Recent Trends in Nanomedicine-Based Strategies to Overcome Multidrug Resistance in Tumors
Abstract
:Simple Summary
Abstract
1. Introduction
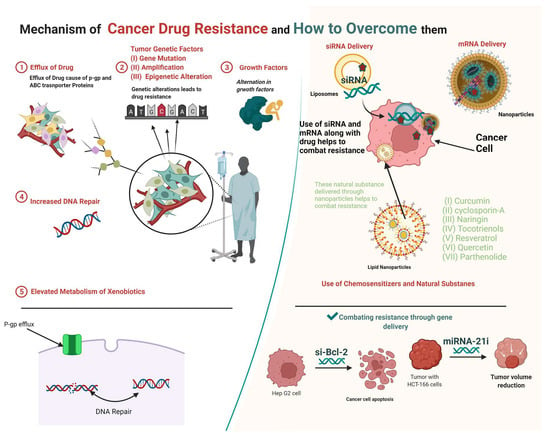
2. Mechanisms of Multidrug Resistance
2.1. Enhanced Efflux of Drugs
2.2. Genetic Factors
2.2.1. Gene Mutations
2.2.2. Amplifications
2.2.3. Epigenetic Alterations
2.3. Growth Factors
2.4. Increased DNA Repair
2.5. Elevated Metabolism of Xenobiotics
3. Strategies to Overcome Multidrug Resistance
3.1. Use of siRNA to Combat Multidrug Resistance
3.2. Use of miRNA to Combat Drug Resistance
3.3. Use of Chemosensitizers and Natural Substances to Combat Resistance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.A.; Fawell, S.; Floc’h, N.; Flemington, V.; McKerrecher, D.; Smith, P.D. Challenges and opportunities in cancer drug resistance. Chem. Rev. 2020, 121, 3297–3351. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, H.; Chen, X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist. 2019, 2, 141–160. [Google Scholar] [CrossRef]
- Vasan, N.; Baselga, J.; Hyman, D.M. A view on drug resistance in cancer. Nature 2019, 575, 299–309. [Google Scholar] [CrossRef]
- Souri, M.; Soltani, M.; Kashkooli, F.M.; Shahvandi, M.K. Engineered strategies to enhance tumor penetration of drug-loaded nanoparticles. J. Control. Release 2022, 341, 227–246. [Google Scholar] [CrossRef]
- Rehman, M.U.; Khan, A.; Imtiyaz, Z.; Ali, S.; Makeen, H.A.; Rashid, S.; Arafah, A. Current Nano-therapeutic Approaches Ameliorating Inflammation in Cancer Progression. Semin. Cancer Biol. 2022. [Google Scholar] [CrossRef]
- Dallavalle, S.; Dobričić, V.; Lazzarato, L.; Gazzano, E.; Machuqueiro, M.; Pajeva, I.; Tsakovska, I.; Zidar, N.; Fruttero, R. Improvement of conventional anti-cancer drugs as new tools against multidrug resistant tumors. Drug Resist. Updates 2020, 50, 100682. [Google Scholar] [CrossRef]
- Alfarouk, K.O.; Stock, C.-M.; Taylor, S.; Walsh, M.; Muddathir, A.K.; Verduzco, D.; Bashir, A.H.; Mohammed, O.Y.; Elhassan, G.O.; Harguindey, S. Resistance to cancer chemotherapy: Failure in drug response from ADME to P-gp. Cancer Cell Int. 2015, 15, 71. [Google Scholar] [CrossRef]
- Wilkens, S. Structure and mechanism of ABC transporters. F1000prime Rep. 2015, 7, 14. [Google Scholar] [CrossRef]
- Wang, J.; Seebacher, N.; Shi, H.; Kan, Q.; Duan, Z. Novel strategies to prevent the development of multidrug resistance (MDR) in cancer. Oncotarget 2017, 8, 84559. [Google Scholar] [CrossRef]
- Satake, K.; Tsukamoto, M.; Mitani, Y.; Regasini, L.O.; da Silva Bolzani, V.; Efferth, T.; Nakagawa, H. Human ABCB1 confers cells resistance to cytotoxic guanidine alkaloids from Pterogyne nitens. Bio-Med. Mater. Eng. 2015, 25, 249–256. [Google Scholar] [CrossRef]
- Lal, S.; Wong, Z.W.; Sandanaraj, E.; Xiang, X.; Ang, P.C.S.; Lee, E.J.; Chowbay, B. Influence of ABCB1 and ABCG2 polymorphisms on doxorubicin disposition in Asian breast cancer patients. Cancer Sci. 2008, 99, 816–823. [Google Scholar] [CrossRef]
- Peng, X.-X.; Tiwari, A.K.; Wu, H.-C.; Chen, Z.-S. Overexpression of P-glycoprotein induces acquired resistance to imatinib in chronic myelogenous leukemia cells. Chin. J. Cancer 2012, 31, 110. [Google Scholar] [CrossRef]
- Mao, Q.; Unadkat, J.D. Role of the breast cancer resistance protein (BCRP/ABCG2) in drug transport—An update. AAPS J. 2015, 17, 65–82. [Google Scholar] [CrossRef]
- Assaraf, Y.G.; Brozovic, A.; Gonçalves, A.C.; Jurkovicova, D.; Linē, A.; Machuqueiro, M.; Saponara, S.; Sarmento-Ribeiro, A.B.; Xavier, C.P.; Vasconcelos, M.H. The multi-factorial nature of clinical multidrug resistance in cancer. Drug Resist. Updates 2019, 46, 100645. [Google Scholar] [CrossRef]
- Duesberg, P.; Stindl, R.; Hehlmann, R. Origin of multidrug resistance in cells with and without multidrug resistance genes: Chromosome reassortments catalyzed by aneuploidy. Proc. Natl. Acad. Sci. USA 2001, 98, 11283–11288. [Google Scholar] [CrossRef]
- Mantovani, F.; Collavin, L.; Del Sal, G. Mutant p53 as a guardian of the cancer cell. Cell Death Differ. 2019, 26, 199–212. [Google Scholar] [CrossRef]
- Chandrasekhar, C.; Kumar, P.S.; Sarma, P.V.G.K. Novel mutations in the kinase domain of BCR-ABL gene causing imatinib resistance in chronic myeloid leukemia patients. Sci. Rep. 2019, 9, 2412. [Google Scholar] [CrossRef]
- Shih, Y.-C.T.; Cortes, J.E.; Kantarjian, H.M. Treatment value of second-generation BCR-ABL1 tyrosine kinase inhibitors compared with imatinib to achieve treatment-free remission in patients with chronic myeloid leukaemia: A modelling study. Lancet Haematol. 2019, 6, e398–e408. [Google Scholar] [CrossRef]
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The different mechanisms of cancer drug resistance: A brief review. Adv. Pharm. Bull. 2017, 7, 339. [Google Scholar] [CrossRef]
- Wahdan-Alaswad, R.; Liu, B.; Thor, A.D. Targeted lapatinib anti-HER2/ErbB2 therapy resistance in breast cancer: Opportunities to overcome a difficult problem. Cancer Drug Resist. 2020, 3, 179–198. [Google Scholar] [CrossRef]
- Ohata, Y.; Shimada, S.; Akiyama, Y.; Mogushi, K.; Nakao, K.; Matsumura, S.; Aihara, A.; Mitsunori, Y.; Ban, D.; Ochiai, T. Acquired resistance with epigenetic alterations under long-term antiangiogenic therapy for hepatocellular carcinoma. Mol. Cancer Ther. 2017, 16, 1155–1165. [Google Scholar] [CrossRef]
- Zhao, Z.; Shilatifard, A. Epigenetic modifications of histones in cancer. Genome Biol. 2019, 20, 245. [Google Scholar] [CrossRef]
- Mohammad, H.P.; Barbash, O.; Creasy, C.L. Targeting epigenetic modifications in cancer therapy: Erasing the roadmap to cancer. Nat. Med. 2019, 25, 403–418. [Google Scholar] [CrossRef]
- Si, W.; Shen, J.; Zheng, H.; Fan, W. The role and mechanisms of action of microRNAs in cancer drug resistance. Clin. Epigenet. 2019, 11, 25. [Google Scholar] [CrossRef]
- Setrerrahmane, S.; Xu, H. Tumor-related interleukins: Old validated targets for new anti-cancer drug development. Mol. Cancer 2017, 16, 153. [Google Scholar] [CrossRef]
- Conze, D.; Weiss, L.; Regen, P.S.; Bhushan, A.; Weaver, D.; Johnson, P.; Rincón, M. Autocrine production of interleukin 6 causes multidrug resistance in breast cancer cells. Cancer Res. 2001, 61, 8851–8858. [Google Scholar]
- Ham, I.-H.; Oh, H.J.; Jin, H.; Bae, C.A.; Jeon, S.-M.; Choi, K.S.; Son, S.-Y.; Han, S.-U.; Brekken, R.A.; Lee, D. Targeting interleukin-6 as a strategy to overcome stroma-induced resistance to chemotherapy in gastric cancer. Mol. Cancer 2019, 18, 68. [Google Scholar] [CrossRef]
- Singh, R.K.; Kumar, S.; Gautam, P.K.; Tomar, M.S.; Verma, P.K.; Singh, S.P.; Acharya, A. Protein kinase C-α and the regulation of diverse cell responses. Biomol. Concepts 2017, 8, 143–153. [Google Scholar] [CrossRef]
- Jena, M.K.; Janjanam, J. Role of extracellular matrix in breast cancer development: A brief update. F1000Research 2018, 7, 274. [Google Scholar] [CrossRef] [PubMed]
- Gentile, F.; Elmenoufy, A.H.; Ciniero, G.; Jay, D.; Karimi-Busheri, F.; Barakat, K.H.; Weinfeld, M.; West, F.G.; Tuszynski, J.A. Computer-aided drug design of small molecule inhibitors of the ERCC1-XPF protein–protein interaction. Chem. Biol. Drug Des. 2020, 95, 460–471. [Google Scholar] [CrossRef] [PubMed]
- Pathania, S.; Bhatia, R.; Baldi, A.; Singh, R.; Rawal, R.K. Drug metabolizing enzymes and their inhibitors’ role in cancer resistance. Biomed. Pharmacother. 2018, 105, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Steppi, A.; Zhou, Y.; Mao, F.; Miller, P.C.; He, M.M.; Zhao, T.; Sun, Q.; Zhang, J. Tumoral expression of drug and xenobiotic metabolizing enzymes in breast cancer patients of different ethnicities with implications to personalized medicine. Sci. Rep. 2017, 7, 4747. [Google Scholar] [CrossRef]
- Riganti, C.; Contino, M. New Strategies to Overcome Resistance to Chemotherapy and Immune System in Cancer. Int. J. Mol. Sci. 2019, 20, 4783. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, S.; Zhi, D.; Cui, J. Cancer treatment with liposomes based drugs and genes co-delivery systems. Curr. Med. Chem. 2018, 25, 3319–3332. [Google Scholar] [CrossRef]
- Zuckerman, J.E.; Davis, M.E. Clinical experiences with systemically administered siRNA-based therapeutics in cancer. Nat. Rev. Drug Discov. 2015, 14, 843–856. [Google Scholar] [CrossRef]
- Ambrosio, L.; Argenziano, M.; Cucci, M.A.; Grattarola, M.; de Graaf, I.A.; Dianzani, C.; Barrera, G.; Sánchez Nieves, J.; Gomez, R.; Cavalli, R. Carbosilane dendrimers loaded with sirna targeting nrf2 as a tool to overcome cisplatin chemoresistance in bladder cancer cells. Antioxidants 2020, 9, 993. [Google Scholar] [CrossRef]
- Byeon, Y.; Lee, J.-W.; Choi, W.S.; Won, J.E.; Kim, G.H.; Kim, M.G.; Wi, T.I.; Lee, J.M.; Kang, T.H.; Jung, I.D. CD44-targeting PLGA nanoparticles incorporating paclitaxel and FAK siRNA overcome chemoresistance in epithelial ovarian cancer. Cancer Res. 2018, 78, 6247–6256. [Google Scholar] [CrossRef]
- Sánchez, C.; Mendoza, P.; Contreras, H.R.; Vergara, J.; McCubrey, J.A.; Huidobro, C.; Castellon, E.A. Expression of multidrug resistance proteins in prostate cancer is related with cell sensitivity to chemotherapeutic drugs. Prostate 2009, 69, 1448–1459. [Google Scholar] [CrossRef]
- Tieu, T.; Wojnilowicz, M.; Huda, P.; Thurecht, K.J.; Thissen, H.; Voelcker, N.H.; Cifuentes-Rius, A. Nanobody-displaying porous silicon nanoparticles for the co-delivery of siRNA and doxorubicin. Biomater. Sci. 2021, 9, 133–147. [Google Scholar] [CrossRef]
- Xiao, Y.-S.; Zeng, D.; Liang, Y.-K.; Wu, Y.; Li, M.-F.; Qi, Y.-Z.; Wei, X.-L.; Huang, W.-H.; Chen, M.; Zhang, G.-J. Major vault protein is a direct target of Notch1 signaling and contributes to chemoresistance in triple-negative breast cancer cells. Cancer Lett. 2019, 440, 156–167. [Google Scholar] [CrossRef]
- Li, X.; Liu, D.; Fan, K.; Qian, M. Cisplatin and si-Notch 1-Folic Acid-Conjugated Mesoporous Silica Nanoparticles Prevent Hepatocellular Carcinoma. J. Biomater. Tissue Eng. 2021, 11, 1284–1292. [Google Scholar] [CrossRef]
- Rodrigues, C.; Pimpão, C.; Mósca, A.F.; Coxixo, A.S.; Lopes, D.; da Silva, I.V.; Pedersen, P.A.; Antunes, F.; Soveral, G. Human aquaporin-5 facilitates hydrogen peroxide permeation affecting adaption to oxidative stress and cancer cell migration. Cancers 2019, 11, 932. [Google Scholar] [CrossRef]
- Jensen, H.H.; Login, F.H.; Koffman, J.S.; Kwon, T.-H.; Nejsum, L.N. The role of aquaporin-5 in cancer cell migration: A potential active participant. Int. J. Biochem. Cell Biol. 2016, 79, 271–276. [Google Scholar] [CrossRef]
- Li, X.; Pei, B.; Wang, H.; Tang, C.; Zhu, W.; Jin, F. Effect of AQP-5 silencing by siRNA interference on chemosensitivity of breast cancer cells. OncoTargets Ther. 2018, 11, 3359. [Google Scholar] [CrossRef]
- Yang, H.; Ding, R.; Tong, Z.; Huang, J.; Shen, L.; Sun, Y.; Liao, J.; Yang, Z.; Hoffman, R.M.; Wang, C. SiRNA targeting of MDR1 reverses multidrug resistance in a nude mouse model of doxorubicin-resistant human hepatocellular carcinoma. Anticancer Res. 2016, 36, 2675–2682. [Google Scholar]
- Pan, J.; Mendes, L.P.; Yao, M.; Filipczak, N.; Garai, S.; Thakur, G.A.; Sarisozen, C.; Torchilin, V.P. Polyamidoamine dendrimers-based nanomedicine for combination therapy with siRNA and chemotherapeutics to overcome multidrug resistance. Eur. J. Pharm. Biopharm. 2019, 136, 18–28. [Google Scholar] [CrossRef]
- Wu, M.; Li, J.; Lin, X.; Wei, Z.; Zhang, D.; Zhao, B.; Liu, X.; Liu, J. Reduction/photo dual-responsive polymeric prodrug nanoparticles for programmed siRNA and doxorubicin delivery. Biomater. Sci. 2018, 6, 1457–1468. [Google Scholar] [CrossRef]
- Sun, W.; Chen, X.; Xie, C.; Wang, Y.; Lin, L.; Zhu, K.; Shuai, X. Co-delivery of doxorubicin and Anti-BCL-2 siRNA by pH-responsive polymeric vector to overcome drug resistance in in vitro and in vivo hepg2 hepatoma model. Biomacromolecules 2018, 19, 2248–2256. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, X.; Xu, X.; Chen, X.; Zhang, X. Doxorubicin@ Bcl-2 siRNA core@ shell nanoparticles for synergistic anticancer chemotherapy. ACS Appl. Bio Mater. 2018, 1, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Han, S.; Kou, Z.; Dai, J.; Liu, J.; Wei, C.; Li, Y.; Jiang, L.; Sun, Y. Lipid nanoparticle-based co-delivery of epirubicin and BCL-2 siRNA for enhanced intracellular drug release and reversing multidrug resistance. Artif. Cells Nanomed. Biotechnol. 2018, 46, 323–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Tang, C.; Yin, C. Co-delivery of doxorubicin and siRNA by all-trans retinoic acid conjugated chitosan-based nanocarriers for multiple synergistic antitumor efficacy. Carbohydr. Polym. 2022, 283, 119097. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.; Lalani, R.; Vhora, I.; Bardoliwala, D.; Patel, A.; Ghosh, S.; Misra, A. Co-delivery of cisplatin and siRNA through hybrid nanocarrier platform for masking resistance to chemotherapy in lung cancer. Drug Deliv. Transl. Res. 2021, 11, 2052–2071. [Google Scholar] [CrossRef]
- Liu, H.; Ma, D.; Chen, J.; Ye, L.; Li, Y.; Xie, Y.; Zhao, X.; Zou, H.; Chen, X.; Pu, J. A targeted nanoplatform co-delivery of pooled siRNA and doxorubicin for reversing of multidrug resistance in breast cancer. Nano Res. 2022, 15, 6306–6314. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, Y.; Li, F.; Bei, S.; Pan, M.; Feng, L. Precise engineering of cholesterol-loaded chitosan micelles as a promising nanocarrier system for co-delivery drug-siRNA for the treatment of gastric cancer therapy. Process Biochem. 2022, 120, 265–274. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, M.; Feng, J.; Qin, B.; Zhang, C.; Zhu, C.; Liu, W.; Wang, Y.; Liu, W.; Huang, L. Multifunctional nanoparticles co-loaded with Adriamycin and MDR-targeting siRNAs for treatment of chemotherapy-resistant esophageal cancer. J. Nanobiotechnol. 2022, 20, 166. [Google Scholar] [CrossRef]
- Nehate, C.; Moothedathu Raynold, A.A.; Koul, V. ATRP fabricated and short chain polyethylenimine grafted redox sensitive polymeric nanoparticles for codelivery of anticancer drug and siRNA in cancer therapy. ACS Appl. Mater. Interfaces 2017, 9, 39672–39687. [Google Scholar] [CrossRef]
- Shakeran, Z.; Varshosaz, J.; Keyhanfar, M.; Mohammad-Beigi, H.; Rahimi, K.; Sutherland, D.S. Co-delivery of STAT3 siRNA and methotrexate in breast cancer cells. Artif. Cells Nanomed. Biotechnol. 2022, 50, 29–39. [Google Scholar] [CrossRef]
- Ngamcherdtrakul, W.; Bejan, D.S.; Cruz-Muñoz, W.; Reda, M.; Zaidan, H.Y.; Siriwon, N.; Marshall, S.; Wang, R.; Nelson, M.A.; Rehwaldt, J.P. Targeted Nanoparticle for Co-delivery of HER2 siRNA and a Taxane to Mirror the Standard Treatment of HER2+ Breast Cancer: Efficacy in Breast Tumor and Brain Metastasis. Small 2022, 18, e2107550. [Google Scholar] [CrossRef]
- Li, M.; Li, S.; Li, Y.; Li, X.; Yang, G.; Li, M.; Xie, Y.; Su, W.; Wu, J.; Jia, L. Cationic liposomes co-deliver chemotherapeutics and siRNA for the treatment of breast cancer. Eur. J. Med. Chem. 2022, 233, 114198. [Google Scholar] [CrossRef]
- Chen, S.-Q.; Li, J.-Q.; Wang, X.-Q.; Lei, W.-j.; Li, H.; Wan, J.; Hu, Z.; Zou, Y.-W.; Wu, X.-Y.; Niu, H.-X. EZH2-inhibitor DZNep enhances apoptosis of renal tubular epithelial cells in presence and absence of cisplatin. Cell Div. 2020, 15, 8. [Google Scholar] [CrossRef]
- Hao, F.; Lee, R.J.; Yang, C.; Zhong, L.; Sun, Y.; Dong, S.; Cheng, Z.; Teng, L.; Meng, Q.; Lu, J. Targeted Co-delivery of siRNA and methotrexate for tumor therapy via mixed micelles. Pharmaceutics 2019, 11, 92. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, F.; Jiang, X.; Zhao, X.; Wang, Y.; Kuai, Q.; Nie, G.; He, M.; Pan, Y.; Shi, W. Co-delivery of gemcitabine and Mcl-1 SiRNA via cationic liposome-based system enhances the efficacy of chemotherapy in pancreatic cancer. J. Biomed. Nanotechnol. 2019, 15, 966–978. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, X.; Cui, C.; Li, J.; Wang, Y. Doxorubicin and anti-VEGF siRNA co-delivery via nano-graphene oxide for enhanced cancer therapy in vitro and in vivo. Int. J. Nanomed. 2018, 13, 3713. [Google Scholar] [CrossRef]
- Liang, G.; Zhu, Y.; Ali, D.J.; Tian, T.; Xu, H.; Si, K.; Sun, B.; Chen, B.; Xiao, Z. Engineered exosomes for targeted co-delivery of miR-21 inhibitor and chemotherapeutics to reverse drug resistance in colon cancer. J. Nanobiotechnol. 2020, 18, 10. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, G.; Luo, X.; Wang, D.; Zhou, W.; Zhang, Y.; Zhang, W.; Chen, J.; Meng, Q.; Chen, E. Co-delivery of 5-fluorouracil and miRNA-34a mimics by host-guest self-assembly nanocarriers for efficacious targeted therapy in colorectal cancer patient-derived tumor xenografts. Theranostics 2021, 11, 2475. [Google Scholar] [CrossRef]
- Wei, S.; Gao, J.; Zhang, M.; Dou, Z.; Li, W.; Zhao, L. Dual delivery nanoscale device for miR-451 and adriamycin co-delivery to combat multidrug resistant in bladder cancer. Biomed. Pharmacother. 2020, 122, 109473. [Google Scholar] [CrossRef]
- Lin, F.; Wen, D.; Wang, X.; Mahato, R.I. Dual responsive micelles capable of modulating miRNA-34a to combat taxane resistance in prostate cancer. Biomaterials 2019, 192, 95–108. [Google Scholar] [CrossRef]
- Wang, H.; Ellipilli, S.; Lee, W.-J.; Li, X.; Vieweger, M.; Ho, Y.-S.; Guo, P. Multivalent rubber-like RNA nanoparticles for targeted co-delivery of paclitaxel and MiRNA to silence the drug efflux transporter and liver cancer drug resistance. J. Control. Release 2021, 330, 173–184. [Google Scholar] [CrossRef]
- Sriram, V.; Lee, J.-Y. Calcium phosphate-polymeric nanoparticle system for co-delivery of microRNA-21 inhibitor and doxorubicin. Colloids Surf. B Biointerfaces 2021, 208, 112061. [Google Scholar] [CrossRef] [PubMed]
- Salzano, G.; Costa, D.F.; Sarisozen, C.; Luther, E.; Mattheolabakis, G.; Dhargalkar, P.P.; Torchilin, V.P. Mixed Nanosized Polymeric Micelles as Promoter of Doxorubicin and miRNA-34a Co-Delivery Triggered by Dual Stimuli in Tumor Tissue. Small 2016, 12, 4837–4848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, C.; Tian, J.; Wang, Z.; Gao, Y.; Wu, X.; Ding, X.; Qiang, L.; Li, G.; Han, Z.; Yuan, Y. Functional exosome-mediated co-delivery of doxorubicin and hydrophobically modified microRNA 159 for triple-negative breast cancer therapy. J. Nanobiotechnology 2019, 17, 93. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaei, S.N.; Derbali, R.M.; Yang, C.; Superstein, R.; Hamel, P.; Chain, J.L.; Hardy, P. Co-delivery of miR-181a and melphalan by lipid nanoparticles for treatment of seeded retinoblastoma. J. Control. Release 2019, 298, 177–185. [Google Scholar] [CrossRef]
- Deng, Y.W.; Hao, W.J.; Li, Y.W.; Li, Y.X.; Zhao, B.C.; Lu, D. Hsa-miRNA-143-3p reverses multidrug resistance of triple-negative breast cancer by inhibiting the expression of its target protein cytokine-induced apoptosis inhibitor 1 in vivo. J. Breast Cancer 2018, 21, 251–258. [Google Scholar] [CrossRef]
- Sun, X.; Xu, H.; Huang, T.; Zhang, C.; Wu, J.; Luo, S. Simultaneous delivery of anti-miRNA and docetaxel with supramolecular self-assembled “chitosome” for improving chemosensitivity of triple negative breast cancer cells. Drug Deliv. Transl. Res. 2021, 11, 192–204. [Google Scholar] [CrossRef]
- Yin, P.T.; Pongkulapa, T.; Cho, H.-Y.; Han, J.; Pasquale, N.J.; Rabie, H.; Kim, J.-H.; Choi, J.-W.; Lee, K.-B. Overcoming Chemoresistance in Cancer via Combined MicroRNA Therapeutics with Anticancer Drugs Using Multifunctional Magnetic Core–Shell Nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 26954–26963. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, L.; Bai, X.; Cao, X.; Jiao, X.; Huang, Y.; Li, Y.; Qin, Y.; Wen, Y. Stimuli-responsive nanocarrier for co-delivery of MiR-31 and doxorubicin to suppress high MtEF4 cancer. ACS Appl. Mater. Interfaces 2018, 10, 22767–22775. [Google Scholar] [CrossRef]
- Gupta, B.; Ruttala, H.B.; Poudel, B.K.; Pathak, S.; Regmi, S.; Gautam, M.; Poudel, K.; Sung, M.H.; Ou, W.; Jin, S.G. Polyamino acid layer-by-layer (LbL) constructed silica-supported mesoporous titania nanocarriers for stimuli-responsive delivery of microRNA 708 and paclitaxel for combined chemotherapy. ACS Appl. Mater. Interfaces 2018, 10, 24392–24405. [Google Scholar] [CrossRef]
- Wang, H.; Huang, Y. Combination therapy based on nano codelivery for overcoming cancer drug resistance. Med. Drug Discov. 2020, 6, 100024. [Google Scholar] [CrossRef]
- Cui, W.; Zhao, H.; Wang, C.; Chen, Y.; Luo, C.; Zhang, S.; Sun, B.; He, Z. Co-encapsulation of docetaxel and cyclosporin A into SNEDDS to promote oral cancer chemotherapy. Drug Deliv. 2019, 26, 542–550. [Google Scholar] [CrossRef]
- Han, W.; Shi, L.; Ren, L.; Zhou, L.; Li, T.; Qiao, Y.; Wang, H. A nanomedicine approach enables co-delivery of cyclosporin A and gefitinib to potentiate the therapeutic efficacy in drug-resistant lung cancer. Signal Transduct. Target. Ther. 2018, 3, 16. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.-T.; Ho, Y.-S. Anticancer effect of curcumin on breast cancer and stem cells. Food Sci. Hum. Wellness 2018, 7, 134–137. [Google Scholar] [CrossRef]
- Mujokoro, B.; Madani, F.; Esnaashari, S.S.; Khosravani, M.; Adabi, M. Combination and co-delivery of methotrexate and curcumin: Preparation and in vitro cytotoxic investigation on glioma cells. J. Pharm. Innov. 2020, 15, 617–626. [Google Scholar] [CrossRef]
- Zhao, M.-D.; Li, J.-Q.; Chen, F.-Y.; Dong, W.; Wen, L.-J.; Fei, W.-D.; Zhang, X.; Yang, P.-L.; Zhang, X.-M.; Zheng, C.-H. Co-delivery of curcumin and paclitaxel by “core-shell” targeting amphiphilic copolymer to reverse resistance in the treatment of ovarian cancer. Int. J. Nanomed. 2019, 14, 9453. [Google Scholar] [CrossRef]
- Jabri, T.; Imran, M.; Aziz, A.; Rao, K.; Kawish, M.; Irfan, M.; Malik, M.I.; Simjee, S.U.; Arfan, M.; Shah, M.R. Design and synthesis of mixed micellar system for enhanced anticancer efficacy of Paclitaxel through its co-delivery with Naringin. Drug Dev. Ind. Pharm. 2019, 45, 703–714. [Google Scholar] [CrossRef]
- Ghanbari-Movahed, M.; Jackson, G.; Farzaei, M.H.; Bishayee, A. A systematic review of the preventive and therapeutic effects of naringin against human malignancies. Front. Pharmacol. 2021, 12, 250. [Google Scholar] [CrossRef]
- Maniam, G.; Mai, C.-W.; Zulkefeli, M.; Fu, J.-Y. Co-encapsulation of gemcitabine and tocotrienols in nanovesicles enhanced efficacy in pancreatic cancer. Nanomedicine 2021, 16, 373–389. [Google Scholar] [CrossRef]
- Raviadaran, R.; Ng, M.H.; Chandran, D.; Ooi, K.K.; Manickam, S. Stable W/O/W multiple nanoemulsion encapsulating natural tocotrienols and caffeic acid with cisplatin synergistically treated cancer cell lines (A549 and HEP G2) and reduced toxicity on normal cell line (HEK 293). Mater. Sci. Eng. 2021, 121, 111808. [Google Scholar] [CrossRef]
- Mohammed, S.; Harikumar, K.B. Role of resveratrol in chemosensitization of cancer. In Role of Nutraceuticals in Cancer Chemosensitization; Elsevier: Amsterdam, The Netherlands, 2018; pp. 61–76. [Google Scholar]
- Venkatadri, R.; Muni, T.; Iyer, A.; Yakisich, J.; Azad, N. Role of apoptosis-related miRNAs in resveratrol-induced breast cancer cell death. Cell Death Dis. 2016, 7, e2104. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, H.; Chen, Y.; Ren, F. Resveratrol chemosensitizes adriamycin-resistant breast cancer cells by modulating miR-122-5p. J. Cell. Biochem. 2019, 120, 16283–16292. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhao, Z.; Chen, D.; Qiao, M.; Wan, F.; Cun, D.; Sun, Y.; Yang, M. Co-delivery of resveratrol and docetaxel via polymeric micelles to improve the treatment of drug-resistant tumors. Asian J. Pharm. Sci. 2019, 14, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Zhang, Y.; Luo, J.; Xiong, K.; Lu, Y.; Wu, Z.; Wang, B.Q.; Wu, J.; Chen, Y.; Fu, S. Therapeutic efficacy of thermosensitive Pluronic hydrogel for codelivery of resveratrol microspheres and cisplatin in the treatment of liver cancer ascites. Int. J. Pharm. 2020, 582, 119334. [Google Scholar] [CrossRef] [PubMed]
- Minaei, A.; Sabzichi, M.; Ramezani, F.; Hamishehkar, H.; Samadi, N. Co-delivery with nano-quercetin enhances doxorubicin-mediated cytotoxicity against MCF-7 cells. Mol. Biol. Rep. 2016, 43, 99–105. [Google Scholar] [CrossRef]
- Fang, J.; Zhang, S.; Xue, X.; Zhu, X.; Song, S.; Wang, B.; Jiang, L.; Qin, M.; Liang, H.; Gao, L. Quercetin and doxorubicin co-delivery using mesoporous silica nanoparticles enhance the efficacy of gastric carcinoma chemotherapy. Int. J. Nanomed. 2018, 13, 5113. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, H.; Wang, S.; Gai, C.; Cui, X.; Xu, Z.; Li, W.; Zhang, W. Targeted delivery of quercetin by nanoparticles based on chitosan sensitizing paclitaxel-resistant lung cancer cells to paclitaxel. Mater. Sci. Eng. C 2021, 119, 111442. [Google Scholar] [CrossRef]
- Shitole, A.A.; Sharma, N.; Giram, P.; Khandwekar, A.; Baruah, M.; Garnaik, B.; Koratkar, S. LHRH-conjugated, PEGylated, poly-lactide-co-glycolide nanocapsules for targeted delivery of combinational chemotherapeutic drugs Docetaxel and Quercetin for prostate cancer. Mater. Sci. Eng. C 2020, 114, 111035. [Google Scholar] [CrossRef]
- Sztiller-Sikorska, M.; Czyz, M. Parthenolide as cooperating agent for anti-cancer treatment of various malignancies. Pharmaceuticals 2020, 13, 194. [Google Scholar] [CrossRef]
- Wu, L.-M.; Liao, X.-Z.; Zhang, Y.; He, Z.-R.; Nie, S.-Q.; Ke, B.; Shi, L.; Zhao, J.-F.; Chen, W.-H. Parthenolide augments the chemosensitivity of non-small-cell lung cancer to cisplatin via the PI3K/AKT signaling pathway. Front. Cell Dev. Biol. 2020, 8, 610097. [Google Scholar] [CrossRef]
- Liang, P.; Wu, H.; Zhang, Z.; Jiang, S.; Lv, H. Preparation and characterization of parthenolide nanocrystals for enhancing therapeutic effects of sorafenib against advanced hepatocellular carcinoma. Int. J. Pharm. 2020, 583, 119375. [Google Scholar] [CrossRef]
- Rad, A.T.; Hargrove, D.; Daneshmandi, L.; Ramsdell, A.; Lu, X.; Nieh, M.-P. Codelivery of Paclitaxel and Parthenolide in Discoidal Bicelles for a Synergistic Anticancer Effect: Structure Matters. Adv. NanoBiomed Res. 2022, 2, 2100080. [Google Scholar] [CrossRef]



| Name of Drug | siRNA | Nanovehicle | Cell Line or Animal Model | Reference |
|---|---|---|---|---|
| Doxorubicin | si-BCL-2 siRNA | (ATRA) double grafted N,N,N-trimethyl chitosan (TMC) nanoparticles | QGY-7703 cells H-22 tumor model | [53] |
| Cisplatin | ABCC3-siRNA | Hybrid nanocarriers (PEG-PLA) | A549 xenograft model of NSCLC | [54] |
| Doxorubicin | P-gp siRNA, Bcl-2 siRNA | Biodegradable boronic-acid-modified ε-polylysine | Breast cancer cell line (MCF-7/ADR) cells | [55] |
| Salinomycin | siRNA | Cholesterol-loaded chitosan nanoparticles (C-SAR) | Gastric carcinoma cells (SNU-668 and SGC-791 | [56] |
| Adriamycin | siRNAs targeting MVP and BCL2 | Multifunctional Carboxymethyl chitosan nanoparticle | Esophageal squamous cell carcinoma mice model | [57] |
| Doxorubicin | P-gp siRNA | GSH reduction- and photoresponsive polymeric nanoparticles | MCF/ADR cells | [49] |
| Doxorubicin | polo-like kinase I (plk1) siRNA | Polyethylenimine-modified ATRP-fabricated Polymeric Nanoparticles | MDA-MB-231 and HeLa cells EAT Tumor-bearing mice | [58] |
| Methotrexate | STAT3 siRNA | Chitosan-modified MSNs | MCF7 cells and breast cancer model | [59] |
| Paclitaxel | siRNA against HER2 (siHER2) | Targeted nanoparticle | Breast tumor and brain tumor | [60] |
| Crizotinib (CRI) | Bcl-xL siRNA | Cationic liposomes | MCF-7 cells and breast cancer model | [61] |
| Name of Drug | miRNA | Nanovehicle | Cell Line or Animal Model | Reference |
|---|---|---|---|---|
| Paclitaxel | miR122 | Multivalent RNA nanoparticle | Hepatocellular carcinoma mice model | [70] |
| Doxorubicin | miR-21 inhibitor | Calcium phosphate-polymeric nanoparticle | MDA-MB-231 and A549 cells | [71] |
| Doxorubicin | miRNA-34a | Mixed nanosized polymeric micelles along with TAT peptide | HT1080 cells | [72] |
| Doxorubicin | miR159 | Exosomes nanovehicle | MDA-MB-231 cells and TNBC breast cancer model | [73] |
| melphalan | miR-181a | Lipid nanoparticles | RB cells and retinoblastoma mice model | [74] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.M.; Torchilin, V.P. Recent Trends in Nanomedicine-Based Strategies to Overcome Multidrug Resistance in Tumors. Cancers 2022, 14, 4123. https://doi.org/10.3390/cancers14174123
Khan MM, Torchilin VP. Recent Trends in Nanomedicine-Based Strategies to Overcome Multidrug Resistance in Tumors. Cancers. 2022; 14(17):4123. https://doi.org/10.3390/cancers14174123
Chicago/Turabian StyleKhan, Muhammad Muzamil, and Vladimir P. Torchilin. 2022. "Recent Trends in Nanomedicine-Based Strategies to Overcome Multidrug Resistance in Tumors" Cancers 14, no. 17: 4123. https://doi.org/10.3390/cancers14174123
APA StyleKhan, M. M., & Torchilin, V. P. (2022). Recent Trends in Nanomedicine-Based Strategies to Overcome Multidrug Resistance in Tumors. Cancers, 14(17), 4123. https://doi.org/10.3390/cancers14174123