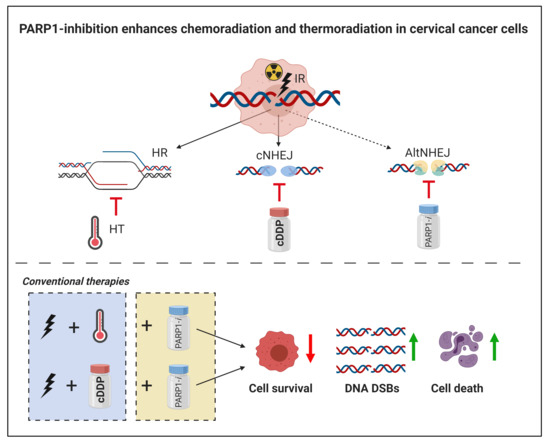
PARP1-Inhibition Sensitizes Cervical Cancer Cell Lines for Chemoradiation and Thermoradiation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. Cellular Survival
2.2. DNA Damage
2.3. Cell Death
3. Discussion
4. Materials and Methods
4.1. Cell Cultures
4.2. Irradiation
4.3. Hyperthermia
4.4. Chemical Agents
4.5. Clonogenic Assay
4.6. Immunocytochemistry
4.7. Live-Cell Imaging
4.8. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Naga Ch, P.; Gurram, L.; Chopra, S.; Mahantshetty, U. The management of locally advanced cervical cancer. Curr. Opin. Oncol. 2018, 30, 323–329. [Google Scholar] [PubMed]
- Small, W., Jr.; Bacon, M.A.; Bajaj, A.; Chuang, L.T.; Fisher, B.J.; Harkenrider, M.M.; Gaffney, D.K. Cervical cancer: A global health crisis. Cancer 2017, 123, 2404–2412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LaVigne, A.W.; Triedman, S.A.; Randall, T.C.; Trimble, E.L.; Viswanathan, A.N. Cervical cancer in low and middle income countries: Addressing barriers to radiotherapy delivery. Gynecol. Oncol. Rep. 2017, 22, 16–20. [Google Scholar] [CrossRef]
- Cohen, P.A.; Jhingran, A.; Oaknin, A.; Denny, L. Cervical cancer. Lancet 2019, 393, 169–182. [Google Scholar] [CrossRef]
- Adam, J.A.; Arkies, H.; Hinnen, K.; Stalpers, L.J.; van Waesberghe, J.H.; Stoker, J.; van Eck-Smit, B.L. 18F-FDG-PET/CT guided external beam radiotherapy volumes in inoperable uterine cervical cancer. Q. J. Nucl. Med. Mol. Imaging 2018, 62, 420–428. [Google Scholar] [PubMed]
- Feng, C.; Ma, F.; Hu, C.; Ma, J.A.; Wang, J.; Zhang, Y.; Feng, Y. SOX9/miR-130a/CTR1 axis modulates DDP-resistance of cervical cancer cell. Cell Cycle 2018, 17, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Crezee, J.; van Leeuwen, C.M.; Oei, A.L.; van Heerden, L.E.; Bel, A.; Stalpers LJ, A.; Kok, H.P. Biological modelling of the radiation dose escalation effect of regional hyperthermia in cervical cancer. Radiat. Oncol. 2016, 11, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Datta, N.R.; Ordóñez, S.G.; Gaipl, U.S.; Paulides, M.M.; Crezee, H.; Gellermann, J.; Bodis, S. Local hyperthermia combined with radiotherapy and-/or chemotherapy: Recent advances and promises for the future. Cancer Treat. Rev. 2015, 41, 742–753. [Google Scholar]
- Lutgens, L.C.; Koper, P.C.; Jobsen, J.J.; van der Steen-Banasik, E.M.; Creutzberg, C.L.; van den Berg, H.A.; van der Zee, J. Radiation therapy combined with hyperthermia versus cisplatin for locally advanced cervical cancer: Results of the randomized RADCHOC trial. Radiother. Oncol. 2016, 120, 378–382. [Google Scholar]
- Burchardt, E.; Roszak, A. Hyperthermia in cervical cancer—Current status. Rep. Pract. Oncol. Radiother. 2018, 23, 595–603. [Google Scholar] [PubMed]
- Santivasi, W.L.; Xia, F. Ionizing radiation-induced DNA damage, response, and repair. Antioxid. Redox. Signal. 2014, 21, 251–259. [Google Scholar] [CrossRef]
- Smith, T.A.; Kirkpatrick, D.R.; Smith, S.; Smith, T.K.; Pearson, T.; Kailasam, A.; Agrawal, D.K. Radioprotective agents to prevent cellular damage due to ionizing radiation. J. Transl. Med. 2017, 15, 232. [Google Scholar] [CrossRef]
- Vignard, J.; Mirey, G.; Salles, B. Ionizing-radiation induced DNA double-strand breaks: A direct and indirect lighting up. Radiother. Oncol. 2013, 108, 362–369. [Google Scholar] [CrossRef] [Green Version]
- Krajewska, M.; Fehrmann, R.S.; de Vries, E.G.; van Vugt, M.A. Regulators of homologous recombination repair as novel targets for cancer treatment. Front. Genet. 2015, 6, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dietlein, F.; Thelen, L.; Reinhardt, H.C. Cancer-specific defects in DNA repair pathways as targets for personalized therapeutic approaches. Trends Genet. 2014, 30, 326–339. [Google Scholar] [CrossRef]
- IJff, M.; Van Oorschot, B.; Oei, A.L.; Krawczyk, P.M.; Rodermond, H.M.; Stalpers, L.J.; Franken, N.A. Enhancement of Radiation Effectiveness in Cervical Cancer Cells by Combining Ionizing Radiation with Hyperthermia and Molecular Targeting Agents. Int. J. Mol. Sci. 2018, 19, 2420. [Google Scholar] [CrossRef] [Green Version]
- Wood, R.D.; Doublie, S. DNA polymerase theta (POLQ), double-strand break repair, and cancer. DNA Repair 2016, 44, 22–32. [Google Scholar] [CrossRef] [Green Version]
- Brandsma, I.; Gent, D.C. Pathway choice in DNA double strand break repair: Observations of a balancing act. Genome Integr. 2012, 3, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, H.H.Y.; Pannunzio, N.R.; Adachi, N.; Lieber, M.R. Non-homologous DNA end joining and alternative pathways to double-strand break repair. Nat. Rev. Mol. Cell Biol. 2017, 18, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhang, Q.; Atsaves, V.; Yang, H.; Claret, F.X. Suppression of Jab1/CSN5 induces radio- and chemo-sensitivity in nasopharyngeal carcinoma through changes to the DNA damage and repair pathways. Oncogene 2013, 32, 2756–2766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toulany, M.; Rodemann, H.P. Phosphatidylinositol 3-kinase/Akt signaling as a key mediator of tumor cell responsiveness to radiation. Semin. Cancer Biol. 2015, 35, 180–190. [Google Scholar] [CrossRef]
- Laan, J.J.; van Lonkhuijzen, L.R.C.W.; van Os, R.M.; Tytgat, K.M.; Fajardo, R.D.; Pieters, B.R.; Westerveld, G.H. Socioeconomic status as an independent risk factor for severe late bowel toxicity after primary radiotherapy for cervical cancer. Gynecol. Oncol. 2017, 147, 684–689. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [Green Version]
- Franckena, M.; van der Zee, J. Use of combined radiation and hyperthermia for gynecological cancer. Curr. Opin. Obstet. Gynecol. 2010, 22, 9–14. [Google Scholar] [CrossRef]
- Elming, P.B.; Sørensen, B.S.; Oei, A.L.; Franken, N.A.; Crezee, J.; Overgaard, J.; Horsman, M.R. Hyperthermia: The Optimal Treatment to Overcome Radiation Resistant Hypoxia. Cancers 2019, 11, 60. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, I.; Emi, Y.; Hasuda, S.; Kakeji, Y.; Maehara, Y.; Sugimachi, K. Clinical application of hyperthermia combined with anticancer drugs for the treatment of solid tumors. Surgery 2002, 131, S78–S84. [Google Scholar] [CrossRef]
- Arslan, S.A.; Ozdemir, N.; Sendur, M.A.; Eren, T.; Ozturk, H.F.; Aral, I.P.; Inan, G.A. Hyperthermia and radiotherapy combination for locoregional recurrences of breast cancer: A review. Breast Cancer Manag. 2018, 6, 117–126. [Google Scholar] [CrossRef] [Green Version]
- Oei, A.L.; Vriend, L.E.; Crezee, J.; Franken, N.A.; Krawczyk, P.M. Effects of hyperthermia on DNA repair pathways: One treatment to inhibit them all. Radiat. Oncol. 2015, 10, 165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van den Tempel, N.; Laffeber, C.; Odijk, H.; van Cappellen, W.A.; van Rhoon, G.C.; Franckena, M.; Kanaar, R. The effect of thermal dose on hyperthermia-mediated inhibition of DNA repair through homologous recombination. Oncotarget 2017, 8, 44593–44604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munroe, M.; Kolesar, J. Olaparib for the treatment of BRCA-mutated advanced ovarian cancer. Am. J. Health Syst. Pharm. 2016, 73, 1037–1041. [Google Scholar] [CrossRef]
- Scott, C.L.; Swisher, E.M.; Kaufmann, S.H. Poly (ADP-ribose) polymerase inhibitors: Recent advances and future development. J. Clin. Oncol. 2015, 33, 1397–1406. [Google Scholar] [CrossRef] [Green Version]
- Robson, M.; Im, S.A.; Senkus, E.; Xu, B.; Domchek, S.M.; Masuda, N.; Conte, P. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N. Engl. J. Med. 2017, 377, 523–533. [Google Scholar] [CrossRef]
- Prasad, C.B.; Prasad, S.B.; Yadav, S.S.; Pandey, L.K.; Singh, S.; Pradhan, S.; Narayan, G. Olaparib modulates DNA repair efficiency, sensitizes cervical cancer cells to cisplatin and exhibits anti-metastatic property. Sci. Rep. 2017, 7, 12876. [Google Scholar] [CrossRef] [PubMed]
- Campillo-Marcos, I.; Lazo, P.A. Olaparib and ionizing radiation trigger a cooperative DNA-damage repair response that is impaired by depletion of the VRK1 chromatin kinase. J. Exp. Clin. Cancer Res. 2019, 38, 203. [Google Scholar] [CrossRef] [Green Version]
- Diaz, J.E.; Ahsen, M.E.; Schaffter, T.; Chen, X.; Realubit, R.B.; Karan, C.; Stolovitzky, G. The transcriptomic response of cells to a drug combination is more than the sum of the responses to the monotherapies. Elife 2020, 9, e52707. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, I.; Wettermark, B.; Bergfeldt, K. Real-World Use and Outcomes of Olaparib: A Population-Based Cohort Study. Target Oncol. 2018, 13, 725–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pujade-Lauraine, E.; Ledermann, J.A.; Selle, F.; Gebski, V.; Penson, R.T.; Oza, A.M.; Vergote, I. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1274–1284. [Google Scholar] [CrossRef] [Green Version]
- Bianchi, A.; Lopez, S.; Altwerger, G.; Bellone, S.; Bonazzoli, E.; Zammataro, L.; Santin, A.D. PARP-1 activity (PAR) determines the sensitivity of cervical cancer to olaparib. Gynecol. Oncol. 2019, 155, 144–150. [Google Scholar] [CrossRef]
- Mann, M.; Kumar, S.; Sharma, A.; Chauhan, S.S.; Bhatla, N.; Kumar, S.; Kumar, L. PARP-1 inhibitor modulate β-catenin signaling to enhance cisplatin sensitivity in cancer cervix. Oncotarget 2019, 10, 4262–4275. [Google Scholar] [CrossRef] [Green Version]
- Moore, K.N.; Sill, M.W.; Miller, D.S.; McCourt, C.; De Geest, K.; Rose, P.G.; Fracasso, P.M. A phase I trial of tailored radiation therapy with concomitant cetuximab and cisplatin in the treatment of patients with cervical cancer: A gynecologic oncology group study. Gynecol. Oncol. 2012, 127, 456–461. [Google Scholar] [CrossRef]
- Arjumand, W.; Merry, C.D.; Wang, C.; Saba, E.; McIntyre, J.B.; Fang, S.; Lees, S.P. Phosphatidyl inositol-3 kinase (PIK3CA) E545K mutation confers cisplatin resistance and a migratory phenotype in cervical cancer cells. Oncotarget 2016, 7, 82424–82439. [Google Scholar] [CrossRef] [Green Version]
- Morgan, E.L.; Macdonald, A. JAK2 Inhibition Impairs Proliferation and Sensitises Cervical Cancer Cells to Cisplatin-Induced Cell Death. Cancers 2019, 11, 1934. [Google Scholar] [CrossRef] [Green Version]
- Anunobi, R.; Boone, B.A.; Cheh, N.; Tang, D.; Kang, R.; Loux, T.; Zeh, H.J. Extracellular DNA promotes colorectal tumor cell survival after cytotoxic chemotherapy. J. Surg. Res. 2018, 226, 181–191. [Google Scholar] [CrossRef] [Green Version]
- Huang, Q.; Shen, H.M. To die or to live: The dual role of poly(ADP-ribose) polymerase-1 in autophagy and necrosis under oxidative stress and DNA damage. Autophagy 2009, 5, 273–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, D.J.; Wilczynski, S.P.; Paquette, R.L.; Miller, C.W.; Koeffler, H.P. p53 mutations in HPV-negative cervical carcinoma. Oncogene 1994, 9, 205–210. [Google Scholar] [PubMed]
- Banáth, J.P.; Macphail, S.H.; Olive, P.L. Radiation sensitivity, H2AX phosphorylation, and kinetics of repair of DNA strand breaks in irradiated cervical cancer cell lines. Cancer Res. 2004, 64, 7144–7149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mei, X.; Ten Cate, R.; van Leeuwen, C.M.; Rodermond, H.M.; de Leeuw, L.; Dimitrakopoulou, D.; Oei, A.L. Radiosensitization by Hyperthermia: The Effects of Temperature, Sequence, and Time Interval in Cervical Cell Lines. Cancers 2020, 12, 582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oei, A.L.; Vriend, L.E.; van Leeuwen, C.M.; Rodermond, H.M.; Ten Cate, R.; Westermann, A.M.; Franken, N.A. Sensitizing thermochemotherapy with a PARP1-inhibitor. Oncotarget 2017, 8, 16303–16312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filippova, M.; Filippov, V.; Williams, V.M.; Zhang, K.; Kokoza, A.; Bashkirova, S.; Duerksen-Hughes, P. Cellular levels of oxidative stress affect the response of cervical cancer cells to chemotherapeutic agents. Biomed. Res. Int. 2014, 2014, 574659. [Google Scholar] [CrossRef]
- Sears, C.R.; Cooney, S.A.; Chin-Sinex, H.; Mendonca, M.S.; Turchi, J.J. DNA damage response (DDR) pathway engagement in cisplatin radiosensitization of non-small cell lung cancer. DNA Repair 2016, 40, 35–46. [Google Scholar] [CrossRef] [Green Version]
- Pauty, J.; Cote, M.F.; Rodrigue, A.; Velic, D.; Masson, J.Y.; Fortin, S. Investigation of the DNA damage response to SFOM-0046, a new small-molecule drug inducing DNA double-strand breaks. Sci. Rep. 2016, 6, 23302. [Google Scholar] [CrossRef]
- Oei, A.L.; van Leeuwen, C.M.; Ahire, V.R.; Rodermond, H.M.; Ten Cate, R.; Westermann, A.M.; Franken, N.A. Enhancing synthetic lethality of PARP-inhibitor and cisplatin in BRCA-proficient tumour cells with hyperthermia. Oncotarget 2017, 8, 28116–28124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moeglin, E.; Desplancq, D.; Conic, S.; Oulad-Abdelghani, M.; Stoessel, A.; Chiper, M.; Weiss, E. Uniform Widespread Nuclear Phosphorylation of Histone H2AX Is an Indicator of Lethal DNA Replication Stress. Cancers 2019, 11, 355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murai, J. Targeting DNA repair and replication stress in the treatment of ovarian cancer. Int. J. Clin. Oncol. 2017, 22, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Ledermann, J.; Harter, P.; Gourley, C.; Friedlander, M.; Vergote, I.; Rustin, G.; Matulonis, U. Olaparib Maintenance Therapy in Platinum-Sensitive Relapsed Ovarian Cancer. N. Engl. J. Med. 2012, 366, 1382–1392. [Google Scholar] [CrossRef] [Green Version]
- Wiggans, A.J.; Cass, G.K.; Bryant, A.; Lawrie, T.A.; Morrison, J. Poly(ADP-ribose) polymerase (PARP) inhibitors for the treatment of ovarian cancer. Cochrane Database Syst. Rev. 2015, 2015, Cd007929. [Google Scholar] [CrossRef]
- O’Neil, N.J.; Bailey, M.L.; Hieter, P. Synthetic lethality and cancer. Nat. Rev. Genet. 2017, 18, 613–623. [Google Scholar]
- Mintz, R.L.; Lao, Y.H.; Chi, C.W.; He, S.; Li, M.; Quek, C.H.; Leong, K.W. CRISPR/Cas9-mediated mutagenesis to validate the synergy between PARP1 inhibition and chemotherapy in BRCA1-mutated breast cancer cells. Bioeng. Transl. Med. 2020, 5, e10152. [Google Scholar] [CrossRef] [Green Version]
- Eppink, B.; Krawczyk, P.M.; Stap, J.; Kanaar, R. Hyperthermia-induced DNA repair deficiency suggests novel therapeutic anti-cancer strategies. Int. J. Hyperth. 2012, 28, 509–517. [Google Scholar] [CrossRef]
- Franken, N.A.; Rodermond, H.M.; Stap, J.; Haveman, J.; van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
IJff, M.; van Bochove, G.G.W.; Whitton, D.; Winiarczyk, R.; Honhoff, C.; Rodermond, H.; Crezee, J.; Stalpers, L.J.A.; Franken, N.A.P.; Oei, A.L. PARP1-Inhibition Sensitizes Cervical Cancer Cell Lines for Chemoradiation and Thermoradiation. Cancers 2021, 13, 2092. https://doi.org/10.3390/cancers13092092
IJff M, van Bochove GGW, Whitton D, Winiarczyk R, Honhoff C, Rodermond H, Crezee J, Stalpers LJA, Franken NAP, Oei AL. PARP1-Inhibition Sensitizes Cervical Cancer Cell Lines for Chemoradiation and Thermoradiation. Cancers. 2021; 13(9):2092. https://doi.org/10.3390/cancers13092092
Chicago/Turabian StyleIJff, Marloes, Gregor G. W. van Bochove, Denise Whitton, Roy Winiarczyk, Celina Honhoff, Hans Rodermond, Johannes Crezee, Lukas J. A. Stalpers, Nicolaas A. P. Franken, and Arlene L. Oei. 2021. "PARP1-Inhibition Sensitizes Cervical Cancer Cell Lines for Chemoradiation and Thermoradiation" Cancers 13, no. 9: 2092. https://doi.org/10.3390/cancers13092092
APA StyleIJff, M., van Bochove, G. G. W., Whitton, D., Winiarczyk, R., Honhoff, C., Rodermond, H., Crezee, J., Stalpers, L. J. A., Franken, N. A. P., & Oei, A. L. (2021). PARP1-Inhibition Sensitizes Cervical Cancer Cell Lines for Chemoradiation and Thermoradiation. Cancers, 13(9), 2092. https://doi.org/10.3390/cancers13092092