The Potential of Raman Spectroscopy in the Diagnosis of Dysplastic and Malignant Oral Lesions
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
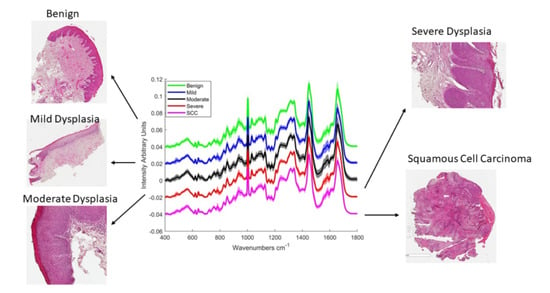
2.1. Epithelial Tissue
2.2. Connective Tissue
2.3. Influence of Patient Factors and Clinical Features on Raman Classification
2.3.1. Smoking
2.3.2. Presence of Inflammation
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
4.2. Instrumentation
4.3. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Pfeifer, G.P.; Denissenko, M.F.; Olivier, M.; Tretyakova, N.; Hecht, S.S.; Hainaut, P. Tobacco smoke carcinogens, DNA damage and p53 mutations in smoking-associated cancers. Oncogene 2002, 21, 7435–7451. [Google Scholar] [CrossRef] [Green Version]
- Boffetta, P.; Hashibe, M. Alcohol and cancer. Lancet Oncol. 2006, 7, 149–156. [Google Scholar] [CrossRef]
- Hashibe, M.; Brennan, P.; Chuang, S.C.; Boccia, S.; Castellsague, X.; Chen, C.; Curado, M.P.; Dal Maso, L.; Daudt, A.W.; Fabianova, E.; et al. Interaction between Tobacco and Alcohol Use and the Risk of Head and Neck Cancer: Pooled Analysis in the International Head and Neck Cancer Epidemiology Consortium. Cancer Epidemiol. Biomark. Prev. 2009, 18, 541–550. [Google Scholar] [CrossRef] [Green Version]
- van der Waal, I. Potentially malignant disorders of the oral and oropharyngeal mucosa; terminology, classification and present concepts of management. Oral Oncol. 2009, 45, 317–323. [Google Scholar] [CrossRef]
- Silverman, S. Demographics and occurrence of oral and pharyngeal cancers—The outcomes, the trends, the challenge. J. Am. Dent. Assoc. 2001, 132, 7S–11S. [Google Scholar] [CrossRef]
- Marur, S.; Forastiere, A.A. Head and neck cancer: Changing epidemiology, diagnosis, and treatment. Mayo Clin. Proc. 2008, 83, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Poh, C.F.; Ng, S.; Berean, K.W.; Williams, P.M.; Rosin, M.P.; Zhang, L.W. Biopsy and histopathologic diagnosis of oral premalignant and malignant lesions. J. Can. Dent. Assoc. 2008, 74, 283–288. [Google Scholar] [PubMed]
- Abbey, L.M.; Kaugars, G.E.; Gunsolley, J.C.; Burns, J.C.; Page, D.G.; Svirsky, J.A.; Eisenberg, E.; Krutchkoff, D.J.; Cushing, M. Intraexaminer and interexaminer reliability in the diagnosis of oral epithelial dysplasia. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1995, 80, 188–191. [Google Scholar] [CrossRef]
- Scully, C. Challenges in predicting which oral mucosal potentially malignant disease will progress to neoplasia. Oral Dis. 2014, 20, 1–5. [Google Scholar] [CrossRef]
- Puppels, G.J.; Demul, F.F.M.; Otto, C.; Greve, J.; Robertnicoud, M.; Arndtjovin, D.J.; Jovin, T.M. Studying single living cells and chromosomes by confocal raman microspectroscopy. Nature 1990, 347, 301–303. [Google Scholar] [CrossRef]
- Jermyn, M.; Desroches, J.; Aubertin, K.; St-Arnaud, K.; Madore, W.J.; De Montigny, E.; Guiot, M.C.; Trudel, D.; Wilson, B.C.; Petrecca, K.; et al. A review of Raman spectroscopy advances with an emphasis on clinical translation challenges in oncology. Phys. Med. Biol. 2016, 61, R370–R400. [Google Scholar] [CrossRef]
- Santos, I.P.; Barroso, E.M.; Schut, T.C.B.; Caspers, P.J.; van Lanschot, C.G.F.; Choi, D.H.; van der Kamp, M.F.; Smits, R.W.H.; van Doorn, R.; Verdijk, R.M.; et al. Raman spectroscopy for cancer detection and cancer surgery guidance: Translation to the clinics. Analyst 2017, 142, 3025–3047. [Google Scholar] [CrossRef]
- Upchurch, E.; Isabelle, M.; Lloyd, G.R.; Kendall, C.; Barr, H. An update on the use of Raman spectroscopy in molecular cancer diagnostics: Current challenges and further prospects. Expert Rev. Mol. Diagn. 2018, 18, 245–258. [Google Scholar] [CrossRef]
- Hiremath, G.; Locke, A.; Sivakumar, A.; Thomas, G.; Mahadevan-Jansen, A. Clinical translational application of Raman spectroscopy to advance Benchside biochemical characterization to bedside diagnosis of esophageal diseases. J. Gastroenterol. Hepatol. 2019, 34, 1911–1921. [Google Scholar] [CrossRef] [Green Version]
- Hubbard, T.J.E.; Shore, A.; Stone, N. Raman spectroscopy for rapid intra-operative margin analysis of surgically excised tumour specimens. Analyst 2019, 144, 6479–6496. [Google Scholar] [CrossRef]
- Kumar, P.; Ingle, A.; Krishna, C.M. In vivo Raman spectroscopy: Monitoring cancer progression post carcinogen withdrawal. In Proceedings of the Conference on Optical Imaging, Therapeutics, and Advanced Technology in Head and Neck Surgery and Otolaryngology, San Francisco, CA, USA, 2 February 2019. [Google Scholar]
- Santana-Codina, N.; Marce-Grau, A.; Muixi, L.; Nieva, C.; Marro, M.; Sebastian, D.; Munoz, J.P.; Zorzano, A.; Sierra, A. GRP94 Is Involved in the Lipid Phenotype of Brain Metastatic Cells. Int. J. Mol. Sci. 2019, 20, 3883. [Google Scholar] [CrossRef] [Green Version]
- Chrabaszcz, K.; Kochan, K.; Fedorowicz, A.; Jasztal, A.; Buczek, E.; Leslie, L.S.; Bhargava, R.; Malek, K.; Chlopicki, S.; Marzec, K.M. FT-IR- and Raman-based biochemical profiling of the early stage of pulmonary metastasis of breast cancer in mice. Analyst 2018, 143, 2042–2050. [Google Scholar] [CrossRef] [PubMed]
- Farhane, Z.; Nawaz, H.; Bonnier, F.; Byrne, H.J. In vitro label-free screening of chemotherapeutic drugs using Raman microspectroscopy: Towards a new paradigm of spectralomics. J. Biophotonics 2018, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahu, A.; Krishna, C.M. Optical diagnostics in oral cancer: An update on Raman spectroscopic applications. J. Cancer Res. Ther. 2017, 13, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, L.; Bonnier, F.; O'Callaghan, K.; O’Sullivan, J.; Flint, S.; Byrne, H.J.; Lyng, F.M. Raman micro-spectroscopy for rapid screening of oral squamous cell carcinoma. Exp. Mol. Pathol. 2015, 98, 502–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, L.; Bonnier, F.; Tellez, C.; dos Santos, L.; O’Callaghan, K.; O’Sullivan, J.; Soares, L.E.S.; Flint, S.; Martin, A.A.; Lyng, F.M.; et al. Raman spectroscopic analysis of oral cells in the high wavenumber region. Exp. Mol. Pathol. 2017, 103, 255–262. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.X.; Yan, H.; Xia, J.B.; Zhu, L.Q.; Zhang, T.; Zhu, Z.H.; Lou, X.P.; Sun, G.K.; Dong, M.L. Deep convolutional neural networks for tongue squamous cell carcinoma classification using Raman spectroscopy. Photodiagnosis Photodyn. Ther. 2019, 26, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Jeng, M.J.; Sharma, M.; Sharma, L.; Chao, T.Y.; Huang, S.F.; Chang, L.B.; Wu, S.L.; Chow, L. Raman Spectroscopy Analysis for Optical Diagnosis of Oral Cancer Detection. J. Clin. Med. 2019, 8, 1313. [Google Scholar] [CrossRef]
- Cals, F.L.J.; Schut, T.C.B.; Caspers, P.J.; de Jong, R.J.B.; Koljenovic, S.; Puppels, G.J. Raman spectroscopic analysis of the molecular composition of oral cavity squamous cell carcinoma and healthy tongue tissue. Analyst 2018, 143, 4090–4102. [Google Scholar] [CrossRef] [PubMed]
- Cals, F.L.J.; Koljenovic, S.; Hardillo, J.A.; de Jong, R.J.B.; Schut, T.C.B.; Puppels, G.J. Development and validation of Raman spectroscopic classification models to discriminate tongue squamous cell carcinoma from non-tumorous tissue. Oral Oncol. 2016, 60, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Barroso, E.M.; ten Hove, I.; Schut, T.C.B.; Mast, H.; van Lanschot, C.G.F.; Smits, R.W.H.; Caspers, P.J.; Verdijk, R.; Hegt, V.N.; de Jong, R.J.B.; et al. Raman spectroscopy for assessment of bone resection margins in mandibulectomy for oral cavity squamous cell carcinoma. Eur. J. Cancer 2018, 92, 77–87. [Google Scholar] [CrossRef]
- Cals, F.L.J.; Schut, T.C.B.; Hardillo, J.A.; de Jong, R.J.B.; Koljenovic, S.; Puppels, G.J. Investigation of the potential of Raman spectroscopy for oral cancer detection in surgical margins. Lab. Investig. 2015, 95, 1186–1196. [Google Scholar] [CrossRef] [Green Version]
- Movasaghi, Z.; Rehman, S.; Rehman, I.U. Raman spectroscopy of biological tissues. Appl. Spectrosc. Rev. 2007, 42, 493–541. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [Green Version]
- Speight, P.M. Update on Oral Epithelial Dysplasia and Progression to Cancer. Head Neck Pathol. 2007, 1, 61–66. [Google Scholar] [CrossRef] [Green Version]
- Sahu, A.; Deshmukh, A.; Ghanate, A.D.; Singh, S.P.; Chaturvedi, P.; Krishna, C.M. Raman Spectroscopy of Oral Buccal Mucosa: A Study on Age-Related Physiological Changes and Tobacco-Related Pathological Changes. Technol. Cancer Res. Treat. 2012, 11, 529–541. [Google Scholar] [CrossRef] [Green Version]
- Depciuch, J.; Sowa-Kucma, M.; Nowak, G.; Dudek, D.; Siwek, M.; Styczen, K.; Parlinska-Wojtan, M. Phospholipid-protein balance in affective disorders: Analysis of human blood serum using Raman and FTIR spectroscopy. A pilot study. J. Pharm. Biomed. Anal. 2016, 131, 287–296. [Google Scholar] [CrossRef]
- Singh, S.P.; Deshmukh, A.; Chaturvedi, P.; Krishna, C.M. In vivo Raman spectroscopic identification of premalignant lesions in oral buccal mucosa. J. Biomed. Opt. 2012, 17. [Google Scholar] [CrossRef] [PubMed]
- Rashid, N.; Nawaz, H.; Poon, K.W.C.; Bonnier, F.; Bakhiet, S.; Martin, C.; O’Leary, J.J.; Byrne, H.J.; Lyng, F.M. Raman microspectroscopy for the early detection of pre-malignant changes in cervical tissue. Exp. Mol. Pathol. 2014, 97, 554–564. [Google Scholar] [CrossRef] [Green Version]
- Sorsa, T.; Tjaderhane, L.; Salo, T. Matrix metalloproteinases (MMPs) in oral diseases. Oral Dis. 2004, 10, 311–318. [Google Scholar] [CrossRef]
- Mashhadiabbas, F.; Fayazi-Boroujeni, M. Correlation of vascularization and inflammation with severity of oral leukoplakia. Iran. J. Pathol. 2017, 12, 225–230. [Google Scholar] [CrossRef]
- Negus, R.P.M.; Stamp, G.W.H.; Hadley, J.; Balkwill, F.R. Quantitative assessment of the leukocyte infiltrate in ovarian cancer and its relationship to the expression of C-C chemokines. Am. J. Pathol. 1997, 150, 1723–1734. [Google Scholar] [PubMed]
- Talmadge, J.E. Immune cell infiltration of primary and metastatic lesions: Mechanisms and clinical impact. Semin. Cancer Biol. 2011, 21, 131–138. [Google Scholar] [CrossRef]
- Takahashi, H.; Ogata, H.; Nishigaki, R.; Broide, D.H.; Karin, M. Tobacco Smoke Promotes Lung Tumorigenesis by Triggering IKK beta- and JNK1-Dependent Inflammation. Cancer Cell 2010, 17, 89–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feller, L.; Altini, M.; Lemmer, J. Inflammation in the context of oral cancer. Oral Oncol. 2013, 49, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, O.; Maguire, A.; Meade, A.D.; Flint, S.; Toner, M.; Byrne, H.J.; Lyng, F.M. Improved protocols for pre-processing Raman spectra of formalin fixed paraffin preserved tissue sections. Anal. Methods 2017, 9, 4709–4717. [Google Scholar] [CrossRef] [Green Version]
- Mandrekar, J.N. Receiver Operating Characteristic Curve in Diagnostic Test Assessment. J. Thorac. Oncol. 2010, 5, 1315–1316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cankovic, M.; Ilic, M.P.; Vuckovic, N.; Bokor-Bratic, M. The histological characteristics of clinically normal mucosa adjacent to oral cancer. J. Cancer Res. Ther. 2013, 9, 240–244. [Google Scholar] [CrossRef] [PubMed]








| Wavenumber (cm−1) | Assignment |
|---|---|
| 484–490 | Glycogen |
| 599/600 | Nucleotide conformation |
| 666 | G, T (ring breathing modes in DNA bases) |
| 752 | Symmetric breathing mode of tryptophan |
| 782 | DNA |
| 811/812 | RNA O-P-O stretch |
| 814 | C-C stretching (collagen assignment) |
| 838 | Deformative vibrations of amine groups |
| 855 | Ring breathing in tyrosine/C-C stretching in proline |
| 919 | C-C stretch of Proline ring/glucose lactic acid C-C, proline ring (collagen assignment) |
| 934/935 | Protein/C-C backbone (collagen assignment) |
| 937/8 | Proline, hydroxyproline (C-C) skeletal of collagen backbone |
| 1001/1002 | Phenylalanine ring breathing |
| 1030–34 | Phenylalanine of collagen |
| 1128/1129 | Skeletal C-C stretch in lipids |
| 1131 | Fatty acid |
| 1237 | Amide III |
| 1245–1248 | Amide III of collagen |
| 1265 | Amide III |
| 1278 | Proteins including collagen I |
| 1285 | Differences in collagen |
| 1315–1317 | Guanine |
| 1333 | Guanine |
| 1336 | Polynucleotide chain (DNA purine bases) |
| 1368 | Guanine TRP protein, porphrin, lipids |
| 1373 | T, A, G (ring breathing modes of the DNA/RNA bases) |
| 1437 | CH2 deformation (lipid) |
| 1441 | Wax |
| 1449/1450 | C-H vibration lipids |
| 1460 | CH2/CH3 deformation in Lipids |
| 1554 | Amide II |
| 1572–1578 | Guanine adenine |
| 1650 | Amide I |
| 1652–1655 | Lipid C=C (lipids)/Amide I |
| 1666–1668 | Protein/collagen |
| 1674 | C=C stretch in cholesterol |
| 1700–1750 | Amino acids aspartic and glutamic acid |
| Pathology | Epithelium | Connective Tissue | ||
|---|---|---|---|---|
| Sensitivity (%) | Specificity (%) | Sensitivity (%) | Specificity (%) | |
| Benign | 74 | 49 | 81 | 44 |
| Mild | 67 | 38 | 67 | 46 |
| Moderate | 39 | 86 | 42 | 61 |
| Severe | 69 | 57 | 59 | 67 |
| SCC | 65 | 76 | 88 | 72 |
| Statistic | Non-Smoker (n = 13) | Ex-Smoker (n = 17) | Smoker (n = 13) |
|---|---|---|---|
| Sensitivity (%) | 83 | 81 | 52 |
| Specificity (%) | 46 | 38 | 88 |
| Class | Benign (n = 17) | Mild (n = 20) | Moderate (n = 20) | Severe (n = 10) | SCC (n = 5) |
|---|---|---|---|---|---|
| Number Inflamed | 2 | 3 | 9 | 7 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, O.; Toner, M.; Flint, S.; Byrne, H.J.; Lyng, F.M. The Potential of Raman Spectroscopy in the Diagnosis of Dysplastic and Malignant Oral Lesions. Cancers 2021, 13, 619. https://doi.org/10.3390/cancers13040619
Ibrahim O, Toner M, Flint S, Byrne HJ, Lyng FM. The Potential of Raman Spectroscopy in the Diagnosis of Dysplastic and Malignant Oral Lesions. Cancers. 2021; 13(4):619. https://doi.org/10.3390/cancers13040619
Chicago/Turabian StyleIbrahim, Ola, Mary Toner, Stephen Flint, Hugh J. Byrne, and Fiona M. Lyng. 2021. "The Potential of Raman Spectroscopy in the Diagnosis of Dysplastic and Malignant Oral Lesions" Cancers 13, no. 4: 619. https://doi.org/10.3390/cancers13040619
APA StyleIbrahim, O., Toner, M., Flint, S., Byrne, H. J., & Lyng, F. M. (2021). The Potential of Raman Spectroscopy in the Diagnosis of Dysplastic and Malignant Oral Lesions. Cancers, 13(4), 619. https://doi.org/10.3390/cancers13040619