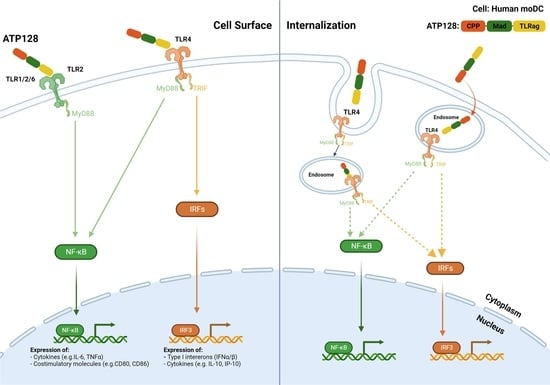
ATP128 Clinical Therapeutic Cancer Vaccine Activates NF-κB and IRF3 Pathways through TLR4 and TLR2 in Human Monocytes and Dendritic Cells
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Vaccines
2.3. Antibodies
2.4. For Western Blot
2.5. TLR Activation Experiment
2.6. Blocking Antibodies Experiments
2.7. Endocytosis Inhibition Experiments
2.8. NF-κB and IRF3 Phosphorylation
2.9. Cell Preparation
2.10. Ex Vivo Human moDCs Activation
2.11. Cytokine Signatures
2.12. Statistics
3. Results
3.1. CPP and TLRag Domains of ATP128 Contribute to Both NF-κB and IRF3 Activation Pathways
3.2. ATP128 Activates NF-κB and IRF3 Pathways Engaging Both TLR2 and TLR4
3.3. Impact of Endocytosis on NF-κB and IRF3 Activaton
3.4. ATP128 Induces IRF3 Phosphorylation with Delayed Kinetics Compared to NF-κB
3.5. TLR4 Is Essential to Activated Human moDCs by ATP128
3.6. CPP and Anaxa Work Synergestically to Activate Human moDCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Belnoue, E.; Mayol, J.F.; Carboni, S.; Besson, W.D.B.; Dupuychaffray, E.; Nelde, A.; Stevanovic, S.; Santiago-Raber, M.L.; Walker, P.R.; Derouazi, M. Targeting Self and Neo-Epitopes with a Modular Self-Adjuvanting Cancer Vaccine. JCI Insight 2019, 4, e127305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Derouazi, M.; Berardino-Besson, W.D.; Belnoue, E.; Hoepner, S.; Walther, R.; Benkhoucha, M.; Teta, P.; Dufour, Y.; Maroun, C.Y.; Salazar, A.M.; et al. Novel Cell-Penetrating Peptide-Based Vaccine Induces Robust CD4+ and CD8+ T Cell-Mediated Antitumor Immunity. Cancer Res. 2015, 75, 3020–3031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopetz, S.; Prenen, H.; Sharma, S.; Cutsem, E.V.; Mayol, J.; Trapani, F.; Bogenrieder, T.; Lenz, H. SO-11 KISIMA-01 Trial: Safety, Tolerability and Immunogenicity of ATP128 with or without Ezabenlimab (BI 754091) in Patients with Stage IV Colorectal Cancer—Preliminary Results from a Phase 1b Study. Ann. Oncol. 2021, 32, S206–S207. [Google Scholar] [CrossRef]
- Segura, E.; Villadangos, J.A. Antigen Presentation by Dendritic Cells in Vivo. Curr. Opin. Immunol. 2009, 21, 105–110. [Google Scholar] [CrossRef]
- Ossendorp, F.; Fu, N.; Camps, M.; Granucci, F.; Gobin, S.J.P.; van den Elsen, P.J.; Schuurhuis, D.; Adema, G.J.; Lipford, G.B.; Chiba, T.; et al. Differential Expression Regulation of the α and β Subunits of the PA28 Proteasome Activator in Mature Dendritic Cells. J. Immunol. 2005, 174, 7815–7822. [Google Scholar] [CrossRef] [Green Version]
- Monie, T.P.; Bryant, C.E.; Gay, N.J. Activating Immunity: Lessons from the TLRs and NLRs. Trends Biochem. Sci. 2009, 34, 553–561. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. TLR Signaling. Cell Death Differ. 2006, 13, 816–825. [Google Scholar] [CrossRef] [Green Version]
- Andersen, B.M.; Xia, J.; Epstein, A.L.; Ohlfest, J.R.; Chen, W.; Blazar, B.R.; Pennell, C.A.; Olin, M.R. Monomeric Annexin A2 Is an Oxygen-Regulated Toll-like Receptor 2 Ligand and Adjuvant. J. Immunother. Cancer 2016, 4, 11. [Google Scholar] [CrossRef] [Green Version]
- Swisher, J.F.A.; Burton, N.; Bacot, S.M.; Vogel, S.N.; Feldman, G.M. Annexin A2 Tetramer Activates Human and Murine Macrophages through TLR4. Blood 2010, 115, 549–558. [Google Scholar] [CrossRef] [Green Version]
- O’Neill, L.A.J.; Bowie, A.G. The Family of Five: TIR-Domain-Containing Adaptors in Toll-like Receptor Signalling. Nat. Rev. Immunol. 2007, 7, 353–364. [Google Scholar] [CrossRef]
- Luchner, M.; Reinke, S.; Milicic, A. TLR Agonists as Vaccine Adjuvants Targeting Cancer and Infectious Diseases. Pharmaceutics 2021, 13, 142. [Google Scholar] [CrossRef] [PubMed]
- Płóciennikowska, A.; Hromada-Judycka, A.; Borzęcka, K.; Kwiatkowska, K. Co-Operation of TLR4 and Raft Proteins in LPS-Induced pro-Inflammatory Signaling. Cell. Mol. Life Sci. 2015, 72, 557–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rothe, R.; Liguori, L.; Villegas-Mendez, A.; Marques, B.; Grunwald, D.; Drouet, E.; Lenormand, J.-L. Characterization of the Cell-Penetrating Properties of the Epstein-Barr Virus ZEBRA Trans-Activator. J. Biol. Chem. 2010, 285, 20224–20233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cluff, C.W. Lipid A in Cancer Therapy. Adv. Exp. Med. Biol. 2009, 667, 111–123. [Google Scholar]
- Ii, M.; Matsunaga, N.; Hazeki, K.; Nakamura, K.; Takashima, K.; Seya, T.; Hazeki, O.; Kitazaki, T.; Iizawa, Y. A Novel Cyclohexene Derivative, Ethyl (6R)-6-[N-(2-Chloro-4-Fluorophenyl)Sulfamoyl]Cyclohex-1-Ene-1-Carboxylate (TAK-242), Selectively Inhibits Toll-Like Receptor 4-Mediated Cytokine Production through Suppression of Intracellular Signaling. Mol. Pharmacol. 2006, 69, 1288–1295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grosche, L.; Knippertz, I.; König, C.; Royzman, D.; Wild, A.B.; Zinser, E.; Sticht, H.; Muller, Y.A.; Steinkasserer, A.; Lechmann, M. The CD83 Molecule—An Important Immune Checkpoint. Front. Immunol. 2020, 11, 721. [Google Scholar] [CrossRef]
- Li, J.; Lee, D.S.W.; Madrenas, J. Evolving Bacterial Envelopes and Plasticity of TLR2-Dependent Responses: Basic Research and Translational Opportunities. Front. Immunol. 2013, 4, 347. [Google Scholar] [CrossRef] [Green Version]
- Guven-Maiorov, E.; Keskin, O.; Gursoy, A.; VanWaes, C.; Chen, Z.; Tsai, C.-J.; Nussinov, R. The Architecture of the TIR Domain Signalosome in the Toll-like Receptor-4 Signaling Pathway. Sci. Rep. 2015, 5, 13128. [Google Scholar] [CrossRef] [Green Version]
- Takeda, K.; Akira, S. Toll-like Receptors in Innate Immunity. Int. Immunol. 2005, 17, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Warger, T.; Osterloh, P.; Rechtsteiner, G.; Fassbender, M.; Heib, V.; Schmid, B.; Schmitt, E.; Schild, H.; Radsak, M.P. Synergistic Activation of Dendritic Cells by Combined Toll-like Receptor Ligation Induces Superior CTL Responses in Vivo. Blood 2006, 108, 544–550. [Google Scholar] [CrossRef] [Green Version]
- Bohnenkamp, H.R.; Papazisis, K.T.; Burchell, J.M.; Taylor-Papadimitriou, J. Synergism of Toll-like Receptor-Induced Interleukin-12p70 Secretion by Monocyte-Derived Dendritic Cells Is Mediated through P38 MAPK and Lowers the Threshold of T-Helper Cell Type I Responses. Cell. Immunol. 2007, 247, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Napolitani, G.; Rinaldi, A.; Bertoni, F.; Sallusto, F.; Lanzavecchia, A. Selected Toll-like Receptor Agonist Combinations Synergistically Trigger a T Helper Type 1–Polarizing Program in Dendritic Cells. Nat. Immunol. 2005, 6, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Madan-Lala, R.; Pradhan, P.; Roy, K. Combinatorial Delivery of Dual and Triple TLR Agonists via Polymeric Pathogen-like Particles Synergistically Enhances Innate and Adaptive Immune Responses. Sci. Rep. 2017, 7, 2530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagchi, A.; Herrup, E.A.; Warren, H.S.; Trigilio, J.; Shin, H.-S.; Valentine, C.; Hellman, J. MyD88-Dependent and MyD88-Independent Pathways in Synergy, Priming, and Tolerance between TLR Agonists. J. Immunol. 2007, 178, 1164–1171. [Google Scholar] [CrossRef] [Green Version]
- Musilova, J.; Mulcahy, M.E.; Kuijk, M.M.; McLoughlin, R.M.; Bowie, A.G. Toll-like Receptor 2–Dependent Endosomal Signaling by Staphylococcus Aureus in Monocytes Induces Type I Interferon and Promotes Intracellular Survival. J. Biol. Chem. 2019, 294, 17031–17042. [Google Scholar] [CrossRef] [Green Version]
- Kawai, T.; Takeuchi, O.; Fujita, T.; Inoue, J.; Mühlradt, P.F.; Sato, S.; Hoshino, K.; Akira, S. Lipopolysaccharide Stimulates the MyD88-Independent Pathway and Results in Activation of IFN-Regulatory Factor 3 and the Expression of a Subset of Lipopolysaccharide-Inducible Genes. J. Immunol. 2001, 167, 5887–5894. [Google Scholar] [CrossRef] [Green Version]
- Kawai, T.; Akira, S. Signaling to NF-ΚB by Toll-like Receptors. Trends Mol. Med. 2007, 13, 460–469. [Google Scholar] [CrossRef]
- Yang, J.; Luo, Y.; Shibu, M.A.; Toth, I.; Skwarczynski, M. Cell-Penetrating Peptides: Efficient Vectors for Vaccine Delivery. Curr. Drug. Deliv. 2019, 16, 430–443. [Google Scholar] [CrossRef]
- Chen, Q.; Zhu, W.; Liu, Z.; Yan, K.; Zhao, S.; Han, D. Toll-Like Receptor 11-Initiated Innate Immune Response in Male Mouse Germ Cells. Biol. Reprod. 2014, 90, 38. [Google Scholar] [CrossRef]
- He, G.; Ma, Y.; Chou, S.; Li, H.; Yang, C.; Chuang, J.; Sung, C.; Ding, A. Role of CLIC4 in the Host Innate Responses to Bacterial Lipopolysaccharide. Eur. J. Immunol. 2011, 41, 1221–1230. [Google Scholar] [CrossRef] [Green Version]
- Nish, S.A.; Schenten, D.; Wunderlich, F.T.; Pope, S.D.; Gao, Y.; Hoshi, N.; Yu, S.; Yan, X.; Lee, H.K.; Pasman, L.; et al. T Cell-Intrinsic Role of IL-6 Signaling in Primary and Memory Responses. Elife 2014, 3, e01949. [Google Scholar] [CrossRef] [PubMed]
- Guinn, Z.; Lampe, A.T.; Brown, D.M.; Petro, T.M. Significant Role for IRF3 in Both T Cell and APC Effector Functions during T Cell Responses. Cell. Immunol. 2016, 310, 141–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanza, L.; Peirano, L.; Bosco, O.; Contini, P.; Filaci, G.; Setti, M.; Puppo, F.; Indiveri, F.; Scudeletti, M. Interferons Up-Regulate with Different Potency HLA Class I Antigen Expression in M14 Human Melanoma Cell Line. Possible Interaction with Glucocorticoid Hormones. Cancer Immunol. Immunother. 1995, 41, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Dunn, I.S.; Haggerty, T.J.; Kono, M.; Durda, P.J.; Butera, D.; Macdonald, D.B.; Benson, E.M.; Rose, L.B.; Kurnick, J.T. Enhancement of Human Melanoma Antigen Expression by IFN-β. J. Immunol. 2007, 179, 2134–2142. [Google Scholar] [CrossRef] [Green Version]
- Giudice, G.D.; Rappuoli, R.; Didierlaurent, A.M. Correlates of Adjuvanticity: A Review on Adjuvants in Licensed Vaccines. Semin. Immunol. 2018, 39, 14–21. [Google Scholar] [CrossRef]
- Didierlaurent, A.M.; Morel, S.; Lockman, L.; Giannini, S.L.; Bisteau, M.; Carlsen, H.; Kielland, A.; Vosters, O.; Vanderheyde, N.; Schiavetti, F.; et al. AS04, an Aluminum Salt- and TLR4 Agonist-Based Adjuvant System, induces a Transient Localized Innate Immune Response Leading to Enhanced Adaptive Immunity. J. Immunol. 2009, 183, 6186–6197. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pascolutti, R.; Yeturu, L.; Philippin, G.; Costa Borges, S.; Dejob, M.; Santiago-Raber, M.-L.; Derouazi, M. ATP128 Clinical Therapeutic Cancer Vaccine Activates NF-κB and IRF3 Pathways through TLR4 and TLR2 in Human Monocytes and Dendritic Cells. Cancers 2022, 14, 5134. https://doi.org/10.3390/cancers14205134
Pascolutti R, Yeturu L, Philippin G, Costa Borges S, Dejob M, Santiago-Raber M-L, Derouazi M. ATP128 Clinical Therapeutic Cancer Vaccine Activates NF-κB and IRF3 Pathways through TLR4 and TLR2 in Human Monocytes and Dendritic Cells. Cancers. 2022; 14(20):5134. https://doi.org/10.3390/cancers14205134
Chicago/Turabian StylePascolutti, Roberta, Lakshmi Yeturu, Géraldine Philippin, Stéphane Costa Borges, Magali Dejob, Marie-Laure Santiago-Raber, and Madiha Derouazi. 2022. "ATP128 Clinical Therapeutic Cancer Vaccine Activates NF-κB and IRF3 Pathways through TLR4 and TLR2 in Human Monocytes and Dendritic Cells" Cancers 14, no. 20: 5134. https://doi.org/10.3390/cancers14205134
APA StylePascolutti, R., Yeturu, L., Philippin, G., Costa Borges, S., Dejob, M., Santiago-Raber, M. -L., & Derouazi, M. (2022). ATP128 Clinical Therapeutic Cancer Vaccine Activates NF-κB and IRF3 Pathways through TLR4 and TLR2 in Human Monocytes and Dendritic Cells. Cancers, 14(20), 5134. https://doi.org/10.3390/cancers14205134