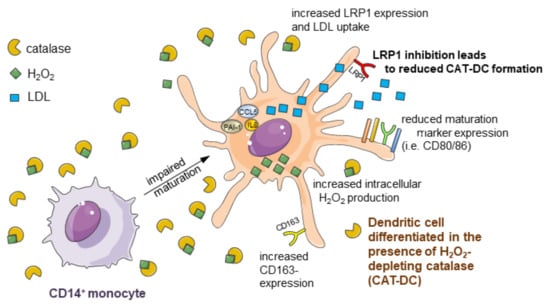
Hydrogen-Peroxide Synthesis and LDL-Uptake Controls Immunosuppressive Properties in Monocyte-Derived Dendritic Cells
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Isolation of Mononuclear Cells from Leukapheresis Concentrates
2.3. Generation of Dendritic Cells from Human Monocytes
2.4. Coculture of DCs and T-Cells
2.5. Measurement of H2O2 and Superoxide Levels
2.6. Proteome Profiling
2.7. Flow Cytometry
2.8. LDL and oxLDL-Uptake Assay
2.9. Statistical Analysis
3. Results
3.1. Catalase-Mediated H2O2 Depletion Impairs Maturation of Monocyte-Derived DCs
3.2. CAT-DCs Are Capable of Suppressing Pan-T-Cell Proliferation
3.3. CAT-DCs Suppress T-Cell Proliferation through a Contact-Dependent, IDO-Mediated Mechanism
3.4. CAT-DCs Skew T-Cell-Polarization towards an Immunosuppressive Phenotype
3.5. Impairment of DC-Maturation by Alternative H2O2 Scavenger Glutathione Peroxidase
3.6. Extracellular Depletion of H2O2 Results in Increased Intracellular NADPH-Driven H2O2 Production in CAT-DCs
3.7. Increased LDL and oxLDL-Uptake and LRP1 Expression Is Associated with the Induction of Immunosuppressive Properties in CAT-DCs
3.8. Interaction between (ox-)LDL-Uptake and Intracellular H2O2 Production Regulates IDO- and CD86-Expression on CAT-DCs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 1-MT | 1-methyl-DL-tryptophan |
| Ab | antibody |
| APC | antigen-presenting cell |
| CAT-DC | DC differentiated and matured in the presence of catalase |
| CCL | C-C motif chemokine ligand |
| CD | cluster of differentiation |
| COX-2 | cyclooxygenase-2 |
| CXCL | C-X-C motif chemokine ligand |
| DC, | dendritic cell |
| DPI | diphenyleneiodonium |
| EDTA | ethylenediaminetetraacetic acid |
| FCS | fetal calf serum |
| FOX-P3 | forkhead box P3 |
| FSC | forward scatter |
| GM-CSF | granulocyte-macrophage colony-stimulating factor |
| GPX | glutathione peroxidase |
| GSH | glutathione |
| H2O2 | hydrogen peroxide |
| HLA | human leukocyte antigen |
| IDO | indoleamine 2,3-dioxygenase |
| IFN | interferon |
| IL | interleukin |
| LDL | low density lipoprotein |
| LDL-R | LDL receptor |
| LPS | lipopolysaccharide |
| LRP1 | LDL related protein 1 |
| LRS | leukoreduction system |
| mAb | monoclonal antibody |
| MDSC | myeloid-derived suppressor cell |
| MHC | major histocompatibility complex |
| MitoQ | mitoquinone mesylate |
| moDC | monocyted-derived dendritic cell |
| NOX | NADPH oxidase |
| oxLDL | oxidized LDL |
| PAI-1 | plasminogen activator inhibitor-1 |
| PBS | phosphate-buffered saline |
| PMA | phorbol 12-myristate 13-acetate |
| RLU | relative luminescent units |
| ROS | reactive oxygen species |
| SD | standard deviation |
| SSC | side scatter |
| TNF | tumor necrosis factor |
References
- Kerkar, S.P.; Restifo, N.P. Cellular constituents of immune escape within the tumor microenvironment. Cancer Res. 2012, 72, 3125–3313. [Google Scholar] [CrossRef] [Green Version]
- Beatty, G.L.; Gladney, W.L. Immune escape mechanisms as a guide for cancer immunotherapy. Clin. Cancer Res. 2015, 21, 687–692. [Google Scholar] [CrossRef] [Green Version]
- Gabrilovich, D.I. Myeloid-derived suppressor cells. Cancer Immunol. Res. 2017, 5, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Seliger, B.; Massa, C. The dark side of dendritic cells: Development and exploitation of tolerogenic activity that favor tumor outgrowth and immune escape. Front. Immunol. 2013, 4, 419. [Google Scholar] [CrossRef] [Green Version]
- De Rosa, V.; Di Rella, F.; Di Giacomo, A.; Matarese, G. Regulatory T cells as suppressors of anti-tumor immunity: Role of metabolism. Cytokine Growth Factor Rev. 2017, 35, 15–25. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, J.; Deng, X.; Xiong, F.; Ge, J.; Xiang, B.; Wu, X.; Ma, J.; Zhou, M.; Li, X.; et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol. Cancer 2019, 18. [Google Scholar] [CrossRef] [Green Version]
- Burkholder, B.; Huang, R.-Y.; Burgess, R.; Luo, S.; Jones, V.S.; Zhang, W.; Lv, Z.-Q.; Gao, C.-Y.; Wang, B.-L.; Zhang, Y.-M.; et al. Tumor-induced perturbations of cytokines and immune cell networks. Biochim. Biophys. Acta (BBA) Bioenerg. 2014, 1845, 182–201. [Google Scholar] [CrossRef] [Green Version]
- Turley, S.J.; Cremasco, V.; Astarita, J.L. Immunological hallmarks of stromal cells in the tumour microenvironment. Nat. Rev. Immunol. 2015, 15, 669–682. [Google Scholar] [CrossRef]
- Shi, Y.; Du, L.; Lin, L.; Wang, Y. Tumour-associated mesenchymal stem/stromal cells: Emerging therapeutic targets. Nat. Rev. Drug Discov. 2017, 16, 35–52. [Google Scholar] [CrossRef]
- Ohta, M.; Ishida, A.; Toda, M.; Akita, K.; Inoue, M.; Yamashita, K.; Watanabe, M.; Murata, T.; Usui, T.; Nakada, H. Immunomodulation of monocyte-derived dendritic cells through ligation of tumor-produced mucins to Siglec-9. Biochem. Biophys. Res. Commun. 2010, 402, 663–669. [Google Scholar] [CrossRef]
- Chow, K.V.; Sutherland, R.M.; Zhan, Y.; Lew, A.M. Heterogeneity, functional specialization and differentiation of monocyte-derived dendritic cells. Immunol. Cell Biol. 2017, 95, 244–251. [Google Scholar] [CrossRef]
- Jürgens, B.; Hainz, U.; Fuchs, D.; Felzmann, T.; Heitger, A. Interferon-γ–triggered indoleamine 2,3-dioxygenase competence in human monocyte-derived dendritic cells induces regulatory activity in allogeneic T cells. Blood 2009, 114, 3235–3243. [Google Scholar] [CrossRef] [Green Version]
- Hazen, S.L.; Zhang, R.; Shen, Z.; Wu, W.; Podrez, E.A.; MacPherson, J.C.; Schmitt, D.; Mitra, S.N.; Mukhopadhyay, C.; Chen, Y.; et al. Formation of nitric oxide-derived oxidants by myeloperoxidase in monocytes: Pathways for monocyte-mediated protein nitration and lipid peroxidation in vivo. Circ. Res. 1999, 85, 950–958. [Google Scholar] [CrossRef] [Green Version]
- Schumacker, P.T. Reactive oxygen species in cancer: A dance with the devil. Cancer Cell 2015, 27, 156–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumari, S.; Badana, A.K.; Murali Mohan, G.; Shailender, G.; Malla, R.R. Reactive oxygen species: A key constituent in cancer survival. Biomark. Insights 2018, 13, 117727191875539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liou, G.-Y.; Storz, P. Reactive oxygen species in cancer. Free. Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef] [Green Version]
- Bauer, G. Increasing the endogenous NO level causes catalase inactivation and reactivation of intercellular apoptosis signaling specifically in tumor cells. Redox Biol. 2015, 6, 353–371. [Google Scholar] [CrossRef] [Green Version]
- Neuzil, J. Vitamin E succinate and cancer treatment: A vitamin E prototype for selective antitumour activity. Br. J. Cancer 2003, 89, 1822–1826. [Google Scholar] [CrossRef] [Green Version]
- Malafa, M.P.; Fokum, F.D.; Mowlavi, A.; Abusief, M.; King, M. Vitamin E inhibits melanoma growth in mice. Surgery 2002, 131, 85–91. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.S.; Suh, N.; Kong, A.-N.T. Does vitamin e prevent or promote cancer? Cancer Prev. Res. 2012, 5, 701–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Resheq, Y.J.; Li, K.-K.; Ward, S.T.; Wilhelm, A.; Garg, A.; Curbishley, S.M.; Blahova, M.; Zimmermann, H.W.; Jitschin, R.; Mougiakakos, D.; et al. Contact-dependent depletion of hydrogen peroxide by catalase is a novel mechanism of myeloid-derived suppressor cell induction operating in human hepatic stellate cells. J. Immunol. 2015, 194, 2578–2586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cella, M.; Sallusto, F.; Lanzavecchia, A. Origin, maturation and antigen presenting function of dendritic cells. Curr. Opin. Immunol. 1997, 9, 10–16. [Google Scholar] [CrossRef]
- Pletjushkina, O.Y.; Fetisova, E.K.; Lyamzaev, K.G.; Ivanova, O.Y.; Domnina, L.V.; Vyssokikh, M.Y.; Pustovidko, A.V.; Alexeevski, A.V.; Alexeevski, D.A.; Vasiliev, J.M.; et al. Hydrogen peroxide produced inside mitochondria takes part in cell-to-cell transmission of apoptotic signal. Biochem. Mosc. 2006, 71, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, D.F.; Helm, K.; DeGregori, J.; Roederer, M.; Majka, S.M. Publishing flow cytometry data. Am. J. Physiol. Cell. Mol. Physiol. 2010, 298, L127–L130. [Google Scholar] [CrossRef]
- Alfonso-Prieto, M.; Biarnés, X.; Vidossich, P.; Rovira, C. The Molecular mechanism of the catalase reaction. J. Am. Chem. Soc. 2009, 131, 11751–11761. [Google Scholar] [CrossRef]
- Italiani, P.; Boraschi, D. From monocytes to M1/M2 macrophages: Phenotypical vs. functional differentiation. Front. Immunol. 2014, 5, 514. [Google Scholar] [CrossRef] [Green Version]
- Zelenay, S.; van der Veen, A.G.; Böttcher, J.P.; Snelgrove, K.J.; Rogers, N.; Acton, S.E.; Chakravarty, P.; Girotti, M.R.; Marais, R.; Quezada, S.A.; et al. Cyclooxygenase-dependent tumor growth through evasion of immunity. Cell 2015, 162, 1257–1270. [Google Scholar] [CrossRef] [Green Version]
- Mannino, M.H.; Zhu, Z.; Xiao, H.; Bai, Q.; Wakefield, M.R.; Fang, Y. The paradoxical role of IL-10 in immunity and cancer. Cancer Lett. 2015, 367, 103–107. [Google Scholar] [CrossRef]
- Huang, B.; Pan, P.-Y.; Li, Q.; Sato, A.I.; Levy, D.E.; Bromberg, J.; Divino, C.M.; Chen, S.-H. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-Induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006, 66, 1123–1131. [Google Scholar] [CrossRef] [Green Version]
- Kubala, M.; Placencio, V.; DeClerck, Y. Plasminogen activator inhibitor-1 increases migration of monocytes to the tumor and skews their differentiation towards M2 macrophage phenotype (TUM6P.1002). J. Immunol. 2015, 194, 141.26. [Google Scholar]
- Gross, S.; Erdmann, M.; Haendle, I.; Voland, S.; Berger, T.; Schultz, E.; Strasser, E.F.; Dankerl, P.; Janka, R.; Schliep, S.; et al. Twelve-year survival and immune correlates in dendritic cell–vaccinated melanoma patients. JCI Insight 2017, 2. [Google Scholar] [CrossRef] [Green Version]
- Fiaschi, T.; Chiarugi, P. Oxidative stress, tumor microenvironment, and metabolic reprogramming: A diabolic liaison. Int. J. Cell Biol. 2012, 2012, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renner, K.; Singer, K.; Koehl, G.E.; Geissler, E.K.; Peter, K.; Siska, P.J.; Kreutz, M. Metabolic hallmarks of tumor and immune cells in the tumor microenvironment. Front. Immunol. 2017, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subauste, C.S.; Malefyt, R.D.W.; Fuh, F. Role of CD80 (B7.1) and CD86 (B7.2) in the immune response to an intracellular pathogen. J. Immunol. 1998, 160, 1831–1840. [Google Scholar] [PubMed]
- Troy, A.J.; Summers, K.L.; Davidson, P.J.T.; Atkinson, C.H.; Hart, D.N.J. Minimal recruitment and activation of dendritic cells within renal cell carcinoma. J. Urol. 1999, 4, 1737–1738. [Google Scholar] [CrossRef]
- Troy, A.; Davidson, P.; Atkinson, C.; Hart, D. Phenotypic characterisation of the dendritic cell infiltrate in prostate cancer. J. Urol. 1998, 160, 214–219. [Google Scholar] [CrossRef]
- Aalamian, M.; Pirtskhalaishvili, G.; Nunez, A.; Esche, C.; Shurin, G.V.; Huland, E.; Huland, H.; Shurin, M.R. Human prostate cancer regulates generation and maturation of monocyte-derived dendritic cells. Prostate 2001, 46, 68–75. [Google Scholar] [CrossRef]
- Collin, M.; Bigley, V. Human dendritic cell subsets: An update. Immunology 2018, 154, 3–20. [Google Scholar] [CrossRef]
- Gay, D.; Maddon, P.; Sékaly, R.; Talle, M.A.; Godfrey, M.; Long, E.; Goldstein, G.; Chess, L.; Axel, R.; Kappler, J.; et al. Functional interaction between human T-cell protein CD4 and the major histocompatibility complex HLA-DR antigen. Nat. Cell Biol. 1987, 328, 626–629. [Google Scholar] [CrossRef]
- Comi, M.; Avancini, D.; De Sio, F.S.; Villa, M.; Uyeda, M.J.; Floris, M.; Tomasoni, D.; Bulfone, A.; Roncarolo, M.G.; Gregori, S. Coexpression of CD163 and CD141 identifies human circulating IL-10-producing dendritic cells (DC-10). Cell. Mol. Immunol. 2020, 17, 95–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalpakcioglu, B.; Senel, K. The interrelation of glutathione reductase, catalase, glutathione peroxidase, superoxide dismutase, and glucose-6-phosphate in the pathogenesis of rheumatoid arthritis. Clin. Rheumatol. 2008, 27, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Braun, D.; Longman, R.S.; Albert, M.L. A two-step induction of indoleamine 2,3 dioxygenase (IDO) activity during dendritic-cell maturation. Blood 2005, 106, 2375–2381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, J.-T.; Deng, Y.-N.; Yi, H.-M.; Wang, G.-Y.; Fu, B.-S.; Chen, W.-J.; Liu, W.; Tai, Y.; Peng, Y.-W.; Zhang, Q. Hepatic carcinoma-associated fibroblasts induce IDO-producing regulatory dendritic cells through IL-6-mediated STAT3 activation. Oncogenesis 2016, 5, e198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nat. Cell Biol. 1998, 392, 245–252. [Google Scholar] [CrossRef]
- Akdis, C.A.; Blaser, K. Mechanisms of interleukin-10-mediated immune suppression. Immunology 2001, 103, 131–136. [Google Scholar] [CrossRef]
- Zou, W.; Restifo, N.P. TH17 cells in tumour immunity and immunotherapy. Nat. Rev. Immunol. 2010, 10, 248–256. [Google Scholar] [CrossRef]
- Musuraca, G.; De Matteis, S.; Napolitano, R.; Papayannidis, C.; Guadagnuolo, V.; Fabbri, F.; Cangini, D.; Ceccolini, M.; Giannini, M.B.; Lucchesi, A.; et al. IL-17/IL-10 double-producing T cells: New link between infections, immunosuppression and acute myeloid leukemia. J. Transl. Med. 2015, 13. [Google Scholar] [CrossRef] [Green Version]
- Kamsler, A.; Segal, M. Hydrogen peroxide as a diffusible signal molecule in synaptic plasticity. Mol. Neurobiol. 2004, 29, 167–178. [Google Scholar] [CrossRef]
- Henriksen, E.J. Effects of H2O2 on insulin signaling the glucose transport system in mammalian skeletal muscle. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2013; pp. 269–278. [Google Scholar]
- Fiévet, C.; Staels, B. Liver X receptor modulators: Effects on lipid metabolism and potential use in the treatment of atherosclerosis. Biochem. Pharmacol. 2009, 77, 1316–1327. [Google Scholar] [CrossRef] [Green Version]
- Beezhold, K.; Byersdorfer, C.A. Targeting immuno-metabolism to improve anti-cancer therapies. Cancer Lett. 2018, 414, 127–135. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, L.A.J.; Pearce, E.J. Immunometabolism governs dendritic cell and macrophage function. J. Exp. Med. 2016, 213, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Herber, D.L.; Cao, W.; Nefedova, Y.; Novitskiy, S.V.; Nagaraj, S.; Tyurin, V.A.; Corzo, A.; Cho, H.-I.; Celis, E.; Lennox, B.; et al. Lipid accumulation and dendritic cell dysfunction in cancer. Nat. Med. 2010, 16, 880–886. [Google Scholar] [CrossRef] [Green Version]
- Cubillos-Ruiz, J.R.; Silberman, P.C.; Rutkowski, M.R.; Chopra, S.; Perales-Puchalt, A.; Song, M.; Zhang, S.; Bettigole, S.E.; Gupta, D.; Holcomb, K.; et al. ER Stress sensor xbp1 controls anti-tumor immunity by disrupting dendritic cell homeostasis. Cell 2015, 161, 1527–1538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lundberg, S.; Lundahl, J.; Gunnarsson, I.; Jacobson, S. Atorvastatin-induced modulation of monocyte respiratory burst in vivo in patients with IgA nephropathy: A chronic inflammatory kidney disease. Clin. Nephrol. 2010, 73, 221–228. [Google Scholar] [CrossRef]
- Visvanathan, K.; Modur, S.; Artama, M.; Murtola, T. Abstract 5782: Lipophilic statins show promise for treatment of epithelial ovarian cancer. In Proceedings of the AACR Annual Meeting 2020, Philadelphia, PA, USA, 27–28 April 2020; p. 5782. [Google Scholar]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menzner, A.-K.; Rottmar, T.; Voelkl, S.; Bosch, J.J.; Mougiakakos, D.; Mackensen, A.; Resheq, Y.J. Hydrogen-Peroxide Synthesis and LDL-Uptake Controls Immunosuppressive Properties in Monocyte-Derived Dendritic Cells. Cancers 2021, 13, 461. https://doi.org/10.3390/cancers13030461
Menzner A-K, Rottmar T, Voelkl S, Bosch JJ, Mougiakakos D, Mackensen A, Resheq YJ. Hydrogen-Peroxide Synthesis and LDL-Uptake Controls Immunosuppressive Properties in Monocyte-Derived Dendritic Cells. Cancers. 2021; 13(3):461. https://doi.org/10.3390/cancers13030461
Chicago/Turabian StyleMenzner, Ann-Katrin, Tanja Rottmar, Simon Voelkl, Jacobus J. Bosch, Dimitrios Mougiakakos, Andreas Mackensen, and Yazid J. Resheq. 2021. "Hydrogen-Peroxide Synthesis and LDL-Uptake Controls Immunosuppressive Properties in Monocyte-Derived Dendritic Cells" Cancers 13, no. 3: 461. https://doi.org/10.3390/cancers13030461
APA StyleMenzner, A. -K., Rottmar, T., Voelkl, S., Bosch, J. J., Mougiakakos, D., Mackensen, A., & Resheq, Y. J. (2021). Hydrogen-Peroxide Synthesis and LDL-Uptake Controls Immunosuppressive Properties in Monocyte-Derived Dendritic Cells. Cancers, 13(3), 461. https://doi.org/10.3390/cancers13030461