Oxidative-Stress-Sensitive microRNAs in UV-Promoted Development of Melanoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Ultraviolet Radiation
3. Beneficial and Adverse Health Effects of Sunlight
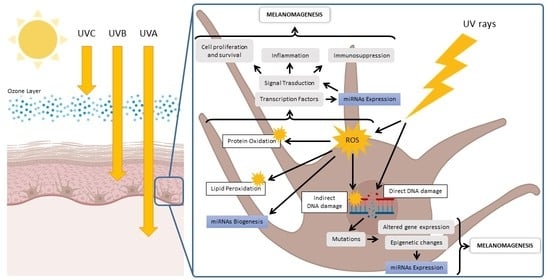
4. Molecular Mechanisms of UV Damage to Skin Tissue
5. UV-Induced Mutations Are Related to Melanoma Development
6. UV-Induced Redox Imbalance Modulates Redox-Sensitive miRNAs
7. UV-Dysregulated Redox-Sensitive miRNAs Involved in Melanoma Development
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Leiter, U.; Keim, U.; Garbe, C. Epidemiology of Skin Cancer: Update 2019. In Sunlight, Vitamin D and Skin Cancer; Advances in Experimental Medicine and Biology; Reichrath, J., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 123–139. ISBN 978-3-030-46227-7. [Google Scholar]
- Urban, K.; Mehrmal, S.; Uppal, P.; Giesey, R.L.; Delost, G.R. The Global Burden of Skin Cancer: A Longitudinal Analysis from the Global Burden of Disease Study, 1990–2017. JAAD Int. 2021, 2, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Dzwierzynski, W.W. Melanoma Risk Factors and Prevention. Clin. Plast. Surg. 2021, 48, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Watson, M.; Holman, D.M.; Maguire-Eisen, M. Ultraviolet Radiation Exposure and Its Impact on Skin Cancer Risk. Semin. Oncol. Nurs. 2016, 32, 241–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skin Cancer. Available online: https://www.aad.org/media/stats-skin-cancer (accessed on 1 June 2022).
- Melanoma of the Skin—Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/html/melan.html (accessed on 1 June 2022).
- Scolyer, R.A.; Prieto, V.G.; Elder, D.E.; Cochran, A.J.; Mihm, M.C. Classification and Histopathology of Melanoma. In Cutaneous Melanoma; Balch, C.M., Atkins, M.B., Garbe, C., Gershenwald, J.E., Halpern, A.C., Kirkwood, J.M., McArthur, G.A., Thompson, J.F., Sober, A.J., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 317–379. ISBN 978-3-030-05070-2. [Google Scholar]
- Liu, Y.; Sheikh, M.S. Melanoma: Molecular Pathogenesis and Therapeutic Management. Mol. Cell Pharmacol. 2014, 6, 228. [Google Scholar] [PubMed]
- Teixido, C.; Castillo, P.; Martinez-Vila, C.; Arance, A.; Alos, L. Molecular Markers and Targets in Melanoma. Cells 2021, 10, 2320. [Google Scholar] [CrossRef]
- Guo, W.; Wang, H.; Li, C. Signal Pathways of Melanoma and Targeted Therapy. Signal Transduct. Target. Ther. 2021, 6, 424. [Google Scholar] [CrossRef]
- Hodis, E.; Watson, I.R.; Kryukov, G.V.; Arold, S.T.; Imielinski, M.; Theurillat, J.-P.; Nickerson, E.; Auclair, D.; Li, L.; Place, C.; et al. A Landscape of Driver Mutations in Melanoma. Cell 2012, 150, 251–263. [Google Scholar] [CrossRef] [Green Version]
- Melanoma: What Do All the Mutations Mean?—PMC. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6191351/ (accessed on 2 June 2022).
- Gracia-Cazaña, T.; González, S.; Parrado, C.; Juarranz, Á.; Gilaberte, Y. Influence of the Exposome on Skin Cancer. Actas Dermo-Sifiliográficas (Engl. Ed.) 2020, 111, 460–470. [Google Scholar] [CrossRef]
- Conforti, C.; Zalaudek, I. Epidemiology and Risk Factors of Melanoma: A Review. Dermatol. Pract. Concept. 2021, 11, e2021161S. [Google Scholar] [CrossRef]
- Landrigan, P.J.; Fuller, R.; Acosta, N.J.R.; Adeyi, O.; Arnold, R.; Basu, N. (Nil); Baldé, A.B.; Bertollini, R.; Bose-O’Reilly, S.; Boufford, J.I.; et al. The Lancet Commission on Pollution and Health. Lancet 2018, 391, 462–512. [Google Scholar] [CrossRef] [Green Version]
- Umar, S.A.; Tasduq, S.A. Ozone Layer Depletion and Emerging Public Health Concerns—An Update on Epidemiological Perspective of the Ambivalent Effects of Ultraviolet Radiation Exposure. Front. Oncol. 2022, 12, 866733. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, D.; Farris, P.; Valacchi, G. Atmospheric Skin Aging—Contributors and Inhibitors. J. Cosmet. Dermatol. 2018, 17, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Zmijewski, M.A.; Plonka, P.M.; Szaflarski, J.P.; Paus, R. How UV Light Touches the Brain and Endocrine System Through Skin, and Why. Endocrinology 2018, 159, 1992–2007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mead, M.N. Benefits of Sunlight: A Bright Spot for Human Health. Environ. Health Perspect. 2008, 116, A160–A167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hart, P.H.; Norval, M.; Byrne, S.N.; Rhodes, L.E. Exposure to Ultraviolet Radiation in the Modulation of Human Diseases. Annu. Rev. Pathol. 2019, 14, 55–81. [Google Scholar] [CrossRef] [PubMed]
- Knuschke, P. Sun Exposure and Vitamin D. Curr. Probl. Dermatol. 2021, 55, 296–315. [Google Scholar] [CrossRef]
- Hoel, D.G.; Berwick, M.; de Gruijl, F.R.; Holick, M.F. The Risks and Benefits of Sun Exposure 2016. Dermato-Endocrinology 2016, 8, e1248325. [Google Scholar] [CrossRef] [Green Version]
- Umar, M.; Sastry, K.S.; Chouchane, A.I. Role of Vitamin D Beyond the Skeletal Function: A Review of the Molecular and Clinical Studies. Int. J. Mol. Sci. 2018, 19, 1618. [Google Scholar] [CrossRef] [Green Version]
- Roy, N.M.; Al-Harthi, L.; Sampat, N.; Al-Mujaini, R.; Mahadevan, S.; Al Adawi, S.; Essa, M.M.; Al Subhi, L.; Al-Balushi, B.; Qoronfleh, M.W. Impact of Vitamin D on Neurocognitive Function in Dementia, Depression, Schizophrenia and ADHD. Front. Biosci. (Landmark Ed.) 2021, 26, 566–611. [Google Scholar] [CrossRef]
- Van der Rhee, H.J.; de Vries, E.; Coebergh, J.W. Regular Sun Exposure Benefits Health. Med. Hypotheses 2016, 97, 34–37. [Google Scholar] [CrossRef]
- Holick, M.F. Biological Effects of Sunlight, Ultraviolet Radiation, Visible Light, Infrared Radiation and Vitamin D for Health. Anticancer Res. 2016, 36, 1345–1356. [Google Scholar] [PubMed]
- Felton, S.; Navid, F.; Schwarz, A.; Schwarz, T.; Gläser, R.; Rhodes, L.E. Ultraviolet Radiation-Induced Upregulation of Antimicrobial Proteins in Health and Disease. Photochem. Photobiol. Sci. 2012, 12, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Rathod, D.G.; Muneer, H.; Masood, S. Phototherapy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Modenese, A.; Korpinen, L.; Gobba, F. Solar Radiation Exposure and Outdoor Work: An Underestimated Occupational Risk. Int. J. Environ. Res. Public Health 2018, 15, 2063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanov, I.V.; Mappes, T.; Schaupp, P.; Lappe, C.; Wahl, S. Ultraviolet Radiation Oxidative Stress Affects Eye Health. J. Biophotonics 2018, 11, e201700377. [Google Scholar] [CrossRef] [Green Version]
- Effects of Changes in Stratospheric Ozone and Global Climate. Available online: http://www.ciesin.columbia.edu/docs/001-535b/001-535b.html (accessed on 31 March 2022).
- Health Effects of Ultraviolet Radiation. Available online: https://www.who.int/teams/environment-climate-change-and-health/radiation-and-health/uv/health-effects (accessed on 31 March 2022).
- US EPA, Health Effects of UV Radiation. Available online: https://www.epa.gov/sunsafety/health-effects-uv-radiation (accessed on 31 March 2022).
- Woodby, B.; Penta, K.; Pecorelli, A.; Lila, M.A.; Valacchi, G. Skin Health from the Inside Out. Annu. Rev. Food Sci. Technol. 2020, 11, 235–254. [Google Scholar] [CrossRef] [Green Version]
- Gallagher, R.P.; Lee, T.K. Adverse Effects of Ultraviolet Radiation: A Brief Review. Prog. Biophys. Mol. Biol. 2006, 92, 119–131. [Google Scholar] [CrossRef]
- Watson, R.E.B.; Gibbs, N.K.; Griffiths, C.E.M.; Sherratt, M.J. Damage to Skin Extracellular Matrix Induced by UV Exposure. Antioxid. Redox Signal. 2014, 21, 1063–1077. [Google Scholar] [CrossRef]
- De Gruijl, F.R. Photocarcinogenesis: UVA vs UVB. Methods Enzymol. 2000, 319, 359–366. [Google Scholar] [CrossRef]
- Schuch, A.P.; Moreno, N.C.; Schuch, N.J.; Menck, C.F.M.; Garcia, C.C.M. Sunlight Damage to Cellular DNA: Focus on Oxidatively Generated Lesions. Free Radic. Biol. Med. 2017, 107, 110–124. [Google Scholar] [CrossRef]
- Svobodova, A.; Walterova, D.; Vostalova, J. Ultraviolet Light Induced Alteration to the Skin. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech. Repub 2006, 150, 25–38. [Google Scholar] [CrossRef] [Green Version]
- De Jager, T.L.; Cockrell, A.E.; Du Plessis, S.S. Ultraviolet Light Induced Generation of Reactive Oxygen Species. In Ultraviolet Light in Human Health, Diseases and Environment; Ahmad, S.I., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 15–23. ISBN 978-3-319-56017-5. [Google Scholar]
- Sun, X.; Zhang, N.; Yin, C.; Zhu, B.; Li, X. Ultraviolet Radiation and Melanomagenesis: From Mechanism to Immunotherapy. Front. Oncol. 2020, 10, 951. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.Q.; Travers, J.B.; Kemp, M.G. Roles of UVA Radiation and DNA Damage Responses in Melanoma Pathogenesis. Environ. Mol. Mutagen. 2018, 59, 438–460. [Google Scholar] [CrossRef]
- Eftekhari, Z.; Fardid, R. The Bystander Effect of Ultraviolet Radiation and Mediators. J. Biomed. Phys. Eng. 2020, 10, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, F.; Woodby, B.; Pecorelli, A.; Schiavone, M.L.; Pambianchi, E.; Messano, N.; Therrien, J.-P.; Choudhary, H.; Valacchi, G. Additive Effect of Combined Pollutants to UV Induced Skin OxInflammation Damage. Evaluating the Protective Topical Application of a Cosmeceutical Mixture Formulation. Redox Biol. 2020, 34, 101481. [Google Scholar] [CrossRef] [PubMed]
- Markiewicz, E.; Idowu, O.C. DNA Damage in Human Skin and the Capacities of Natural Compounds to Modulate the Bystander Signalling. Open Biol. 2019, 9, 190208. [Google Scholar] [CrossRef] [Green Version]
- Ferrara, F.; Pambianchi, E.; Woodby, B.; Messano, N.; Therrien, J.-P.; Pecorelli, A.; Canella, R.; Valacchi, G. Evaluating the Effect of Ozone in UV Induced Skin Damage. Toxicol Lett 2021, 338, 40–50. [Google Scholar] [CrossRef]
- Mohania, D.; Chandel, S.; Kumar, P.; Verma, V.; Digvijay, K.; Tripathi, D.; Choudhury, K.; Mitten, S.K.; Shah, D. Ultraviolet Radiations: Skin Defense-Damage Mechanism. In Ultraviolet Light in Human Health, Diseases and Environment; Advances in Experimental Medicine and Biology; Ahmad, S.I., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 71–87. ISBN 978-3-319-56017-5. [Google Scholar]
- Emri, G.; Paragh, G.; Tósaki, Á.; Janka, E.; Kollár, S.; Hegedűs, C.; Gellén, E.; Horkay, I.; Koncz, G.; Remenyik, É. Ultraviolet Radiation-Mediated Development of Cutaneous Melanoma: An Update. J. Photochem. Photobiol. B Biol. 2018, 185, 169–175. [Google Scholar] [CrossRef]
- Valacchi, G.; Sticozzi, C.; Pecorelli, A.; Cervellati, F.; Cervellati, C.; Maioli, E. Cutaneous Responses to Environmental Stressors. Ann. N. Y. Acad. Sci. 2012, 1271, 75–81. [Google Scholar] [CrossRef]
- Bernard, J.J.; Gallo, R.L.; Krutmann, J. Photoimmunology: How Ultraviolet Radiation Affects the Immune System. Nat. Rev. Immunol. 2019, 19, 688–701. [Google Scholar] [CrossRef] [PubMed]
- Passeron, T.; Krutmann, J.; Andersen, M.L.; Katta, R.; Zouboulis, C.C. Clinical and Biological Impact of the Exposome on the Skin. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 4–25. [Google Scholar] [CrossRef]
- Scatena, C.; Murtas, D.; Tomei, S. Cutaneous Melanoma Classification: The Importance of High-Throughput Genomic Technologies. Front. Oncol. 2021, 11, 635488. [Google Scholar] [CrossRef] [PubMed]
- Viros, A.; Sanchez-Laorden, B.; Pedersen, M.; Furney, S.J.; Rae, J.; Hogan, K.; Ejiama, S.; Girotti, M.R.; Cook, M.; Dhomen, N.; et al. Ultraviolet Radiation Accelerates BRAF-Driven Melanomagenesis by Targeting TP53. Nature 2014, 511, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Laughery, M.F.; Brown, A.J.; Bohm, K.A.; Sivapragasam, S.; Morris, H.S.; Tchmola, M.; Washington, A.D.; Mitchell, D.; Mather, S.; Malc, E.P.; et al. Atypical UV Photoproducts Induce Non-Canonical Mutation Classes Associated with Driver Mutations in Melanoma. Cell Rep. 2020, 33, 108401. [Google Scholar] [CrossRef] [PubMed]
- Nishigori, C.; Wang, S.; Miyakoshi, J.; Sato, M.; Tsukada, T.; Yagi, T.; Imamura, S.; Takebe, H. Mutations in Ras Genes in Cells Cultured from Mouse Skin Tumors Induced by Ultraviolet Irradiation. Proc. Natl. Acad. Sci. USA 1994, 91, 7189–7193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Elsas, A.; Zerp, S.F.; van der Flier, S.; Krüse, K.M.; Aarnoudse, C.; Hayward, N.K.; Ruiter, D.J.; Schrier, P.I. Relevance of Ultraviolet-Induced N-Ras Oncogene Point Mutations in Development of Primary Human Cutaneous Melanoma. Am. J. Pathol. 1996, 149, 883–893. [Google Scholar]
- Handolias, D.; Salemi, R.; Murray, W.; Tan, A.; Liu, W.; Viros, A.; Dobrovic, A.; Kelly, J.; McArthur, G.A. Mutations in KIT Occur at Low Frequency in Melanomas Arising from Anatomical Sites Associated with Chronic and Intermittent Sun Exposure. Pigment Cell Melanoma Res. 2010, 23, 210–215. [Google Scholar] [CrossRef]
- Curtin, J.A.; Busam, K.; Pinkel, D.; Bastian, B.C. Somatic Activation of KIT in Distinct Subtypes of Melanoma. JCO 2006, 24, 4340–4346. [Google Scholar] [CrossRef]
- Wang, Y.; DiGiovanna, J.J.; Stern, J.B.; Hornyak, T.J.; Raffeld, M.; Khan, S.G.; Oh, K.-S.; Hollander, M.C.; Dennis, P.A.; Kraemer, K.H. Evidence of Ultraviolet Type Mutations in Xeroderma Pigmentosum Melanomas. Proc. Natl. Acad. Sci. USA 2009, 106, 6279–6284. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.S.; Fisher, D.E. UV and Melanoma: The TP53 Link. Cell Res. 2014, 24, 1157–1158. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, B.; Handoko, H.Y.; Mukhopadhyay, P.; Chitsazan, A.; Balmer, L.; Morahan, G.; Walker, G.J. Different Genetic Mechanisms Mediate Spontaneous versus UVR-Induced Malignant Melanoma. eLife 2019, 8, e42424. [Google Scholar] [CrossRef]
- Alexandrov, L.B.; Nik-Zainal, S.; Wedge, D.C.; Aparicio, S.A.J.R.; Behjati, S.; Biankin, A.V.; Bignell, G.R.; Bolli, N.; Borg, A.; Børresen-Dale, A.-L.; et al. Signatures of Mutational Processes in Human Cancer. Nature 2013, 500, 415–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, B.-R.; Xu, Y.; Luo, D. Effect of UVB Irradiation on MicroRNA Expression in Mouse Epidermis. Oncol. Lett. 2012, 3, 560–564. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Ponandai-Srinivasan, S.; Nandakumar, K.S.; Fabre, S.; Xu Landén, N.; Mavon, A.; Khmaladze, I. Targeting MicroRNA for Improved Skin Health. Health Sci. Rep. 2021, 4, e374. [Google Scholar] [CrossRef] [PubMed]
- Pothof, J.; Verkaik, N.S.; van IJcken, W.; Wiemer, E.A.C.; Ta, V.T.B.; van der Horst, G.T.J.; Jaspers, N.G.J.; van Gent, D.C.; Hoeijmakers, J.H.J.; Persengiev, S.P. MicroRNA-Mediated Gene Silencing Modulates the UV-Induced DNA-Damage Response. EMBO J. 2009, 28, 2090–2099. [Google Scholar] [CrossRef] [Green Version]
- Syed, D.N.; Khan, M.I.; Shabbir, M.; Mukhtar, H. MicroRNAs in Skin Response to UV Radiation. Curr. Drug Targets 2013, 14, 1128–1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valacchi, G.; Pambianchi, E.; Coco, S.; Pulliero, A.; Izzotti, A. MicroRNA Alterations Induced in Human Skin by Diesel Fumes, Ozone, and UV Radiation. J. Pers. Med. 2022, 12, 176. [Google Scholar] [CrossRef]
- Bell, A.; Bell, D.; Chakravarti, N.; Ma, J.; Henton, N.; Prieto, V.G. Detection of a MicroRNA Molecular Signature of Ultraviolet Radiation in the Superficial Regions of Melanocytic Nevi on Sun-Exposed Skin. Mod. Pathol. 2018, 31, 1744–1755. [Google Scholar] [CrossRef]
- Mohr, A.M.; Mott, J.L. Overview of MicroRNA Biology. Semin. Liver Dis. 2015, 35, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Beermann, J.; Piccoli, M.-T.; Viereck, J.; Thum, T. Non-Coding RNAs in Development and Disease: Background, Mechanisms, and Therapeutic Approaches. Physiol. Rev. 2016, 96, 1297–1325. [Google Scholar] [CrossRef] [Green Version]
- Varrone, F.; Caputo, E. The MiRNAs Role in Melanoma and in Its Resistance to Therapy. Int. J. Mol. Sci. 2020, 21, 878. [Google Scholar] [CrossRef] [Green Version]
- Iyer, N.J.; Jia, X.; Sunkar, R.; Tang, G.; Mahalingam, R. MicroRNAs Responsive to Ozone-Induced Oxidative Stress in Arabidopsis Thaliana. Plant. Signal. Behav. 2012, 7, 484–491. [Google Scholar] [CrossRef] [Green Version]
- Venza, I.; Venza, M.; Visalli, M.; Lentini, G.; Teti, D.; d’Alcontres, F.S. ROS as Regulators of Cellular Processes in Melanoma. Oxid. Med. Cell. Longev. 2021, 2021, e1208690. [Google Scholar] [CrossRef] [PubMed]
- Pecorelli, A.; Woodby, B.; Prieux, R.; Valacchi, G. Involvement of 4-Hydroxy-2-Nonenal in Pollution-Induced Skin Damage. BioFactors 2019, 45, 536–547. [Google Scholar] [CrossRef] [Green Version]
- Emanuele, S.; D’Anneo, A.; Calvaruso, G.; Cernigliaro, C.; Giuliano, M.; Lauricella, M. The Double-Edged Sword Profile of Redox Signaling: Oxidative Events as Molecular Switches in the Balance between Cell Physiology and Cancer. Chem. Res. Toxicol. 2018, 31, 201–210. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Jiang, B.-H. Interplay between Reactive Oxygen Species and MicroRNAs in Cancer. Curr. Pharmacol. Rep. 2016, 2, 82–90. [Google Scholar] [CrossRef] [Green Version]
- Akbari, A.; Majd, H.M.; Rahnama, R.; Heshmati, J.; Morvaridzadeh, M.; Agah, S.; Amini, S.M.; Masoodi, M. Cross-Talk between Oxidative Stress Signaling and MicroRNA Regulatory Systems in Carcinogenesis: Focused on Gastrointestinal Cancers. Biomed. Pharmacother. 2020, 131, 110729. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-H. MicroRNA Networks Modulate Oxidative Stress in Cancer. Int. J. Mol. Sci 2019, 20, 4497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, C.; Zhou, D.; Wang, Q.; Liu, W.; Yu, F.; Wu, F.; Chen, C. Crosstalk of MicroRNAs and Oxidative Stress in the Pathogenesis of Cancer. Oxid. Med. Cell. Longev. 2020, 2020, e2415324. [Google Scholar] [CrossRef]
- Sand, M.; Skrygan, M.; Sand, D.; Georgas, D.; Gambichler, T.; Hahn, S.A.; Altmeyer, P.; Bechara, F.G. Comparative Microarray Analysis of MicroRNA Expression Profiles in Primary Cutaneous Malignant Melanoma, Cutaneous Malignant Melanoma Metastases, and Benign Melanocytic Nevi. Cell Tissue Res. 2013, 351, 85–98. [Google Scholar] [CrossRef]
- Tan, G.; Shi, Y.; Wu, Z.-H. MicroRNA-22 Promotes Cell Survival upon UV Radiation by Repressing PTEN. Biochem. Biophys. Res. Commun. 2012, 417, 546–551. [Google Scholar] [CrossRef] [Green Version]
- Blignaut, M.; Harries, S.; Lochner, A.; Huisamen, B. Ataxia Telangiectasia Mutated Protein Kinase: A Potential Master Puppeteer of Oxidative Stress-Induced Metabolic Recycling. Oxid. Med. Cell. Longev. 2021, 2021, e8850708. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Zhang, Y.; Wang, Z.; Wang, S.; Jiang, X.; Cui, H.; Zhou, T.; He, Z.; Feng, H.; Guo, Q.; et al. ATM at the Crossroads of Reactive Oxygen Species and Autophagy. Int. J. Biol. Sci. 2021, 17, 3080–3090. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hu, M.; Liu, P.; Jiao, M.; Zhou, M.; Lee, A.K.; Li, F.; Li, C.-Y. ATM Paradoxically Promotes Oncogenic Transformation via Transcriptional Reprogramming. Cancer Res. 2020, 80, 1669–1680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Z.; Kozlov, S.; Lavin, M.F.; Person, M.D.; Paull, T.T. ATM Activation by Oxidative Stress. Science 2010, 330, 517–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhandaru, M.; Martinka, M.; McElwee, K.J.; Rotte, A. Prognostic Significance of Nuclear Phospho-ATM Expression in Melanoma. PLoS ONE 2015, 10, e0134678. [Google Scholar] [CrossRef]
- Boohaker, R.J.; Xu, B. The Versatile Functions of ATM Kinase. Biomed. J. 2014, 37, 3–9. [Google Scholar] [CrossRef]
- Wandler, A.; Riber-Hansen, R.; Hager, H.; Hamilton-Dutoit, S.J.; Schmidt, H.; Nielsen, B.S.; Stougaard, M.; Steiniche, T. Quantification of MicroRNA-21 and MicroRNA-125b in Melanoma Tissue. Melanoma Res. 2017, 27, 417–428. [Google Scholar] [CrossRef]
- Nyholm, A.M.; Lerche, C.M.; Manfé, V.; Biskup, E.; Johansen, P.; Morling, N.; Thomsen, B.M.; Glud, M.; Gniadecki, R. MiR-125b Induces Cellular Senescence in Malignant Melanoma. BMC Dermatol. 2014, 14, 8. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.; Jiang, Q.; Lu, H.; Na, S.; Long, S.; Xin, Y.; Zhang, C.; Zhang, J. Association between MicroRNA-125b Expression in Formalin-fixed Paraffin-embedded Tumor Tissues and Prognosis in Patients with Melanoma. Oncol. Lett. 2019, 18, 1856–1862. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Sendra, B.; Martinez-Ciarpaglini, C.; González-Muñoz, J.F.; Murgui, A.; Terrádez, L.; Monteagudo, C. Downregulation of Intratumoral Expression of MiR-205, MiR-200c and MiR-125b in Primary Human Cutaneous Melanomas Predicts Shorter Survival. Sci. Rep. 2018, 8, 17076. [Google Scholar] [CrossRef]
- Glud, M.; Rossing, M.; Hother, C.; Holst, L.; Hastrup, N.; Nielsen, F.C.; Gniadecki, R.; Drzewiecki, K.T. Downregulation of MiR-125b in Metastatic Cutaneous Malignant Melanoma. Melanoma Res. 2010, 20, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Kappelmann, M.; Kuphal, S.; Meister, G.; Vardimon, L.; Bosserhoff, A.-K. MicroRNA MiR-125b Controls Melanoma Progression by Direct Regulation of c-Jun Protein Expression. Oncogene 2013, 32, 2984–2991. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Xu, Q.; Jing, Y.; Agani, F.; Qian, X.; Carpenter, R.; Li, Q.; Wang, X.-R.; Peiper, S.S.; Lu, Z.; et al. Reactive Oxygen Species Regulate ERBB2 and ERBB3 Expression via MiR-199a/125b and DNA Methylation. EMBO Rep. 2012, 13, 1116–1122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.-Y.; Lee, D.H.; Shin, M.H.; Shin, H.S.; Kim, M.-K.; Chung, J.H. UV-Induced DNA Methyltransferase 1 Promotes Hypermethylation of Tissue Inhibitor of Metalloproteinase 2 in the Human Skin. J. Dermatol. Sci. 2018, 91, 19–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazar, J.; Khaitan, D.; DeBlasio, D.; Zhong, C.; Govindarajan, S.S.; Kopanathi, S.; Zhang, S.; Ray, A.; Perera, R.J. Epigenetic Regulation of MicroRNA Genes and the Role of MiR-34b in Cell Invasion and Motility in Human Melanoma. PLoS ONE 2011, 6, e24922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Lei, S.; Long, J.; Liu, X.; Wu, Q. MicroRNA-199a-5p Inhibits Tumor Proliferation in Melanoma by Mediating HIF-1α. Mol. Med. Rep. 2016, 13, 5241–5247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lujambio, A.; Calin, G.A.; Villanueva, A.; Ropero, S.; Sánchez-Céspedes, M.; Blanco, D.; Montuenga, L.M.; Rossi, S.; Nicoloso, M.S.; Faller, W.J.; et al. A MicroRNA DNA Methylation Signature for Human Cancer Metastasis. Proc. Natl. Acad. Sci. USA 2008, 105, 13556–13561. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Chen, X.; He, Q.; Luo, C. MicroRNA-9 Suppresses the Growth, Migration, and Invasion of Malignant Melanoma Cells via Targeting NRP1. OncoTargets Ther. 2016, 9, 7047–7057. [Google Scholar] [CrossRef] [Green Version]
- Su, M.; Yi, H.; He, X.; Luo, L.; Jiang, S.; Shi, Y. MiR-9 Regulates Melanocytes Adhesion and Migration during Vitiligo Repigmentation Induced by UVB Treatment. Exp. Cell Res. 2019, 384, 111615. [Google Scholar] [CrossRef]
- Tian, R.; Liu, T.; Qiao, L.; Gao, M.; Li, J. Decreased Serum MicroRNA-206 Level Predicts Unfavorable Prognosis in Patients with Melanoma. Int. J. Clin. Exp. Pathol. 2015, 8, 3097–3103. [Google Scholar]
- Georgantas, R.W., III; Streicher, K.; Luo, X.; Greenlees, L.; Zhu, W.; Liu, Z.; Brohawn, P.; Morehouse, C.; Higgs, B.W.; Richman, L.; et al. MicroRNA-206 Induces G1 Arrest in Melanoma by Inhibition of CDK4 and Cyclin D. Pigment. Cell Melanoma Res. 2014, 27, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Happel, C.; Manna, S.K.; Acquaah-Mensah, G.; Carrerero, J.; Kumar, S.; Nasipuri, P.; Krausz, K.W.; Wakabayashi, N.; Dewi, R.; et al. Transcription Factor NRF2 Regulates MiR-1 and MiR-206 to Drive Tumorigenesis. J. Clin. Investig. 2013, 123, 2921–2934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carpenter, E.L.; Becker, A.L.; Indra, A.K. NRF2 and Key Transcriptional Targets in Melanoma Redox Manipulation. Cancers 2022, 14, 1531. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.M.; Rushworth, S.A.; Murray, M.Y.; Bowles, K.M.; MacEwan, D.J. Understanding the Role of NRF2-Regulated MiRNAs in Human Malignancies. Oncotarget 2013, 4, 1130–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Zhang, X.; Lentz, C.; Abi-Daoud, M.; Paré, G.C.; Yang, X.; Feilotter, H.E.; Tron, V.A. MiR-193b Regulates Mcl-1 in Melanoma. Am. J. Pathol. 2011, 179, 2162–2168. [Google Scholar] [CrossRef]
- Andrews, M.C.; Cursons, J.; Hurley, D.G.; Anaka, M.; Cebon, J.S.; Behren, A.; Crampin, E.J. Systems Analysis Identifies MiR-29b Regulation of Invasiveness in Melanoma. Mol. Cancer 2016, 15, 72. [Google Scholar] [CrossRef] [Green Version]
- Satzger, I.; Mattern, A.; Kuettler, U.; Weinspach, D.; Niebuhr, M.; Kapp, A.; Gutzmer, R. MicroRNA-21 Is Upregulated in Malignant Melanoma and Influences Apoptosis of Melanocytic Cells. Exp. Dermatol. 2012, 21, 509–514. [Google Scholar] [CrossRef]
- Melnik, B.C. MiR-21: An Environmental Driver of Malignant Melanoma? J. Transl. Med. 2015, 13, 202. [Google Scholar] [CrossRef] [Green Version]
- Melnik, B.C.; John, S.M.; Carrera-Bastos, P.; Schmitz, G. MicroRNA-21-Enriched Exosomes as Epigenetic Regulators in Melanomagenesis and Melanoma Progression: The Impact of Western Lifestyle Factors. Cancers 2020, 12, 2111. [Google Scholar] [CrossRef]
- Wäster, P.; Eriksson, I.; Vainikka, L.; Öllinger, K. Extracellular Vesicles Released by Melanocytes after UVA Irradiation Promote Intercellular Signaling via MiR21. Pigment Cell Melanoma Res. 2020, 33, 542–555. [Google Scholar] [CrossRef]
- Cooper, S.J.; Bowden, G.T. Ultraviolet B Regulation of Transcription Factor Families: Roles of Nuclear Factor-Kappa B (NF-KappaB) and Activator Protein-1 (AP-1) in UVB-Induced Skin Carcinogenesis. Curr. Cancer Drug Targets 2007, 7, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Gaziel-Sovran, A.; Segura, M.F.; Di Micco, R.; Collins, M.K.; Hanniford, D.; de Miera, E.V.-S.; Rakus, J.F.; Dankert, J.F.; Shang, S.; Kerbel, R.S.; et al. MiR-30b/30d Regulation of GalNAc Transferases Enhances Invasion and Immunosuppression during Metastasis. Cancer Cell 2011, 20, 104–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pu, W.; Shang, Y.; Shao, Q.; Yuan, X. MiR-146a Promotes Cell Migration and Invasion in Melanoma by Directly Targeting SMAD4. Oncol. Lett. 2018, 15, 7111–7117. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Usatorre, A.; Sempere, L.F.; Carmona, S.J.; Carretero-Iglesia, L.; Monnot, G.; Speiser, D.E.; Rufer, N.; Donda, A.; Zehn, D.; Jandus, C.; et al. MicroRNA-155 Expression Is Enhanced by T-Cell Receptor Stimulation Strength and Correlates with Improved Tumor Control in Melanoma. Cancer Immunol. Res. 2019, 7, 1013–1024. [Google Scholar] [CrossRef] [Green Version]
- Ding, J.; Zhu, X.; Chen, X.; Guan, J.; Li, H. MicroRNA-182 Suppresses Malignant Melanoma Proliferation by Targeting RECK. Clin. Lab. 2020, 66. [Google Scholar] [CrossRef]
- Liu, X.; Li, H.; Wu, G.; Cui, S. MiR-182 Promotes Cell Proliferation and Invasion by Inhibiting APC in Melanoma. Int. J. Clin. Exp. Pathol. 2018, 11, 1900–1908. [Google Scholar]
- Chiang, C.-H.; Hou, M.-F.; Hung, W.-C. Up-Regulation of MiR-182 by β-Catenin in Breast Cancer Increases Tumorigenicity and Invasiveness by Targeting the Matrix Metalloproteinase Inhibitor RECK. Biochim. Biophys Acta 2013, 1830, 3067–3076. [Google Scholar] [CrossRef]
- Liu, Y.; Qiang, W.; Xu, X.; Dong, R.; Karst, A.M.; Liu, Z.; Kong, B.; Drapkin, R.I.; Wei, J.-J. Role of MiR-182 in Response to Oxidative Stress in the Cell Fate of Human Fallopian Tube Epithelial Cells. Oncotarget 2015, 6, 38983–38998. [Google Scholar] [CrossRef]
- Bertrand, J.U.; Petit, V.; Hacker, E.; Berlin, I.; Hayward, N.K.; Pouteaux, M.; Sage, E.; Whiteman, D.C.; Larue, L. UVB Represses Melanocyte Cell Migration and Acts through β-Catenin. Exp. Dermatol. 2017, 26, 875–882. [Google Scholar] [CrossRef]
- Liu, S.; Howell, P.M.; Riker, A.I. Up-Regulation of MiR-182 Expression after Epigenetic Modulation of Human Melanoma Cells. Ann. Surg. Oncol. 2013, 20, 1745–1752. [Google Scholar] [CrossRef]
| UV Radiation Spectrum | UVA | UVB | UVC |
|---|---|---|---|
| Wavelength | 320–400 nm | 280–320 nm | 100–280 nm |
| Energy level | Lowest | Medium | Highest |
| Ozone layer absorption level | Not absorbed | Mostly absorbed | Completely absorbed |
| Percent reaching the ground | >95% | <5% | 0% |
| Skin penetrance | Epidermis, dermis, and subcutaneous layer | Epidermis and marginally into the papillary dermis | * Uppermost, nonliving cornified layer of epidermis |
| Molecular cutaneous effects | ROS formation; indirect DNA damage (i.e., oxidized DNA bases such as 8-oxoG); protein and lipid oxidation | ROS formation; direct DNA damage (i.e., CPDs and 6–4 PPs); protein and lipid oxidation | Direct DNA damage (i.e., CPDs and 6–4 PPs); oxidative stress |
| Biological effects | Immediate tanning; sunburn; photoaging, wrinkles and loss of elasticity; some skin cancers | Delayed tanning; sunburn; erythema, edema, immunosuppression and skin cancer; premature aging | Redness; ulcers; skin cancer; premature aging |
| miRNAs | Expression | Mechanism of UV-Related ROS Regulation | Physiopathologic Changes |
|---|---|---|---|
| miR-22 | ↑ * | ATM phosphorylation/activation/PTEN repression | Decreased apoptosis; progression to metastatic phenotype |
| miR-9, miR-29c, miR-34b, miR-34c, miR-125b, miR-148, and miR-199a | ↓ * | Promoter hypermethylation via DNMT activation | Increased cell proliferation, migration, and motility; progression to metastatic phenotype |
| miR-206, miR-200c, and miR-193b | ↓ | NRF2-dependent transcriptional regulation | Increased cell proliferation; progression to metastatic phenotype |
| miR-21 | ↑ | STAT3-, AP-1-, and NF-kB-dependent transcriptional regulation | Increased cell proliferation, migration, and invasiveness, as well as increases in tumor cell survival and redox imbalance |
| miR-9, miR-30b, miR-146a, and miR-155 | ↑ | NF-kB-dependent transcriptional regulation | Increased cell migration and invasion |
| miR-182 | ↑ | Wnt/β-catenin-dependent transcriptional regulation | Increased cell proliferation, migration, and invasion; inhibition of cell apoptosis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pecorelli, A.; Valacchi, G. Oxidative-Stress-Sensitive microRNAs in UV-Promoted Development of Melanoma. Cancers 2022, 14, 3224. https://doi.org/10.3390/cancers14133224
Pecorelli A, Valacchi G. Oxidative-Stress-Sensitive microRNAs in UV-Promoted Development of Melanoma. Cancers. 2022; 14(13):3224. https://doi.org/10.3390/cancers14133224
Chicago/Turabian StylePecorelli, Alessandra, and Giuseppe Valacchi. 2022. "Oxidative-Stress-Sensitive microRNAs in UV-Promoted Development of Melanoma" Cancers 14, no. 13: 3224. https://doi.org/10.3390/cancers14133224
APA StylePecorelli, A., & Valacchi, G. (2022). Oxidative-Stress-Sensitive microRNAs in UV-Promoted Development of Melanoma. Cancers, 14(13), 3224. https://doi.org/10.3390/cancers14133224