Laser Interstitial Thermal Therapy for Posterior Fossa Lesions: A Systematic Review and Analysis of Multi-Institutional Outcomes
Abstract
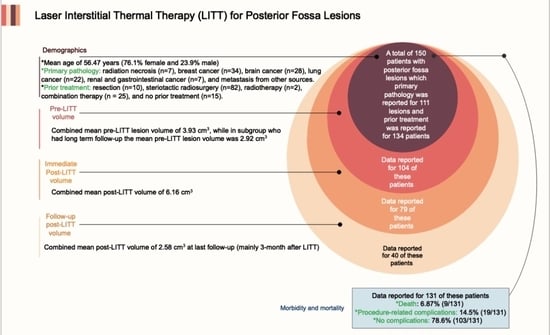
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search and Selection Criteria
2.2. Data Extraction
3. Results
4. Discussion
Limitations and Future Research
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Remick, M.; McDowell, M.M.; Gupta, K.; Felker, J.; Abel, T.J. Emerging indications for stereotactic laser interstitial thermal therapy in pediatric neurosurgery. Int. J. Hyperth. 2020, 37, 84–93. [Google Scholar] [CrossRef]
- Medvid, R.; Ruiz, A.; Komotar, R.J.; Jagid, J.; Ivan, M.; Quencer, R.; Desai, M. Current applications of MRI-guided laser interstitial thermal therapy in the treatment of brain neoplasms and epilepsy: A radiologic and neurosurgical overview. Am. J. Neuroradiol. 2015, 36, 1998–2006. [Google Scholar] [CrossRef] [Green Version]
- Munier, S.M.; Hargreaves, E.L.; Patel, N.V.; Danish, S.F. Ablation dynamics of subsequent thermal doses delivered to previously heat-damaged tissue during magnetic resonance–guided laser-induced thermal therapy. J. Neurosurg. 2018, 131, 1958–1965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salem, U.; Kumar, V.A.; Madewell, J.E.; Schomer, D.F.; de Almeida Bastos, D.C.; Zinn, P.O.; Weinberg, J.S.; Rao, G.; Prabhu, S.S.; Colen, R.R. Neurosurgical applications of MRI guided laser interstitial thermal therapy (LITT). Cancer Imaging 2019, 19, 65. [Google Scholar] [CrossRef] [Green Version]
- de Almeida Bastos, D.C.; Weinberg, J.; Kumar, V.A.; Fuentes, D.T.; Stafford, J.; Li, J.; Rao, G.; Prabhu, S.S. Laser Interstitial Thermal Therapy in the treatment of brain metastases and radiation necrosis. Cancer Lett. 2020, 489, 9–18. [Google Scholar] [CrossRef]
- Leuthardt, E.C.; Duan, C.; Kim, M.J.; Campian, J.L.; Kim, A.H.; Miller-Thomas, M.M.; Shimony, J.S.; Tran, D.D. Hyperthermic laser ablation of recurrent glioblastoma leads to temporary disruption of the peritumoral blood brain barrier. PLoS ONE 2016, 11, e0148613. [Google Scholar]
- Appelboom, G.; Detappe, A.; LoPresti, M.; Kunjachan, S.; Mitrasinovic, S.; Goldman, S.; Chang, S.D.; Tillement, O. Stereotactic modulation of blood-brain barrier permeability to enhance drug delivery. Neuro-Oncology 2016, 18, 1601–1609. [Google Scholar] [CrossRef] [PubMed]
- Schott, M.; Suhr, D.; Jantzen, J.-P.A. Perioperative challenges during posterior fossa surgery. In Essentials of Neurosurgical Anesthesia & Critical Care; Springer: Berlin/Heidelberg, Germany, 2020; pp. 193–199. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- Ashraf, O.; Arzumanov, G.; Luther, E.; McMahon, J.T.; Malcolm, J.G.; Mansour, S.; Lee, I.Y.; Willie, J.T.; Komotar, R.J.; Danish, S.F. Magnetic resonance-guided laser interstitial thermal therapy for posterior fossa neoplasms. J. Neuro-Oncol. 2020, 149, 533–542. [Google Scholar] [CrossRef]
- Luther, E.; Lu, V.M.; Morell, A.A.; Elarjani, T.; Mansour, S.; Echeverry, N.; Gaztanaga, W.; King, H.; McCarthy, D.; Eichberg, D.G. Supralesional Ablation Volumes Are Feasible in the Posterior Fossa and May Provide Enhanced Symptomatic Relief. Oper. Neurosurg. 2021, 21, 418–425. [Google Scholar] [CrossRef]
- Beechar, V.B.; Prabhu, S.S.; Bastos, D.; Weinberg, J.S.; Stafford, R.J.; Fuentes, D.; Hess, K.R.; Rao, G. Volumetric response of progressing post-SRS lesions treated with laser interstitial thermal therapy. J. Neuro-Oncol. 2018, 137, 57–65. [Google Scholar] [CrossRef]
- Borghei-Razavi, H.; Koech, H.; Sharma, M.; Krivosheya, D.; Lee, B.S.; Barnett, G.H.; Mohammadi, A.M. Laser interstitial thermal therapy for posterior fossa lesions: An initial experience. World Neurosurg. 2018, 117, e146–e153. [Google Scholar] [CrossRef]
- Traylor, J.I.; Patel, R.; Habib, A.; Muir, M.; de Almeida Bastos, D.C.; Rao, G.; Prabhu, S.S. Laser interstitial thermal therapy to the posterior fossa: Challenges and nuances. World Neurosurg. 2019, 132, e124–e132. [Google Scholar] [CrossRef] [PubMed]
- Eichberg, D.G.; VanDenBerg, R.; Komotar, R.J.; Ivan, M.E. Quantitative volumetric analysis following magnetic resonance–guided laser interstitial thermal ablation of cerebellar metastases. World Neurosurg. 2018, 110, e755–e765. [Google Scholar] [CrossRef] [PubMed]
- Gamboa, N.T.; Karsy, M.; Iyer, R.R.; Bollo, R.J.; Schmidt, R.H. Stereotactic laser interstitial thermal therapy for brainstem cavernous malformations: Two preliminary cases. Acta Neurochir. 2020, 162, 1771–1775. [Google Scholar] [CrossRef]
- Tan, S.K.; Luther, E.; Eichberg, D.; Shah, A.; Khan, K.; Jamshidi, A.; Ivan, M.; Gultekin, S.H.; Komotar, R. Complete regression of a solitary cholangiocarcinoma brain metastasis following laser interstitial thermal therapy. World Neurosurg. 2020, 144, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.Y.; Tran, D.K.T.; Gill, A.S.; Hsu, F.P.; Vadera, S. Stereotactic robot-assisted MRI-guided laser thermal ablation of radiation necrosis in the posterior cranial fossa. Neurosurg. Focus 2016, 41, E5. [Google Scholar] [CrossRef] [Green Version]
- Eliyas, J.K.; Bailes, J.; Merrell, R.; O’Leary, S. NT-15stereotactic laser thermal ablation of recurrent posterior fossa metastatic lesion: Description of new technology FOR infratentorial tumors refractory to conventional therapies. Neuro-Oncology 2014, 16, v162. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, J.D.; Rehman, A.; Lee, M. Treatment of a Pontine Cavernoma with Laser Interstitial Thermal Therapy: Case Report. J. Neurol. Surg. Part B Skull Base 2021, 82, P177. [Google Scholar]
- Kozlowski, J.; VanKoevering, K.; Heth, J.A. A customized 3D implant to target laser interstitial thermal therapy ablation of a posterior fossa mass. J. Clin. Neurosci. 2021, 90, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Dadey, D.Y.; Kamath, A.A.; Smyth, M.D.; Chicoine, M.R.; Leuthardt, E.C.; Kim, A.H. Utilizing personalized stereotactic frames for laser interstitial thermal ablation of posterior fossa and mesiotemporal brain lesions: A single-institution series. Neurosurg. Focus 2016, 41, E4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahluwalia, M.; Barnett, G.H.; Deng, D.; Tatter, S.B.; Laxton, A.W.; Mohammadi, A.M.; Leuthardt, E.; Chamoun, R.; Judy, K.; Asher, A. Laser ablation after stereotactic radiosurgery: A multicenter prospective study in patients with metastatic brain tumors and radiation necrosis. J. Neurosurg. 2018, 130, 804–811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaye, J.; Patel, N.V.; Danish, S.F. Laser interstitial thermal therapy for in-field recurrence of brain metastasis after stereotactic radiosurgery: Does treatment with LITT prevent a neurologic death? Clin. Exp. Metastasis 2020, 37, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Radakovich, N.R.; Grabowski, M.; Borghei-Razavi, H.; Knusel, K.; Joshi, K.C.; Baha’eddin, A.M.; Hwang, L.; Barnett, G.H.; Mohammadi, A.M. Lessons learned in using laser interstitial thermal therapy for treatment of brain tumors: A case series of 238 Patients from a single institution. World Neurosurg. 2020, 139, e345–e354. [Google Scholar] [CrossRef]
- Rao, M.S.; Hargreaves, E.L.; Khan, A.J.; Haffty, B.G.; Danish, S.F. Magnetic resonance-guided laser ablation improves local control for postradiosurgery recurrence and/or radiation necrosis. Neurosurgery 2014, 74, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Patel, N.V.; Danish, S.F. Intracranial MR-guided laser-induced thermal therapy: Single-center experience with the Visualase thermal therapy system. J. Neurosurg. 2016, 125, 853–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashraf, O.; Patel, N.V.; Hanft, S.; Danish, S.F. Laser-induced thermal therapy in neuro-oncology: A review. World Neurosurg. 2018, 112, 166–177. [Google Scholar] [CrossRef]
- Hernandez, R.N.; Carminucci, A.; Patel, P.; Hargreaves, E.L.; Danish, S.F. Magnetic resonance-guided laser-induced thermal therapy for the treatment of progressive enhancing inflammatory reactions following stereotactic radiosurgery, or PEIRs, for metastatic brain disease. Neurosurgery 2019, 85, 84–90. [Google Scholar] [CrossRef]
- Jeon, C.; Choi, J.W.; Kong, D.-S.; Nam, D.-H.; Lee, J.-I.; Seol, H.J. Treatment Strategy for Giant Solid Hemangioblastomas in the Posterior Fossa: A Retrospective Review of 13 Consecutive Cases. World Neurosurg. 2021. online ahead of print. [Google Scholar] [CrossRef]
- Siomin, V.E.; Vogelbaum, M.A.; Kanner, A.A.; Lee, S.-Y.; Suh, J.H.; Barnett, G.H. Posterior fossa metastases: Risk of leptomeningeal disease when treated with stereotactic radiosurgery compared to surgery. J. Neuro-Oncol. 2004, 67, 115–121. [Google Scholar] [CrossRef] [PubMed]
- McTyre, E.R.; Johnson, A.G.; Ruiz, J.; Isom, S.; Lucas Jr, J.T.; Hinson, W.H.; Watabe, K.; Laxton, A.W.; Tatter, S.B.; Chan, M.D. Predictors of neurologic and nonneurologic death in patients with brain metastasis initially treated with upfront stereotactic radiosurgery without whole-brain radiation therapy. Neuro-Oncology 2017, 19, 558–566. [Google Scholar] [CrossRef] [Green Version]
- Khan, R.B.; Patay, Z.; Klimo, P.; Huang, J.; Kumar, R.; Boop, F.A.; Raches, D.; Conklin, H.M.; Sharma, R.; Simmons, A. Clinical features, neurologic recovery, and risk factors of post-operative posterior fossa syndrome and delayed recovery: A prospective study. Neuro-Oncology 2021, 23, 1586–1596. [Google Scholar] [CrossRef] [PubMed]
- Gentile, M.S.; Yeap, B.Y.; Paganetti, H.; Goebel, C.P.; Gaudet, D.E.; Gallotto, S.L.; Weyman, E.A.; Morgan, M.L.; MacDonald, S.M.; Giantsoudi, D. Brainstem injury in pediatric patients with posterior fossa tumors treated with proton beam therapy and associated dosimetric factors. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 719–729. [Google Scholar] [CrossRef] [PubMed]

| Author, Year, (Ref.) | Sample Size | Mean Age | Histology | Location | Prior Treatment | Volume (Mean ± SD) | Complications | KPS | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-LITT Tumor Volume | Post-LITT Cavity Volume | Tumor Volume at Last Follow-Up | Pre-LITT | Post-LITT | |||||||
| Traylor, 2019, [15] | 13 | 58 | RN: 5, breast: 4, lung †: 2, kidney and GI ‡: 2 | NR | SRS: 8, RT: 1, Co $:4 | 4.63 ± 2.85 cm3 | 6.90 ± 3.42 cm3 | 3.14 ± 1.39 cm3 | CN7&8 palsy: 1 | 90 | 80 |
| Borghei-Razavi, 2018, [14] | 8 | 53.87 | RN: 2, brain *: 3, lung †: 1, others §: 2 | Cerebellum: 6 Cerebellar peduncle: 2 | Res:1, SRS: 2, N: 5 | 5.58 ± 5.27 cm3 | 9.64 ± 7.33 cm3 | 5.67 ± 8.39 cm3 | Wound infection: 1, ataxia and hydrocephalus: 1, and CN6 palsy: 1 | 90 | 80 |
| Eichberg, 2017, [16] | 4 | 54.25 | Breast: 3, others §: 1 | Cerebellum: 4 | SRS: 1, Co $: 3 | 3.35 ± 2.72 cm3 | NR | NR | Diplopia: 1,dysarthria due to a new lesion: 1 | NR | NR |
| Ashraf, 2020, [11] | 58 ¶ | 56.4 | Breast: 19 #, brain *: 16, lung †: 17, kidney and GI ‡: 2, others §: 6 | Cerebellum: 52 Brain stem: 7 Pineal region: 1 | Res: 6, SRS: 34, RT: 1, Co $: 12, N: 4 | 2.24 ± 0.21 cm3 | 3.92 ± 0.28 cm3 | NR | Hemiparesis: 1, CN7 palsy: 1, facial droop and hemiparesis: 2, arm weakness: 1, dysmetria and slurred speech: 2, diplopia: 1, refractory cerebral edema: 1, hearing loss: 2, truncal ataxia and scanning speech: 1, and death: 1 | NR | NR |
| Luther, 2021, [12] | 17 | 57.9 | Breast: 8, brain *: 3, lung †: 1, kidney and GI ‡: 2, others §: 3 | Cerebellum: 16 Vermis: 1 | Res: 1, SRS: 10, Co $: 5, N: 1 | 2.0 ± 1.5 cm3 | 4.8 ± 2.2 cm3 | 1.7 ± 0.9 cm3 | Diplopia: 1 and speech impairment: 1 | 91.2 | NS |
| Gamboa, 2020, [17] | 2 | 57.5 | Brain *: 2 | Brain stem: 2 | Res: 2 | 1.8 and 1.6 cm | NR | NR | Left-sided weakness and ataxia: 1 | NR | NR |
| Tan, 2020, [18] | 1 | 71 | Kidney and GI ‡: 1 | Cerebellum: 1 | N: 1 | 0.7 cm3 | NR | Resolve | NR | NR | NR |
| Chan, 2016, [19] | 1 | 60 | Brain *: 1 | Cerebellar peduncle: 1 | SRS: 1 | 2.4 × 2.7 (cm2) | NR | Resolve | NR | NR | NR |
| Eliyas, 2014, [20] | 1 | 67 | Lung †: 1 | Cerebellum: 1 | N: 1 | 3.23 cm3 | NR | NR | NR | NR | NR |
| Lawrence, 2021, [21] | 1 | 20 | Brain *: 1 | Brain stem: 1 | N: 1 | 2.4 × 2.6 (cm2) | NR | 1.3 × 1.2 (cm2) | Diplopia secondary to CN6 palsy: 1 | NR | NR |
| Kozlowski, 2021, [22] | 1 | 75 | Others §: 1 | Cerebellum: 1 | Co $: 1 | NR | NR | NR | NR | NR | NR |
| Dadey, 2016, [23] | 2 | 45.5 | Brain *: 2 | Cerebellum: 1 Brain stem: 1 | N: 2 | 12.85 ± 5.8 cm3 | NR | NR | Internuclear ophthalmoplegia, right eye ophthalmoplegia, dysarthria, and reduced sensation on left side: 1 | NR | NR |
| Beechar, 2018, [13] | 4 | NS | NS | Cerebellum: 4 | SRS: 4 | NS | NS | NS | Neurological complication: 2 | NS | NS |
| Ahluwali, 2019, [24] | 6 | NS | NS | Cerebellum: 6 | NS | NS | NS | NS | NS | NS | NS |
| Kaye, 2020, [25] | 22 | NS | NS | Cerebellum: 20 Brain stem: 2 | SRS: 22 | NS | NS | NS | Neurologic death: 8 Non-neurologic death: 10 | NS | NS |
| Shao, 2020, [26] | 9 | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabahi, M.; Bordes, S.J.; Najera, E.; Mohammadi, A.M.; Barnett, G.H.; Adada, B.; Borghei-Razavi, H. Laser Interstitial Thermal Therapy for Posterior Fossa Lesions: A Systematic Review and Analysis of Multi-Institutional Outcomes. Cancers 2022, 14, 456. https://doi.org/10.3390/cancers14020456
Sabahi M, Bordes SJ, Najera E, Mohammadi AM, Barnett GH, Adada B, Borghei-Razavi H. Laser Interstitial Thermal Therapy for Posterior Fossa Lesions: A Systematic Review and Analysis of Multi-Institutional Outcomes. Cancers. 2022; 14(2):456. https://doi.org/10.3390/cancers14020456
Chicago/Turabian StyleSabahi, Mohammadmahdi, Stephen J. Bordes, Edinson Najera, Alireza M. Mohammadi, Gene H. Barnett, Badih Adada, and Hamid Borghei-Razavi. 2022. "Laser Interstitial Thermal Therapy for Posterior Fossa Lesions: A Systematic Review and Analysis of Multi-Institutional Outcomes" Cancers 14, no. 2: 456. https://doi.org/10.3390/cancers14020456
APA StyleSabahi, M., Bordes, S. J., Najera, E., Mohammadi, A. M., Barnett, G. H., Adada, B., & Borghei-Razavi, H. (2022). Laser Interstitial Thermal Therapy for Posterior Fossa Lesions: A Systematic Review and Analysis of Multi-Institutional Outcomes. Cancers, 14(2), 456. https://doi.org/10.3390/cancers14020456