PSMA-PET/MRI-Based Focal Dose Escalation in Patients with Primary Prostate Cancer Treated with Stereotactic Body Radiation Therapy (HypoFocal-SBRT): Study Protocol of a Randomized, Multicentric Phase III Trial
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods and Design
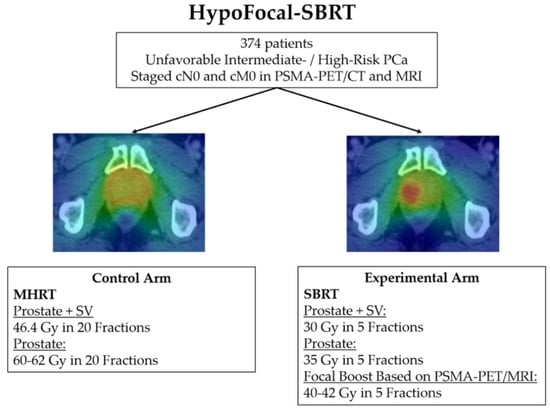
2.1. Study Design and Legal Aspects
2.2. Justification of the Treatment Choice for the Experimental and the Control Arm
2.3. Patients—Eligibility Criteria
2.4. Primary and Secondary Endpoints
2.5. Randomization
2.6. Recruitment and Sample Size Considerations
2.7. Imaging
3. Radiotherapy
3.1. Contouring
3.2. Planning Procedures
3.3. Image-Guided RT (IGRT) and Adaptive Planning
3.4. Androgen Deprivation Therapy
3.5. Quality Assurance (QA) and Safety
4. Data Analysis
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Incrocci, L.; Wortel, R.C.; Alemayehu, W.G.; Aluwini, S.; Schimmel, E.; Krol, S.; van der Toorn, P.-P.; de Jager, H.; Heemsbergen, W.; Heijmen, B.; et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with localised prostate cancer (HYPRO): Final efficacy results from a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2016, 17, 1061–1069. [Google Scholar] [CrossRef]
- Bruner, D.; Pugh, S.L.; Lee, W.R.; Hall, W.A.; Dignam, J.J.; Low, D.; Swanson, G.; Shah, A.B.; Malone, S.; Michalski, J.M.; et al. Quality of Life in Patients with Low-Risk Prostate Cancer Treated with Hypofractionated vs. Conventional Radiotherapy: A Phase 3 Randomized Clinical Trial. JAMA Oncol. 2019, 5, 664–670. [Google Scholar] [CrossRef] [Green Version]
- Dearnaley, D.; Syndikus, I.; Mossop, H.; Khoo, V.; Birtle, A.; Bloomfield, D.; Graham, J.; Kirkbride, P.; Logue, J.; Malik, Z.; et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016, 17, 1047–1060. [Google Scholar] [CrossRef] [Green Version]
- Catton, C.N.; Lukka, H.; Gu, C.-S.; Martin, J.M.; Supiot, S.; Chung, P.W.M.; Bauman, G.S.; Bahary, J.-P.; Ahmed, S.; Cheung, P.; et al. Randomized Trial of a Hypofractionated Radiation Regimen for the Treatment of Localized Prostate Cancer. J. Clin. Oncol. 2017, 35, 1884–1890. [Google Scholar] [CrossRef]
- Arcangeli, G.; Saracino, B.; Arcangeli, S.; Gomellini, S.; Petrongari, M.G.; Sanguineti, G.; Strigari, L. Moderate Hypofractionation in High-Risk, Organ-Confined Prostate Cancer: Final Results of a Phase III Randomized Trial. J. Clin. Oncol. 2017, 35, 1891–1897. [Google Scholar] [CrossRef]
- Vogelius, I.R.; Bentzen, S.M. Diminishing Returns from Ultrahypofractionated Radiation Therapy for Prostate Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2020, 107, 299–304. [Google Scholar] [CrossRef]
- Brand, D.H.; Tree, A.C.; Ostler, P.; Van Der Voet, H.; Loblaw, A.; Chu, W.; Ford, D.; Tolan, S.; Jain, S.; Martin, A.; et al. Intensity-modulated fractionated radiotherapy versus stereotactic body radiotherapy for prostate cancer (PACE-B): Acute toxicity findings from an international, randomised, open-label, phase 3, non-inferiority trial. Lancet Oncol. 2019, 20, 1531–1543. [Google Scholar] [CrossRef]
- Widmark, A.; Gunnlaugsson, A.; Beckman, L.; Thellenberg-Karlsson, C.; Hoyer, M.; Lagerlund, M.; Kindblom, J.; Ginman, C.; Johansson, B.; Björnlinger, K.; et al. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5-year outcomes of the HYPO-RT-PC randomised, non-inferiority, phase 3 trial. Lancet 2019, 394, 385–395. [Google Scholar] [CrossRef]
- Viani, G.A.; Stefano, E.J.; Afonso, S.L. Higher-than-conventional radiation doses in localized prostate cancer treatment: A meta-analysis of randomized, controlled trials. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 1405–1418. [Google Scholar] [CrossRef] [PubMed]
- Spohn, S.K.B.; Sachpazidis, I.; Wiehle, R.; Thomann, B.; Sigle, A.; Bronsert, P.; Ruf, J.; Benndorf, M.; Nicolay, N.H.; Sprave, T.; et al. Influence of Urethra Sparing on Tumor Control Probability and Normal Tissue Complication Probability in Focal Dose Escalated Hypofractionated Radiotherapy: A Planning Study Based on Histopathology Reference. Front. Oncol. 2021, 11, 652678. [Google Scholar] [CrossRef] [PubMed]
- Zamboglou, C.; Thomann, B.; Koubar, K.; Bronsert, P.; Krauss, T.; Rischke, H.C.; Sachpazidis, I.; Drendel, V.; Salman, N.; Reichel, K.; et al. Focal dose escalation for prostate cancer using (68)Ga-HBED-CC PSMA PET/CT and MRI: A planning study based on histology reference. Radiat. Oncol. 2018, 13, 81. [Google Scholar] [CrossRef] [Green Version]
- Kerkmeijer, L.G.W.; Groen, V.H.; Pos, F.J.; Haustermans, K.; Monninkhof, E.M.; Smeenk, R.J.; Kunze-Busch, M.; de Boer, J.C.J.; Zijp, J.V.D.V.V.; van Vulpen, M.; et al. Focal Boost to the Intraprostatic Tumor in External Beam Radiotherapy for Patients with Localized Prostate Cancer: Results from the FLAME Randomized Phase III Trial. J. Clin. Oncol. 2021, 39, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Kasivisvanathan, V.; Rannikko, A.S.; Borghi, M.; Panebianco, V.; Mynderse, L.A.; Vaarala, M.H.; Moore, C.M. MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. N. Engl. J. Med. 2018, 378, 1767–1777. [Google Scholar] [CrossRef] [PubMed]
- Rischke, H.C.; Nestle, U.; Fechter, T.; Doll, C.; Volegova-Neher, N.; Henne, K.; Scholber, J.; Knippen, S.; Kirste, S.; Grosu, A.L.; et al. 3 Tesla multiparametric MRI for GTV-definition of Dominant Intraprostatic Lesions in patients with Prostate Cancer—An interobserver variability study. Radiat. Oncol. 2013, 8, 183. [Google Scholar] [CrossRef] [Green Version]
- Priester, A.; Natarajan, S.; Khoshnoodi, P.; Margolis, D.J.; Raman, S.S.; Reiter, R.E.; Huang, J.; Grundfest, W.; Marks, L.S. Magnetic Resonance Imaging Underestimation of Prostate Cancer Geometry: Use of Patient Specific Molds to Correlate Images with Whole Mount Pathology. J. Urol. 2017, 197, 320–326. [Google Scholar] [CrossRef] [Green Version]
- Kramer, M.; Spohn, S.K.B.; Kiefer, S.; Ceci, L.; Sigle, A.; Oerther, B.; Schultze-Seemann, W.; Gratzke, C.; Bock, M.; Bamberg, F.; et al. Isotropic Expansion of the Intraprostatic Gross Tumor Volume of Primary Prostate Cancer Patients Defined in MRI-A Correlation Study with Whole Mount Histopathological Information as Reference. Front. Oncol. 2020, 10, 596756. [Google Scholar] [CrossRef]
- Hofman, M.S.; Lawrentschuk, N.; Francis, R.; Tang, C.; Vela, I.; Thomas, P.; Rutherford, N.; Martin, J.M.; Frydenberg, M.; Shakher, R.; et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): A prospective, randomised, multicentre study. Lancet 2020, 395, 1208–1216. [Google Scholar] [CrossRef]
- Draulans, C.; De Roover, R.; van der Heide, U.A.; Kerkmeijer, L.; Smeenk, R.J.; Pos, F.; Vogel, W.V.; Nagarajah, J.; Janssen, M.; Isebaert, S.; et al. Optimal (68)Ga-PSMA and (18)F-PSMA PET window levelling for gross tumour volume delineation in primary prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Kuten, J.; Fahoum, I.; Savin, Z.; Shamni, O.; Gitstein, G.; Hershkovitz, D.; Mabjeesh, N.J.; Yossepowitch, O.; Mishani, E.; Even-Sapir, E. Head-to-Head Comparison of (68)Ga-PSMA-11 with (18)F-PSMA-1007 PET/CT in Staging Prostate Cancer Using Histopathology and Immunohistochemical Analysis as a Reference Standard. J. Nucl. Med. 2020, 61, 527–532. [Google Scholar] [CrossRef] [Green Version]
- Spohn, S.; Jaegle, C.; Fassbender, T.F.; Sprave, T.; Gkika, E.; Nicolay, N.H.; Bock, M.; Ruf, J.; Benndorf, M.; Gratzke, C.; et al. Intraindividual comparison between (68)Ga-PSMA-PET/CT and mpMRI for intraprostatic tumor delineation in patients with primary prostate cancer: A retrospective analysis in 101 patients. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2796–2803. [Google Scholar] [CrossRef]
- Spohn, S.K.B.; Kramer, M.; Kiefer, S.; Bronsert, P.; Sigle, A.; Schultze-Seemann, W.; Jilg, C.A.; Sprave, T.; Ceci, L.; Fassbender, T.F.; et al. Comparison of Manual and Semi-Automatic [(18)F]PSMA-1007 PET Based Contouring Techniques for Intraprostatic Tumor Delineation in Patients with Primary Prostate Cancer and Validation with Histopathology as Standard of Reference. Front. Oncol. 2020, 10, 600690. [Google Scholar] [CrossRef]
- Zamboglou, C.; Fassbender, T.F.; Steffan, L.; Schiller, F.; Fechter, T.; Carles, M.; Kiefer, S.; Rischke, H.C.; Reichel, K.; Schmidt-Hegemann, N.-S.; et al. Validation of different PSMA-PET/CT-based contouring techniques for intraprostatic tumor definition using histopathology as standard of reference. Radiother. Oncol. 2019, 141, 208–213. [Google Scholar] [CrossRef]
- Zamboglou, C.; Kramer, M.; Kiefer, S.; Bronsert, P.; Ceci, L.; Sigle, A.; Schultze-Seemann, W.; Jilg, C.A.; Sprave, T.; Fassbender, T.F.; et al. The impact of the co-registration technique and analysis methodology in comparison studies between advanced imaging modalities and whole-mount-histology reference in primary prostate cancer. Sci. Rep. 2021, 11, 5836. [Google Scholar] [CrossRef]
- Bettermann, A.S.; Zamboglou, C.; Kiefer, S.; Jilg, C.A.; Spohn, S.; Kranz-Rudolph, J.; Fassbender, T.F.; Bronsert, P.; Nicolay, N.H.; Gratzke, C.; et al. [(68)Ga-]PSMA-11 PET/CT and multiparametric MRI for gross tumor volume delineation in a slice by slice analysis with whole mount histopathology as a reference standard—Implications for focal radiotherapy planning in primary prostate cancer. Radiother. Oncol. 2019, 141, 214–219. [Google Scholar] [CrossRef]
- Eiber, M.; Weirich, G.; Holzapfel, K.; Souvatzoglou, M.; Haller, B.; Rauscher, I.; Beer, A.J.; Wester, H.-J.; Gschwend, J.; Schwaiger, M.; et al. Simultaneous (68)Ga-PSMA HBED-CC PET/MRI Improves the Localization of Primary Prostate Cancer. Eur. Urol. 2016, 70, 829–836. [Google Scholar] [CrossRef]
- Jackson, W.C.; Silva, J.; Hartman, H.E.; Dess, R.T.; Kishan, A.U.; Beeler, W.H.; Gharzai, L.A.; Jaworski, E.M.; Mehra, R.; Hearn, J.W.D.; et al. Stereotactic Body Radiation Therapy for Localized Prostate Cancer: A Systematic Review and Meta-Analysis of Over 6000 Patients Treated On Prospective Studies. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 778–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamboglou, C.; Klein, C.M.; Thomann, B.; Fassbender, T.F.; Rischke, H.C.; Kirste, S.; Henne, K.; Volegova-Neher, N.; Bock, M.; Langer, M.; et al. The dose distribution in dominant intraprostatic tumour lesions defined by multiparametric MRI and PSMA PET/CT correlates with the outcome in patients treated with primary radiation therapy for prostate cancer. Radiat. Oncol. 2018, 13, 65. [Google Scholar] [CrossRef] [PubMed]
- Coen, J.J.; Zietman, A.L.; Thakral, H.; Shipley, W.U. Radical radiation for localized prostate cancer: Local persistence of disease results in a late wave of metastases. J. Clin. Oncol. 2002, 20, 3199–3205. [Google Scholar] [CrossRef] [PubMed]
- Kupelian, P.A.; Ciezki, J.; Reddy, C.A.; Klein, E.A.; Mahadevan, A. Effect of increasing radiation doses on local and distant failures in patients with localized prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 16–22. [Google Scholar] [CrossRef]
- Marks, L.B.; Yorke, E.D.; Jackson, A.; Haken, R.K.T.; Constine, L.S.; Eisbruch, A.; Bentzen, S.M.; Nam, J.; Deasy, J.O. Use of normal tissue complication probability models in the clinic. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76 (Suppl. 3), S10–S19. [Google Scholar] [CrossRef] [Green Version]
- Zamboglou, C.; Sachpazidis, I.; Koubar, K.; Drendel, V.; Wiehle, R.; Kirste, S.; Mix, M.; Schiller, F.; Mavroidis, P.; Meyer, P.T.; et al. Evaluation of intensity modulated radiation therapy dose painting for localized prostate cancer using (68)Ga-HBED-CC PSMA-PET/CT: A planning study based on histopathology reference. Radiother. Oncol. 2017, 123, 472–477. [Google Scholar] [CrossRef] [Green Version]
- Draulans, C.; van der Heide, U.A.; Haustermans, K.; Pos, F.J.; Zyp, J.V.D.V.V.; De Boer, H.; Groen, V.H.; Monninkhof, E.M.; Smeenk, R.J.; Kunze-Busch, M.; et al. Primary endpoint analysis of the multicentre phase II hypo-FLAME trial for intermediate and high risk prostate cancer. Radiother. Oncol. 2020, 147, 92–98. [Google Scholar] [CrossRef]
- Herrera, F.G.; Valerio, M.; Berthold, D.; Tawadros, T.; Meuwly, J.-Y.; Vallet, V.; Baumgartner, P.; Thierry, A.-C.; De Bari, B.; Jichlinski, P.; et al. 50-Gy Stereotactic Body Radiation Therapy to the Dominant Intraprostatic Nodule: Results from a Phase 1a/b Trial. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 320–334. [Google Scholar] [CrossRef] [Green Version]
- Nicholls, L.; Suh, Y.-E.; Chapman, E.; Henderson, D.; Jones, C.; Morrison, K.; Sohaib, A.; Taylor, H.; Tree, A.; van As, N. Stereotactic radiotherapy with focal boost for intermediate and high-risk prostate cancer: Initial results of the SPARC trial. Clin. Transl. Radiat. Oncol. 2020, 25, 88–93. [Google Scholar] [CrossRef]
- Johnson, D.C.; Raman, S.S.; Mirak, S.A.; Kwan, L.; Bajgiran, A.M.; Hsu, W.; Maehara, C.K.; Ahuja, P.; Faiena, I.; Pooli, A.; et al. Detection of Individual Prostate Cancer Foci via Multiparametric Magnetic Resonance Imaging. Eur. Urol. 2019, 75, 712–720. [Google Scholar] [CrossRef] [Green Version]
- Zamboglou, C.; Drendel, V.; Jilg, C.A.; Rischke, H.C.; Beck, T.I.; Schultze-Seemann, W.; Krauss, T.; Mix, M.; Schiller, F.; Wetterauer, U.; et al. Comparison of (68)Ga-HBED-CC PSMA-PET/CT and multiparametric MRI for gross tumour volume detection in patients with primary prostate cancer based on slice by slice comparison with histopathology. Theranostics 2017, 7, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Zamboglou, C.; Schiller, F.; Fechter, T.; Wieser, G.; Jilg, C.A.; Chirindel, A.; Salman, N.; Drendel, V.; Werner, M.; Mix, M.; et al. (68)Ga-HBED-CC-PSMA PET/CT Versus Histopathology in Primary Localized Prostate Cancer: A Voxel-Wise Comparison. Theranostics 2016, 6, 1619–1628. [Google Scholar] [CrossRef] [Green Version]
- Zamboglou, C.; Wieser, G.; Hennies, S.; Rempel, I.; Kirste, S.; Soschynski, M.; Rischke, H.C.; Fechter, T.; Jilg, C.A.; Langer, M.; et al. MRI versus 68Ga-PSMA PET/CT for gross tumour volume delineation in radiation treatment planning of primary prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Grosu, A.-L.; Weirich, G.; Wendl, C.; Prokić, V.; Kirste, S.; Geinitz, H.; Souvatzoglou, M.; Gschwend, J.E.; Schwaiger, M.; Molls, M.; et al. 11C-Choline PET/pathology image coregistration in primary localized prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 2242–2248. [Google Scholar] [CrossRef] [PubMed]
- de Vries, K.C.; Wortel, R.C.; Oomen-de Hoop, E.; Heemsbergen, W.D.; Pos, F.J.; Incrocci, L. Hyprofractionated Versus Conventionally Fractionated Radiation Therapy for Patients with Intermediate- or High-Risk, Localized, Prostate Cancer: 7-Year Outcomes from the Randomized, Multicenter, Open-Label, Phase 3 HYPRO Trial. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 108–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mottet, N.; van den Bergh, R.C.; Briers, E.; van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, A.; Naismith, O.; Brand, D.; Fernandez, K.; Hall, E.; Dearnaley, D.; Gulliford, S. Derivation of Dose/Volume Constraints for the Anorectum from Clinician- and Patient-Reported Outcomes in the CHHiP Trial of Radiation Therapy Fractionation. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 928–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roach, M., 3rd; Hanks, G.; Thames, H., Jr.; Schellhammer, P.; Shipley, W.U.; Sokol, G.H.; Sandler, H. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: Recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 965–974. [Google Scholar] [CrossRef]
- Dignam, J.J.; Hamstra, D.A.; Lepor, H.; Grignon, D.; Brereton, H.; Currey, A.; Rosenthal, S.; Zeitzer, K.L.; Venkatesan, V.M.; Horwitz, E.M.; et al. Time Interval to Biochemical Failure as a Surrogate End Point in Locally Advanced Prostate Cancer: Analysis of Randomized Trial NRG/RTOG 9202. J. Clin. Oncol. 2019, 37, 213–221. [Google Scholar] [CrossRef]
- Dearnaley, D.P.; Sydes, M.R.; Graham, J.; Aird, E.G.; Bottomley, D.; Cowan, R.A.; Huddart, R.A.; Jose, C.C.; Matthews, J.H.; Millar, J.; et al. Escalated-dose versus standard-dose conformal radiotherapy in prostate cancer: First results from the MRC RT01 randomised controlled trial. Lancet Oncol. 2007, 8, 475–487. [Google Scholar] [CrossRef]
- Michalski, J.M.; Moughan, J.; Purdy, J.; Bosch, W.; Bruner, D.; Bahary, J.-P.; Lau, H.; Duclos, M.; Parliament, M.; Morton, G.; et al. Effect of Standard vs. Dose-Escalated Radiation Therapy for Patients with Intermediate-Risk Prostate Cancer: The NRG Oncology RTOG 0126 Randomized Clinical Trial. JAMA Oncol. 2018, 4, e180039. [Google Scholar] [CrossRef]
- Morris, W.J.; Tyldesley, S.; Rodda, S.; Halperin, R.; Pai, H.; McKenzie, M.; Duncan, G.; Morton, G.; Hamm, J.; Murray, N. Androgen Suppression Combined with Elective Nodal and Dose Escalated Radiation Therapy (the ASCENDE-RT Trial): An Analysis of Survival Endpoints for a Randomized Trial Comparing a Low-Dose-Rate Brachytherapy Boost to a Dose-Escalated External Beam Boost for High- and Intermediate-risk Prostate Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 275–285. [Google Scholar]
- Turkbey, B.; Rosenkrantz, A.B.; Haider, M.A.; Padhani, A.; Villeirs, G.; Macura, K.J.; Weinreb, J.C. Prostate Imaging Reporting and Data System Version 2.1: 2019 Update of Prostate Imaging Reporting and Data System Version 2. Eur. Urol. 2019, 76, 340–351. [Google Scholar] [CrossRef]
- Salembier, C.; Villeirs, G.; De Bari, B.; Hoskin, P.; Pieters, B.R.; Van Vulpen, M.; Khoo, V.; Henry, A.; Bossi, A.; De Meerleer, G.; et al. ESTRO ACROP consensus guideline on CT- and MRI-based target volume delineation for primary radiation therapy of localized prostate cancer. Radiother. Oncol. 2018, 127, 49–61. [Google Scholar] [CrossRef]
- Zamboglou, C.; Carles, M.; Fechter, T.; Kiefer, S.; Reichel, K.; Fassbender, T.F.; Bronsert, P.; Köber, G.; Schilling, O.; Ruf, J.; et al. Radiomic features from PSMA PET for non-invasive intraprostatic tumor discrimination and characterization in patients with intermediate- and high-risk prostate cancer—A comparison study with histology reference. Theranostics 2019, 9, 2595–2605. [Google Scholar] [CrossRef] [PubMed]
- Kostyszyn, D.; Fechter, T.; Bartl, N.; Grosu, A.L.; Gratzke, C.; Sigle, A.; Mix, M.; Ruf, J.; Fassbender, T.F.; Kiefer, S.; et al. Intraprostatic Tumor Segmentation on PSMA PET Images in Patients with Primary Prostate Cancer with a Convolutional Neural Network. J. Nucl. Med. 2021, 62, 823–828. [Google Scholar] [CrossRef]
- NRG-GU005. Available online: https://clinicaltrialsgov/ct2/show/NCT03367702 (accessed on 1 October 2021).
- Draulans, C.; De Roover, R.; van der Heide, U.A.; Haustermans, K.; Pos, F.; Smeenk, R.J.; De Boer, H.; Depuydt, T.; Kunze-Busch, M.; Isebaert, S.; et al. Stereotactic body radiation therapy with optional focal lesion ablative microboost in prostate cancer: Topical review and multicenter consensus. Radiother. Oncol. 2019, 140, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Alayed, Y.; Cheung, P.; Vesprini, D.; Liu, S.; Chu, W.; Chung, H.; Musunuru, H.B.; Davidson, M.; Ravi, A.; Ho, L.; et al. SABR in High-Risk Prostate Cancer: Outcomes from 2 Prospective Clinical Trials with and without Elective Nodal Irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 36–41. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Djajaputra, D.; King, C.R.; Hossain, S.; Ma, L.; Xing, L. Intrafractional motion of the prostate during hypofractionated radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 236–246. [Google Scholar] [CrossRef] [Green Version]
- Pisansky, T.M.; Hunt, D.; Gomella, L.G.; Amin, M.B.; Balogh, A.G.; Chinn, D.M.; Seider, M.J.; Duclos, M.; Rosenthal, S.A.; Bauman, G.S.; et al. Duration of Androgen Suppression Before Radiotherapy for Localized Prostate Cancer: Radiation Therapy Oncology Group Randomized Clinical Trial 9910. J. Clin. Oncol. 2015, 33, 332–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, C.U.; Pugh, S.L.; Sandler, H.M.; Chetner, M.P.; Amin, M.B.; Bruner, D.W.; Zietman, A.L.; Den, R.B.; Leibenhaut, M.H.; Longo, J.M.; et al. Adding Short-Term Androgen Deprivation Therapy to Radiation Therapy in Men with Localized Prostate Cancer: Long-Term Update of the NRG/RTOG 9408 Randomized Clinical Trial. Int. J. Radiat. Oncol. Biol. Phys. 2021, S0360–S3016. [Google Scholar] [CrossRef]
- Nabid, A.; Carrier, N.; Martin, A.-G.; Bahary, J.-P.; Lemaire, C.; Vass, S.; Bahoric, B.; Archambault, R.; Vincent, F.; Bettahar, R.; et al. Duration of Androgen Deprivation Therapy in High-risk Prostate Cancer: A Randomized Phase III Trial. Eur. Urol. 2018, 74, 432–441. [Google Scholar] [CrossRef]
- Moon, D.H.; Basak, R.S.; Usinger, D.S.; Dickerson, G.A.; Morris, D.E.; Perman, M.; Lim, M.; Wibbelsman, T.; Chang, J.; Crawford, Z.; et al. Patient-reported Quality of Life Following Stereotactic Body Radiotherapy and Conventionally Fractionated External Beam Radiotherapy Compared with Active Surveillance Among Men with Localized Prostate Cancer. Eur. Urol. 2019, 76, 391–397. [Google Scholar] [CrossRef]
- Vogelius, I.R.; Bentzen, S.M. Dose Response and Fractionation Sensitivity of Prostate Cancer after External Beam Radiation Therapy: A Meta-analysis of Randomized Trials. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 858–865. [Google Scholar] [CrossRef]
- Kishan, A.U.; Cook, R.; Ciezki, J.P.; Ross, A.E.; Pomerantz, M.M.; Nguyen, P.L.; Shaikh, T.; Tran, P.T.; Sandler, K.A.; Stock, R.G.; et al. Radical Prostatectomy, External Beam Radiotherapy, or External Beam Radiotherapy with Brachytherapy Boost and Disease Progression and Mortality in Patients with Gleason Score 9-10 Prostate Cancer. JAMA 2018, 319, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.A.; Gonzalez, J.; Ye, H.; Ghilezan, M.; Shetty, S.; Kernen, K.; Gustafson, G.; Krauss, D.; Vicini, F.; Kestin, L. Dose escalation improves cancer-related events at 10 years for intermediate-and high-risk prostate cancer patients treated with hypofractionated high-dose-rate boost and external beam radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Vogelius, I.R.; Bentzen, S.M. Radiation Dose Escalation for Early Prostate Cancer: Reigniting the FLAME? J. Clin. Oncol. 2021, 39, 3085–3086. [Google Scholar] [CrossRef] [PubMed]



| Inclusion Criteria: |
| Histologically confirmed adenocarcinoma of the prostate (histological confirmation can be based on tissue taken at any time, but a re-biopsy should be considered if the biopsy is more than 12 months old) |
| Primary localized PCa (cN0 and cM0 in mpMRI and PSMA-PET): high- or very high-risk according to NCCN v2.2021 OR unfavorable intermediate-risk disease according to NCCN v2.2021 |
| Signed written informed consent for this study |
| Age > 18 years |
| Previously conducted PSMA-PET/CT and mpMRI scans or PSMA-PET/MR for staging, fulfilling standard requirements for PCa |
| ECOG performance score 0 or 1 |
| IPSS score ≤ 15 |
| Prostate volume ≤ 75 mL at RT planning |
| Exclusion criteria: |
| Evidence of neuroendocrine tumor cells |
| Prior radiotherapy to the prostate or pelvis |
| Prior radical prostatectomy |
| Prior focal therapy approaches to the prostate |
| Time gap between the beginning of ADT and conduction of initial mpMRI and PSMA-PET scans is >1 month |
| Radiologically suspicious or pathologically confirmed lymph node involvement (cN+) in mpMRI and/or PSMA-PET/CT |
| Evidence of metastatic disease (cM+) in mpMRI and/or PSMA-PET/CT |
| Evidence of cT4 disease in mpMRI and/or PSMA-PET/CT |
| PSA > 30 ng/mL prior to starting ADT |
| Expected patient survival <5 years |
| Bilateral hip prostheses or any other implants/hardware that would introduce substantial CT artefacts |
| Contraindication to undergo a mpMRI scan |
| Prostate surgery (TURP or HOLEP) with a significant tissue cavity or prostate surgery (TURP or HOLEP) within the last 6 months prior to randomization |
| Medical conditions likely to make radiotherapy inadvisable, e.g., acute inflammatory bowel disease, hemiplegia or paraplegia |
| Previous malignancy within the last 2 years (except basal cell carcinoma or squamous cell carcinoma of the skin), or if previous malignancy is expected to significantly compromise 5 year survival |
| Any other contraindication to external beam radiotherapy (EBRT) to the pelvis |
| In mpMRI and PSMA-PET/CT or PSMA-PET/MRI scans, no visible tumor |
| Participation in any other interventional clinical trial within the last 30 days before the start of this trial |
| Simultaneous participation in other interventional trials that could interfere with this trial; simultaneous participation in registry and diagnostic trials is allowed |
| Patient without legal capacity who is unable to understand the nature, significance and consequences of the trial |
| Known or persistent abuse of medication, drugs or alcohol |
| Patients expected to have severe set up problems |
| Dose constraints for organs at risk cannot be adhered to |
| PTV1 (PTV1–PTV3) | CTV1 | PTV2 (PTV2–PTV3) | CTV2 | PTV3 | ||||
|---|---|---|---|---|---|---|---|---|
| D50% | D98% | D99% | D50% | D98% | D99% | D50% | D98% | D0.01cc |
| ≥30 Gy | ≥28.5 Gy | ≥28.5 Gy | ≥35 Gy | ≥33.25 Gy (minor deviation ≥ 31 Gy) | ≥34.5 Gy (minor deviation: ≥34 Gy) | 40–42 Gy | ≥95% of prescribed dose (minor deviation: up to 36.25 Gy) | ≤105% of prescribed dose (minor deviation up to 110%) |
| OAR | Constraint |
|---|---|
| PRV-Rectum or PRV-Rectum adapt | D0.03cc (near Dmax): <38 Gy (minor deviation: 38–40 Gy) |
| Rectum | D1cc: <36 Gy (minor deviation: 36–38 Gy) |
| D2cc: <35 Gy | |
| D20%: ≤28 Gy | |
| PRV-Urethra | D0.01cc (near Dmax): <40 Gy (minor deviation: 40–42 Gy) |
| Urethra | D50%: <36 Gy (minor deviation: 36–39 Gy) |
| Bladder | D0.03cc: <38.06 Gy (minor deviation: 38.06–40 Gy) |
| D5cc: <37 Gy | |
| D15%: ≤32 Gy: | |
| D20%: ≤28 Gy | |
| D50%: <18.12 Gy (minor deviation: 18.12–20 Gy) | |
| Penile bulb (facultative) | D90%: ≤20 Gy |
| D50%: ≤29.5 Gy | |
| Femoral head (left or right) | D5%: ≤28 Gy |
| Small bowel | D < 5cc: 18.1 Gy |
| D0.01cc (near Dmax): <30 Gy (minor deviation: 30–33 Gy) |
| PTV1 | PTV2 | CTV2 | ||||
|---|---|---|---|---|---|---|
| Prescription dose | D95% | D98% | D95% | D98% | D2% | D98% |
| 60 Gy | 95% | ≥42 Gy | 95% | ≥55 Gy | ≤61.2 Gy (minor deviation up to 63 Gy) | ≥58.8 Gy |
| 62 Gy | 95% | ≥42 Gy | 95% | ≥57 Gy | ≤63.2 Gy (minor deviation up to 65 Gy) | ≥58.8 Gy |
| Organ | Dose for 20 Fractions (Gy) | Max. Volume (%) Optimal | Max. Volume (%) Mandatory |
|---|---|---|---|
| Rectum or Rectum Adapt | Dose for 20 Fractions (Gy) | Max Volume (%) | Max Volume (%) |
| 20 | 85 | ||
| 25 | 80 | ||
| 30 | 57 | ||
| 40 | 38 | 50 | |
| 50 | 22 | ||
| 57 | 5 | 10 | |
| 60 | 0.01 | 0.3 | |
| Bladder | Dose for 20 fractions (Gy) | Max volume (%) | Max volume (%) |
| 40 | 30 | 40 | |
| 50 | 15 | 25 | |
| 60 | 3 | 5 | |
| Femoral head left and right | Dose for 20 fractions (Gy) | Max volume (%) | |
| 41 | 50 | ||
| Penile bulb | Dose for 20 fractions (Gy) | Max volume (%) | Max volume (%) |
| 41 | 50 | ||
| 49 | 20 | ||
| Sigmoid | Dose for 20 fractions (Gy) | Max volume (%) | Max volume (cc) |
| 53 | 3 | ||
| Small bowel | Dose for 20 fractions (Gy) | Max volume (cc) | |
| 41 | 17 | ||
| 47 | Dmax | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamboglou, C.; Spohn, S.K.B.; Adebahr, S.; Huber, M.; Kirste, S.; Sprave, T.; Gratzke, C.; Chen, R.C.; Carl, E.G.; Weber, W.A.; et al. PSMA-PET/MRI-Based Focal Dose Escalation in Patients with Primary Prostate Cancer Treated with Stereotactic Body Radiation Therapy (HypoFocal-SBRT): Study Protocol of a Randomized, Multicentric Phase III Trial. Cancers 2021, 13, 5795. https://doi.org/10.3390/cancers13225795
Zamboglou C, Spohn SKB, Adebahr S, Huber M, Kirste S, Sprave T, Gratzke C, Chen RC, Carl EG, Weber WA, et al. PSMA-PET/MRI-Based Focal Dose Escalation in Patients with Primary Prostate Cancer Treated with Stereotactic Body Radiation Therapy (HypoFocal-SBRT): Study Protocol of a Randomized, Multicentric Phase III Trial. Cancers. 2021; 13(22):5795. https://doi.org/10.3390/cancers13225795
Chicago/Turabian StyleZamboglou, Constantinos, Simon K. B. Spohn, Sonja Adebahr, Maria Huber, Simon Kirste, Tanja Sprave, Christian Gratzke, Ronald C. Chen, Ernst Günther Carl, Wolfgang A. Weber, and et al. 2021. "PSMA-PET/MRI-Based Focal Dose Escalation in Patients with Primary Prostate Cancer Treated with Stereotactic Body Radiation Therapy (HypoFocal-SBRT): Study Protocol of a Randomized, Multicentric Phase III Trial" Cancers 13, no. 22: 5795. https://doi.org/10.3390/cancers13225795
APA StyleZamboglou, C., Spohn, S. K. B., Adebahr, S., Huber, M., Kirste, S., Sprave, T., Gratzke, C., Chen, R. C., Carl, E. G., Weber, W. A., Mix, M., Benndorf, M., Wiegel, T., Baltas, D., Jenkner, C., & Grosu, A. L. (2021). PSMA-PET/MRI-Based Focal Dose Escalation in Patients with Primary Prostate Cancer Treated with Stereotactic Body Radiation Therapy (HypoFocal-SBRT): Study Protocol of a Randomized, Multicentric Phase III Trial. Cancers, 13(22), 5795. https://doi.org/10.3390/cancers13225795