biosynthesis of the selectin-ligand sialyl Lewis x in colorectal cancer tissues is regulated by fucosyltransferase VI and can be inhibited by an RNA interference-based approach. Int. J. Biochem. Cell Biol. 2011, 43, 130–139. [Google Scholar] [CrossRef]
- Balog, C.I.; Stavenhagen, K.; Fung, W.L.; Koeleman, C.A.; McDonnell, L.A.; Verhoeven, A.; Mesker, W.E.; Tollenaar, R.A.; Deelder, A.M.; Wuhrer, M. N-glycosylation of Colorectal Cancer Tissues: A liquid chromatography and mass spectrometry-based investigation. Mol. Cell Proteom. 2012, 11, 571–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holst, S.; Stavenhagen, K.; Balog, C.I.; Koeleman, C.A.; McDonnell, L.M.; Mayboroda, O.A.; Verhoeven, A.; Mesker, W.E.; Tollenaar, R.A.; Deelder, A.M.; et al. Investigations on aberrant glycosylation of glycosphingolipids in colorectal cancer tissues using liquid chromatography and matrix-assisted laser desorption time-of-flight mass spectrometry (MALDI-TOF-MS). Mol. Cell Proteom. 2013, 12, 3081–3093. [Google Scholar] [CrossRef] [Green Version]
- Holst, S.; Deuss, A.J.; van Pelt, G.W.; van Vliet, S.J.; Garcia-Vallejo, J.J.; Koeleman, C.A.; Deelder, A.M.; Mesker, W.E.; Tollenaar, R.A.; Rombouts, Y.; et al. N-glycosylation Profiling of Colorectal Cancer Cell Lines Reveals Association of Fucosylation with Differentiation and Caudal Type Homebox 1 (CDX1)/Villin mRNA Expression. Mol. Cell Proteom. 2016, 15, 124–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madunic, K.; Zhang, T.; Mayboroda, O.A.; Holst, S.; Stavenhagen, K.; Jin, C.; Karlsson, N.G.; Lageveen-Kammeijer, G.S.M.; Wuhrer, M. Colorectal cancer cell lines show striking diversity of their O-glycome reflecting the cellular differentiation phenotype. Cell Mol. Life Sci. 2021, 78, 337–350. [Google Scholar] [CrossRef] [Green Version]
- Kaprio, T.; Satomaa, T.; Heiskanen, A.; Hokke, C.H.; Deelder, A.M.; Mustonen, H.; Hagstrom, J.; Carpen, O.; Saarinen, J.; Haglund, C. N-glycomic Profiling as a Tool to Separate Rectal Adenomas from Carcinomas. Mol. Cell Proteom. 2015, 14, 277–288. [Google Scholar] [CrossRef] [Green Version]
- Kikuchi, D.; Saito, M.; Saito, K.; Watanabe, Y.; Matsumoto, Y.; Kanke, Y.; Onozawa, H.; Hayase, S.; Sakamoto, W.; Ishigame, T.; et al. Upregulated solute carrier family 37 member 1 in colorectal cancer is associated with poor patient outcome and metastasis. Oncol. Lett. 2018, 15, 2065–2072. [Google Scholar] [CrossRef]
- Yamadera, M.; Shinto, E.; Tsuda, H.; Kajiwara, Y.; Naito, Y.; Hase, K.; Yamamoto, J.; Ueno, H. Sialyl Lewis(x) expression at the invasive front as a predictive marker of liver recurrence in stage II colorectal cancer. Oncol. Lett. 2018, 15, 221–228. [Google Scholar] [CrossRef]
- Pothuraju, R.; Krishn, S.R.; Gautam, S.K.; Pai, P.; Ganguly, K.; Chaudhary, S.; Rachagani, S.; Kaur, S.; Batra, S.K. Mechanistic and Functional Shades of Mucins and Associated Glycans in Colon Cancer. Cancers 2020, 12, 649. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, A.S.; Harduin-Lepers, A.; Magalhaes, A.; Machado, E.; Mendes, N.; Costa, L.T.; Matthiesen, R.; Almeida, R.; Costa, J.; Reis, C.A. Differential expression of a-2,3-sialyltransferases and a-1,3/4-fucosyltransferases regulates the levels of sialyl Lewis a and sialyl Lewis x in gastrointestinal carcinoma cells. Int. J. Biochem. Cell Biol. 2010, 42, 80–89. [Google Scholar] [CrossRef]
- Perez-Garay, M.; Arteta, B.; Pages, L.; De Llorens, R.; de Bolos, C.; Vidal-Vanaclocha, F.; Peracaula, R. a2,3-sialyltransferase ST3Gal III modulates pancreatic cancer cell motility and adhesion in vitro and enhances its metastatic potential in vivo. PLoS ONE 2010, 5, e12524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimitroff, C.J.; Pera, P.; Dall’Olio, F.; Matta, K.L.; Chandrasekaran, E.V.; Lau, J.T.; Bernacki, R.J. Cell surface n-acetylneuraminic acid a2,3-galactoside-dependent intercellular adhesion of human colon cancer cells. Biochem. Biophys. Res. Commun. 1999, 256, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.; Osorio, H.; Pinto, M.T.; Campos, D.; Oliveira, M.J.; Reis, C.A. Expression of ST3GAL4 leads to SLex expression and induces c-Met activation and an invasive phenotype in gastric carcinoma cells. PLoS ONE 2013, 8, e66737. [Google Scholar] [CrossRef] [PubMed]
- Perez-Garay, M.; Arteta, B.; Llop, E.; Cobler, L.; Pages, L.; Ortiz, R.; Ferri, M.J.; de Bolos, C.; Figueras, J.; De Llorens, R.; et al. a2,3-Sialyltransferase ST3Gal IV promotes migration and metastasis in pancreatic adenocarcinoma cells and tends to be highly expressed in pancreatic adenocarcinoma tissues. Int. J. Biochem. Cell Biol. 2013, 45, 1748–1757. [Google Scholar] [CrossRef]
- Colomb, F.; Krzewinski-Recchi, M.A.; El Machhour, F.; Mensier, E.; Jaillard, S.; Steenackers, A.; Harduin-Lepers, A.; Lafitte, J.J.; Delannoy, P.; Groux-Degroote, S. TNF regulates sialyl-Lewisx and 6-sulfo-sialyl-Lewisx expression in human lung through up-regulation of ST3GAL4 transcript isoform BX. Biochimie 2012, 94, 2045–2053. [Google Scholar] [CrossRef]
- Hiller, K.M.; Mayben, J.P.; Bendt, K.M.; Manousos, G.A.; Senger, K.; Cameron, H.S.; Weston, B.W. Transfection of a1,3 fucosyltransferase antisense sequences impairs the proliferative and tumorigenic ability of human colon carcinoma cells. Mol. Carcinog. 2000, 27, 280–288. [Google Scholar] [CrossRef]
- Weston, B.W.; Hiller, K.M.; Mayben, J.P.; Manousos, G.A.; Bendt, K.M.; Liu, R.; Cusack, J.C., Jr. Expression of human a1,3 fucosyltransferase antisense sequences inhibits selectin-mediated adhesion and liver metastasis of colon carcinoma cells. Cancer Res. 1999, 59, 2127–2135. [Google Scholar] [PubMed]
- Pan, S.; Liu, Y.; Liu, Q.; Xiao, Y.; Liu, B.; Ren, X.; Qi, X.; Zhou, H.; Zeng, C.; Jia, L. HOTAIR/miR-326/FUT6 axis facilitates colorectal cancer progression through regulating fucosylation of CD44 via PI3K/AKT/mTOR pathway. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Kudo, T.; Ikehara, Y.; Togayachi, A.; Morozumi, K.; Watanabe, M.; Nakamura, M.; Nishihara, S.; Narimatsu, H. Up-regulation of a set of glycosyltransferase genes in human colorectal cancer. Lab. Investig. 1998, 78, 797–811. [Google Scholar] [PubMed]
- Holmes, E.H.; Hakomori, S.; Ostrander, G.K. Synthesis of type 1 and 2 lacto series glycolipid antigens in human colonic adenocarcinoma and derived cell lines is due to activation of a normally unexpressed b1,3N-acetylglucosaminyltransferase. J. Biol. Chem. 1987, 262, 15649–15658. [Google Scholar] [CrossRef]
- Marcos, N.T.; Magalhaes, A.; Ferreira, B.; Oliveira, M.J.; Carvalho, A.S.; Mendes, N.; Gilmartin, T.; Head, S.R.; Figueiredo, C.; David, L.; et al. Helicobacter pylori induces b3GnT5 in human gastric cell lines, modulating expression of the SabA ligand sialyl-Lewis x. J. Clin. Investig. 2008, 118, 2325–2336. [Google Scholar]
- Lu, C.H.; Wu, W.Y.; Lai, Y.J.; Yang, C.M.; Yu, L.C. Suppression of B3GNT7 gene expression in colon adenocarcinoma and its potential effect in the metastasis of colon cancer cells. Glycobiology 2014, 24, 359–367. [Google Scholar] [CrossRef] [Green Version]
- Shiozaki, K.; Yamaguchi, K.; Takahashi, K.; Moriya, S.; Miyagi, T. Regulation of Sialyl Lewis Antigen Expression in Colon Cancer Cells by Sialidase NEU4. J. Biol. Chem. 2011, 286, 21052–21061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izawa, M.; Kumamoto, K.; Mitsuoka, C.; Kanamori, C.; Kanamori, A.; Ohmori, K.; Ishida, H.; Nakamura, S.; Kurata-Miura, K.; Sasaki, K.; et al. Expression of sialyl 6-sulfo Lewis X is inversely correlated with conventional sialyl Lewis X expression in human colorectal cancer. Cancer Res. 2000, 60, 1410–1416. [Google Scholar]
- Yusa, A.; Miyazaki, K.; Kimura, N.; Izawa, M.; Kannagi, R. Epigenetic silencing of the sulfate transporter gene DTDST induces sialyl Lewisx expression and accelerates proliferation of colon cancer cells. Cancer Res. 2010, 70, 4064–4073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyazaki, K.; Ohmori, K.; Izawa, M.; Koike, T.; Kumamoto, K.; Furukawa, K.; Ando, T.; Kiso, M.; Yamaji, T.; Hashimoto, Y.; et al. Loss of disialyl Lewisa the ligand for lymphocyte inhibitory receptor sialic acid-binding immunoglobulin-like lectin-7 (Siglec-7) associated with increased sialyl Lewis a expression on human colon cancers. Cancer Res. 2004, 64, 4498–4505. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.C.; Chao, C.C.; Wu, P.H.; Chung, H.Y.; Lee, H.Y.; Suen, C.S.; Hwang, M.J.; Cai, B.H.; Kannagi, R. Epigenetic silencing of the synthesis of immunosuppressive Siglec ligand glycans by NF-kappaB/EZH2/YY1 axis in early-stage colon cancers. Biochim. Biophys. Acta Gene Regul. Mech. 2019, 1862, 173–183. [Google Scholar] [CrossRef]
- Huang, H.C.; Cai, B.H.; Suen, C.S.; Lee, H.Y.; Hwang, M.J.; Liu, F.T.; Kannagi, R. BGN/TLR4/NF-B Mediates Epigenetic Silencing of Immunosuppressive Siglec Ligands in Colon Cancer Cells. Cells 2020, 9, 397. [Google Scholar] [CrossRef] [Green Version]
- Kawamura, Y.I.; Kawashima, R.; Fukunaga, R.; Hirai, K.; Toyama-Sorimachi, N.; Tokuhara, M.; Shimizu, T.; Dohi, T. Introduction of Sda carbohydrate antigen in gastrointestinal cancer cells eliminates selectin ligands and inhibits metastasis. Cancer Res. 2005, 65, 6220–6227. [Google Scholar] [CrossRef] [Green Version]
- Capon, C.; Maes, E.; Michalski, J.C.; Leffler, H.; Kim, Y.S. Sda-antigen-like structures carried on core 3 are prominent features of glycans from the mucin of normal human descending colon. Biochem. J. 2001, 358, 657–664. [Google Scholar] [CrossRef]
- Foxall, C.; Watson, S.R.; Dowbenko, D.; Fennie, C.; Lasky, L.A.; Kiso, M.; Hasegawa, A.; Asa, D.; Brandley, B.K. The three members of the selectin receptor family recognize a common carbohydrate epitope, the sialyl Lewisx oligosaccharide. J. Cell Biol. 1992, 117, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.X.; Liang, Y.; Gao, W. Clinicopathological and prognostic significance of sialyl Lewis X overexpression in patients with cancer: A meta-analysis. OncoTargets Ther. 2016, 9, 3113–3125. [Google Scholar] [CrossRef] [Green Version]
- Nakamori, S.; Kameyama, M.; Imaoka, S.; Furukawa, H.; Ishikawa, O.; Sasaki, Y.; Izumi, Y.; Irimura, T. Involvement of carbohydrate antigen sialyl Lewisx in colorectal cancer metastasis. Dis. Colon Rectum 1997, 40, 420–431. [Google Scholar] [CrossRef]
- Terraneo, L.; Avagliano, L.; Caretti, A.; Bianciardi, P.; Tosi, D.; Bulfamante, G.P.; Samaja, M.; Trinchera, M. Expression of carbohydrate-antigen sialyl-Lewis a on colon cancer cells promotes xenograft growth and angiogenesis in nude mice. Int. J. Biochem. Cell Biol. 2013, 45, 2796–2800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerrero, P.E.; Miro, L.; Wong, B.S.; Massaguer, A.; Martinez-Bosch, N.; Llorens, R.; Navarro, P.; Konstantopoulos, K.; Llop, E.; Peracaula, R. Knockdown of a2,3-Sialyltransferases Impairs Pancreatic Cancer Cell Migration, Invasion and E-selectin-Dependent Adhesion. Int. J. Mol. Sci. 2020, 21, 6239. [Google Scholar] [CrossRef] [PubMed]
- Carrascal, M.A.; Silva, M.; Ramalho, J.S.; Pen, C.; Martins, M.; Pascoal, C.; Amaral, C.; Serrano, I.; Oliveira, M.J.; Sackstein, R.; et al. Inhibition of fucosylation in human invasive ductal carcinoma reduces E-selectin ligand expression, cell proliferation, and ERK1/2 and p38 MAPK activation. Mol. Oncol. 2018, 12, 579–593. [Google Scholar] [CrossRef] [Green Version]
- Yoshihama, N.; Yamaguchi, K.; Chigita, S.; Mine, M.; Abe, M.; Ishii, K.; Kobayashi, Y.; Akimoto, N.; Mori, Y.; Sugiura, T. A Novel Function of CD82/KAI1 in Sialyl Lewis Antigen-Mediated Adhesion of Cancer Cells: Evidence for an Anti-Metastasis Effect by Down-Regulation of Sialyl Lewis Antigens. PLoS ONE 2015, 10, e0124743. [Google Scholar] [CrossRef] [Green Version]
- Kohler, S.; Ullrich, S.; Richter, U.; Schumacher, U. E-/P-selectins and colon carcinoma metastasis: First in vivo evidence for their crucial role in a clinically relevant model of spontaneous metastasis formation in the lung. Br. J. Cancer 2010, 102, 602–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gebauer, F.; Wicklein, D.; Stubke, K.; Nehmann, N.; Schmidt, A.; Salamon, J.; Peldschus, K.; Nentwich, M.F.; Adam, G.; Tolstonog, G.; et al. Selectin binding is essential for peritoneal carcinomatosis in a xenograft model of human pancreatic adenocarcinoma in pfp--/rag2--mice. Gut 2013, 62, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Stubke, K.; Wicklein, D.; Herich, L.; Schumacher, U.; Nehmann, N. Selectin-deficiency reduces the number of spontaneous metastases in a xenograft model of human breast cancer. Cancer Lett. 2012, 321, 89–99. [Google Scholar] [CrossRef]
- Esposito, M.; Mondal, N.; Greco, T.M.; Wei, Y.; Spadazzi, C.; Lin, S.C.; Zheng, H.; Cheung, C.; Magnani, J.L.; Lin, S.H.; et al. Bone vascular niche E-selectin induces mesenchymal-epithelial transition and Wnt activation in cancer cells to promote bone metastasis. Nat. Cell Biol. 2019, 21, 627–639. [Google Scholar] [CrossRef]
- Kelm, M.; Quiros, M.; Azcutia, V.; Boerner, K.; Cummings, R.D.; Nusrat, A.; Brazil, J.C.; Parkos, C.A. Targeting epithelium-expressed sialyl Lewis glycans improves colonic mucosal wound healing and protects against colitis. JCI Insight 2020, 5, 135843. [Google Scholar] [CrossRef]
- Bordon, Y. Inflammation: Live long and prosper with Siglecs. Nat. Rev. Immunol. 2015, 15, 266–267. [Google Scholar] [CrossRef] [PubMed]
- Deschepper, F.M.; Zoppi, R.; Pirro, M.; Hensbergen, P.J.; Dall’Olio, F.; Kotsias, M.; Gardner, R.A.; Spencer, D.I.R.; Videira, P.A. L1CAM as an E-selectin Ligand in Colon Cancer. Int. J. Mol. Sci. 2020, 21, 8286. [Google Scholar] [CrossRef] [PubMed]
- Pucci, M.; Gomes, F.I.; Malagolini, N.; Ferracin, M.; Dall’Olio, F. The Sda Synthase B4GALNT2 Reduces Malignancy and Stemness in Colon Cancer Cell Lines Independently of Sialyl Lewis X Inhibition. Int. J. Mol. Sci. 2020, 21, 6558. [Google Scholar] [CrossRef] [PubMed]
- Zhan, L.; Chen, L.; Chen, Z. Knockdown of FUT3 disrupts the proliferation, migration, tumorigenesis and TGF-beta induced EMT in pancreatic cancer cells. Oncol. Lett. 2018, 16, 924–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawamura, Y.I.; Adachi, Y.; Curiel, D.T.; Kawashima, R.; Kannagi, R.; Nishimoto, N.; Dohi, T. Therapeutic adenoviral gene transfer of a glycosyltransferase for prevention of peritoneal dissemination and metastasis of gastric cancer. Cancer Gene Ther. 2014, 21, 427–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Gene Symbol | Ratio | Function | PMID | |
|---|---|---|---|---|
| KRT20 | 494 | Cytokeratin 20, associated with worse prognosis | 22493626 | |
| CEACAM6 | 11 | Member of the CEA family. Associated with worse prognosis | 24186057 | |
| CRISP3 | 10 | High expression correlates with malignancy | 30609035 | |
| ABCC2 | 10 | Multidrug resistance-associated protein 2 | 26499806 | |
| LRRC31 | 9 | Inhibitor of DNA repair | 33005030 | |
| MUC21 | 8.5 | Associated with incohesive growth of lung cancer | 31301084 | |
| CEACAM5 | 8 | Member of the CEA family. Driver of breast cancer metastasis | 29736411 | |
| TRIM72 | 8 | Lower expression predicts recurrence in colon cancer | 30852740 | |
| PNMT | 7.8 | Co-amplified with ERBB2 | 12727839 | |
| CXCL17 | 7 | Promotes proliferation and invasion of breast cancer cells | 28943434 | |
| AMER3 | −8.7 | Enhances β-catenin signaling in CRC | 24251807 | |
| KCNC1 | −8.8 | Its inhibition is associated with poor survival in seminoma | 34105734 | |
| INSM1 | −9.3 | In SCLC, low expression associated with better prognosis | 32118626 | |
| SLIT1 | −9 | Suppresses breast cancer growth | 18829537 | |
| RUNDC3A | −10.6 | High expression correlates with shorter survival in rectal cancer | 29050227 | |
| HDC | −13 | Increased expression associated with better survival | 31748740 | |
| ECEL1 | −14 | Associated with good prognosis in neuroblastoma | 12632073 | |
| CHRNA9 | −19 | High expression associated with poor survival in BRCA | 20953833 | |
| PCSK1 | −23 | Reduces growth of breast cancer cells | 11241161 | |
| NELL1 | −27 | Down-regulated in kidney cancer | 25726761 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dall’Olio, F.; Pucci, M.; Malagolini, N. The Cancer-Associated Antigens Sialyl Lewisa/x and Sda: Two Opposite Faces of Terminal Glycosylation. Cancers 2021, 13, 5273. https://doi.org/10.3390/cancers13215273
Dall’Olio F, Pucci M, Malagolini N. The Cancer-Associated Antigens Sialyl Lewisa/x and Sda: Two Opposite Faces of Terminal Glycosylation. Cancers. 2021; 13(21):5273. https://doi.org/10.3390/cancers13215273
Chicago/Turabian StyleDall’Olio, Fabio, Michela Pucci, and Nadia Malagolini. 2021. "The Cancer-Associated Antigens Sialyl Lewisa/x and Sda: Two Opposite Faces of Terminal Glycosylation" Cancers 13, no. 21: 5273. https://doi.org/10.3390/cancers13215273
APA StyleDall’Olio, F., Pucci, M., & Malagolini, N. (2021). The Cancer-Associated Antigens Sialyl Lewisa/x and Sda: Two Opposite Faces of Terminal Glycosylation. Cancers, 13(21), 5273. https://doi.org/10.3390/cancers13215273