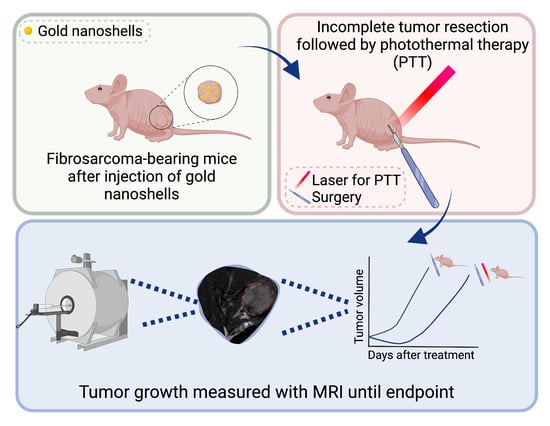
Photothermal Therapy as Adjuvant to Surgery in an Orthotopic Mouse Model of Human Fibrosarcoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Gold Nanoshells
2.2. Cell Line and Animal Model
2.3. Surgical Resection Followed by Photothermal Therapy
2.4. Tumor Size Monitoring with CT
2.5. Tumor Size Monitoring with MRI
2.6. Histology
2.7. Biodistribution of Gold Nanoshells
2.8. Statistics and Data Analysis
3. Results
3.1. Subtotal Tumor Resection Followed by Photothermal Therapy in a Subcutaneous Fibrosarcoma Model
3.2. Characterization of the Orthotopic Fibrosarcoma Model
3.3. Biodistribution of Gold Nanoshells
3.4. Subtotal Tumor Resection Followed by Photothermal Therapy in an Orthotopic Fibrosarcoma Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moore, L.S.; de Boer, E.; Warram, J.M.; Tucker, M.D.; Carroll, W.R.; Korb, M.L.; Brandwein-Gensler, M.S.; van Dam, G.M.; Rosenthal, E.L. Photoimmunotherapy of residual disease after incomplete surgical resection in head and neck cancer models. Cancer Med. 2016, 5, 1526–1534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer, A.; Sorger, P.K. Combination Cancer Therapy Can Confer Benefit via Patient-to-Patient Variability without Drug Additivity or Synergy. Cell 2017, 171, 1678–1691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mokhtari, R.B.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef] [Green Version]
- Tohme, S.; Simmons, R.L.; Tsung, A. Surgery for cancer: A trigger for metastases. Cancer Res. 2017, 77, 1548–1552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, T.L.; Patel, N.; Faiena, I.; Radadia, K.D.; Moore, D.F.; Elsamra, S.E.; Singer, E.A.; Stein, M.N.; Eastham, J.A.; Scardino, P.T.; et al. Comparative effectiveness of radical prostatectomy with adjuvant radiotherapy versus radiotherapy plus androgen deprivation therapy for men with advanced prostate cancer. Cancer 2018, 124, 4010–4022. [Google Scholar] [CrossRef] [Green Version]
- Jaque, D.; Martínez Maestro, L.; del Rosal, B.; Haro-Gonzalez, P.; Benayas, A.; Plaza, J.L.; Rodríguez, E.M.; García Solé, J. Nanoparticles for photothermal therapies. Nanoscale 2014, 6, 9494–9530. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, J.T.; Norregaard, K.; Tian, P.; Bendix, P.M.; Kjaer, A.; Oddershede, L.B. Single Particle and PET-based Platform for Identifying Optimal Plasmonic Nano-Heaters for Photothermal Cancer Therapy. Sci. Rep. 2016, 6, 30076. [Google Scholar] [CrossRef]
- Diogo, D.M.D.M.; Pais-Silva, C.; Dias, D.R.; Moreira, A.F.; Correia, I.J. Strategies to Improve Cancer Photothermal Therapy Mediated by Nanomaterials. Adv. Heal. Mater. 2017, 6, 1700073. [Google Scholar] [CrossRef]
- Norregaard, K.; Jørgensen, J.T.; Simon, M.; Melander, F.; Kristensen, L.K.; Bendix, P.M.; Andresen, T.L.; Oddershede, L.B.; Kjaer, A. 18F-FDG PET/CT-based early treatment response evaluation of nanoparticle-assisted photothermal cancer therapy. PLoS ONE 2017, 12, e0177997. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, D.K.; Diagaradjane, P.; Krishnan, S. Nanoparticle-mediated hyperthermia in cancer therapy. Ther. Deliv. 2011, 2, 1001–1014. [Google Scholar] [CrossRef] [Green Version]
- Ayala-Orozco, C.; Urban, C.; Bishnoi, S.; Urban, A.; Charron, H.; Mitchell, T.; Shea, M.; Nanda, S.; Schiff, R.; Halas, N.; et al. Sub-100nm gold nanomatryoshkas improve photo-thermal therapy efficacy in large and highly aggressive triple negative breast tumors. J. Control Release 2014, 191, 90–97. [Google Scholar] [CrossRef] [Green Version]
- Simón, M.; Nørregaard, K.; Jørgensen, J.T.; Oddershede, L.B.; Kjaer, A. Fractionated photothermal therapy in a murine tumor model: Comparison with single dose. Int. J. Nanomed. 2019, 14, 5369–5379. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Ma, X.; Hong, X.; Cheng, Y.; Tian, Y.; Zhao, S.; Liu, W.; Tang, Y.; Zhao, R.; Song, L.; et al. Adjuvant Photothermal Therapy Inhibits Local Recurrences after Breast-Conserving Surgery with Little Skin Damage. ACS Nano 2017, 12, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhang, L.; Yi, H.; Huang, J.; Zhang, N.; Zhong, Y.; Hao, L.; Yang, K.; Wang, Z.; Wang, D.; et al. NIR-responsive nanoplatform for pre/intraoperative image-guided carcinoma surgery and photothermal ablation of residual tumor tissue. Nanomed. Nanotechnol. Biol. Med. 2019, 20, 102020. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.R.K.; Farghali, H.A.M.; Wu, Y.; El-Sayed, I.; Osman, A.H.; Selim, S.A.; El-Sayed, M.A. Gold Nanorod-Assisted Photothermal Therapy Decreases Bleeding during Breast Cancer Surgery in Dogs and Cats. Cancers 2019, 11, 851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daigeler, A.; Zmarsly, I.; Hirsch, S.T.F.; Goertz, O.; Steinau, H.-U.; Lehnhardt, M.; Harati, K. Long-term outcome after local recurrence of soft tissue sarcoma: A retrospective analysis of factors predictive of survival in 135 patients with locally recurrent soft tissue sarcoma. Br. J. Cancer 2014, 110, 1456–1464. [Google Scholar] [CrossRef] [Green Version]
- Augsburger, D.; Nelson, P.J.; Kalinski, T.; Udelnow, A.; Knösel, T.; Hofstetter, M.; Qin, J.W.; Wang, Y.; Gupta, A.S.; Bonifatius, S.; et al. Current diagnostics and treatment of fibrosarcoma -perspectives for future therapeutic targets and strategies. Oncotarget 2017, 8, 104638–104653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrison, B.A. Soft Tissue Sarcomas of the Extremities. Bayl. Univ. Med Cent. Proc. 2003, 16, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Nie, P.; Dong, C.; Li, J.; Huang, Y.; Hao, D.; Xu, W. CT and MRI Findings of Soft Tissue Adult Fibrosarcoma in Extremities. BioMed. Res. Int. 2018, 2018, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Yano, S.; Miwa, S.; Kishimoto, H.; Uehara, F.; Tazawa, H.; Toneri, M.; Hiroshima, Y.; Yamamoto, M.; Urata, Y.; Kagawa, S.; et al. Targeting tumors with a killer-reporter adenovirus for curative fluorescence-guided surgery of soft-tissue sarcoma. Oncotarget 2015, 6, 13133–13148. [Google Scholar] [CrossRef] [Green Version]
- Sorace, A.G.; Korb, M.; Warram, J.M.; Umphrey, H.; Zinn, K.R.; Rosenthal, E.; Hoyt, K. Ultrasound-Stimulated Drug Delivery for Treatment of Residual Disease after Incomplete Resection of Head and Neck Cancer. Ultrasound Med. Biol. 2014, 40, 755–764. [Google Scholar] [CrossRef] [Green Version]
- Misra, R.M.; Bajaj, M.S.; Kale, V.P. Vasculogenic Mimicry of HT1080 Tumour Cells In Vivo: Critical Role of HIF-1α-Neuropilin-1 Axis. PLoS ONE 2012, 7, e50153. [Google Scholar] [CrossRef] [PubMed]
- Gad, S.C.; Sharp, K.L.; Montgomery, C.; Payne, J.D.; Goodrich, G.P. Evaluation of the Toxicity of Intravenous Delivery of Auroshell Particles (Gold–Silica Nanoshells). Int. J. Toxicol. 2012, 31, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Hervey-Jumper, S.L.; Berger, M.S. Maximizing safe resection of low- and high-grade glioma. J. Neuro Oncol. 2016, 130, 269–282. [Google Scholar] [CrossRef]
- Riley, R.S.; Day, E.S. Gold nanoparticle-mediated photothermal therapy: Applications and opportunities for multimodal cancer treatment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1449. [Google Scholar] [CrossRef] [PubMed]
- Norum, O.-J.; Giercksky, K.-E.; Berg, K. Photochemical internalization as an adjunct to marginal surgery in a human sarcoma model. Photochem. Photobiol. Sci. 2009, 8, 758–762. [Google Scholar] [CrossRef]
- Broomfield, S.; Currie, A.; Van Der Most, R.G.; Brown, M.; Van Bruggen, I.; Robinson, B.W.; Lake, R.A. Partial, but not Complete, Tumor-Debulking Surgery Promotes Protective Antitumor Memory when Combined with Chemotherapy and Adjuvant Immunotherapy. Cancer Res. 2005, 65, 7580–7584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Fan, W.; Rachagani, S.; Zhou, Z.; Lele, S.M.; Batra, S.K.; Garrison, J.C. Comparative Study of Subcutaneous and Orthotopic Mouse Models of Prostate Cancer: Vascular Perfusion, Vasculature Density, Hypoxic Burden and BB2r-Targeting Efficacy. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Rastinehad, A.R.; Anastos, H.; Wajswol, E.; Winoker, J.S.; Sfakianos, J.P.; Doppalapudi, S.K.; Carrick, M.R.; Knauer, C.J.; Taouli, B.; Lewis, S.C.; et al. Gold nanoshell-localized photothermal ablation of prostate tumors in a clinical pilot device study. Proc. Natl. Acad. Sci. USA 2019, 116, 18590–18596. [Google Scholar] [CrossRef] [Green Version]
- Fang, J.; Nakamura, H.; Maeda, H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv. Drug Deliv. Rev. 2011, 63, 136–151. [Google Scholar] [CrossRef]
- Shi, Y.; Van Der Meel, R.; Chen, X.; Lammers, T. The EPR effect and beyond: Strategies to improve tumor targeting and cancer nanomedicine treatment efficacy. Theranostics 2020, 10, 7921–7924. [Google Scholar] [CrossRef] [PubMed]
- Juhl, K.; Christensen, A.; Persson, M.; Ploug, M.; Kjaer, A. Peptide-Based Optical uPAR Imaging for Surgery: In Vivo Testing of ICG-Glu-Glu-AE105. PLoS ONE 2016, 11, e0147428. [Google Scholar] [CrossRef]
- Juhl, K.; Christensen, A.; Rubek, N.; Karnov, K.K.S.; Von Buchwald, C.; Kjaer, A. Improved surgical resection of metastatic pancreatic cancer using uPAR targeted in vivo fluorescent guidance: Comparison with traditional white light surgery. Oncotarget 2019, 10, 6308–6316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simón, M.; Jørgensen, J.T.; Juhl, K.; Kjaer, A. The use of a uPAR-targeted probe for photothermal cancer therapy prolongs survival in a xenograft mouse model of glioblastoma. Oncotarget 2021, 12, 1366–1376. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simón, M.; Jørgensen, J.T.; Melander, F.; Andresen, T.L.; Christensen, A.; Kjaer, A. Photothermal Therapy as Adjuvant to Surgery in an Orthotopic Mouse Model of Human Fibrosarcoma. Cancers 2021, 13, 5820. https://doi.org/10.3390/cancers13225820
Simón M, Jørgensen JT, Melander F, Andresen TL, Christensen A, Kjaer A. Photothermal Therapy as Adjuvant to Surgery in an Orthotopic Mouse Model of Human Fibrosarcoma. Cancers. 2021; 13(22):5820. https://doi.org/10.3390/cancers13225820
Chicago/Turabian StyleSimón, Marina, Jesper Tranekjær Jørgensen, Fredrik Melander, Thomas Lars Andresen, Anders Christensen, and Andreas Kjaer. 2021. "Photothermal Therapy as Adjuvant to Surgery in an Orthotopic Mouse Model of Human Fibrosarcoma" Cancers 13, no. 22: 5820. https://doi.org/10.3390/cancers13225820
APA StyleSimón, M., Jørgensen, J. T., Melander, F., Andresen, T. L., Christensen, A., & Kjaer, A. (2021). Photothermal Therapy as Adjuvant to Surgery in an Orthotopic Mouse Model of Human Fibrosarcoma. Cancers, 13(22), 5820. https://doi.org/10.3390/cancers13225820