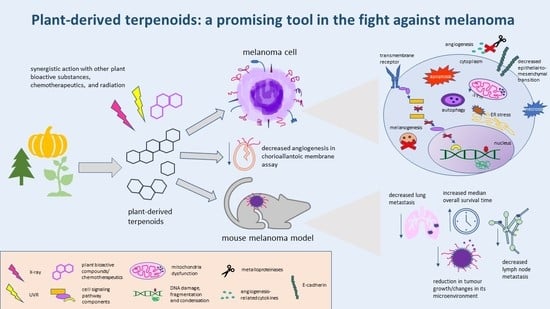
Plant-Derived Terpenoids: A Promising Tool in the Fight against Melanoma
Abstract
:Simple Summary
Abstract
1. Introduction
1.1. Melanoma: Incidence, Staging, and Genetic Background
1.2. Melanoma: Currently Used Therapies
2. Terpenoids as Anti-Melanoma Agents
2.1. Chemical Structure and Function of Terpenes/Terpenoids of Plant Origin
2.2. Monoterpenoids
2.2.1. Thymoquinone
2.2.2. Terpineols
2.2.3. Borneol and Its Esters
2.2.4. Other Monoterpenoids
2.3. Sesquiterpenoids
2.3.1. β-Elemene
2.3.2. Other Sesquiterpenoids
2.4. Diterpenoids
2.4.1. Andrographolide
2.4.2. Other Diterpenoids
2.5. Triterpenoids
2.5.1. Ursolic and Oleanolic Acid
2.5.2. Other Triterpenoids
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Matthews, N.H.; Li, W.-Q.; Quereshi, A.A.; Weinstock, M.A.; Cho, E. Epidemiology of melanoma. In Cutaneous Melanoma: Etiology and Therapy; Ward, W.H., Farma, J.M., Eds.; Codon Publications: Brisbane, Australia, 2017; pp. 3–22. [Google Scholar]
- Moan, J.; Grigalavicius, M.; Baturaite, Z.; Dahlback, A.; Juzeniene, A. The Relationship between UV Exposure and Incidence of Skin Cancer. Photodermatol. Photoimmunol. Photomed. 2015, 31, 26–35. [Google Scholar] [CrossRef]
- Sample, A.; He, Y.Y. Mechanisms and prevention of UV-induced melanoma. Photodermatol. Photoimmunol. Photomed. 2018, 34, 13–24. [Google Scholar] [CrossRef]
- Radiation: Ultraviolet (UV) Radiation and Skin Cancer. Available online: https://www.who.int/news-room/questions-and-answers/item/radiation-ultraviolet-(uv)-radiation-and-skin-cancer (accessed on 30 November 2021).
- Weir, H.K.; Marrett, L.D.; Cokkinides, V.; Barnholtz-Sloan, J.; Patel, P.; Tai, E.; Jemal, A.; Li, J.; Kim, J.; Ekwueme, D.U. Melanoma in Adolescents and Young Adults (Ages 15–39 Years): United States, 1999–2006. J. Am. Acad. Dermatol. 2011, 65 (Suppl. S1), S38–S49. [Google Scholar] [CrossRef] [Green Version]
- Colantonio, S.; Bracken, M.B.; Beecker, J. The Association of Indoor Tanning and Melanoma in Adults: Systematic Review and Meta-Analysis. J. Am. Acad. Dermatol. 2014, 70, 847–857.e1-18. [Google Scholar] [CrossRef] [PubMed]
- Cancer Statistics Review, 1975–2013-Previous Version-SEER Cancer Statistics Review. Available online: https://seer.cancer.gov/archive/csr/1975_2013/index.html (accessed on 30 November 2021).
- Keung, E.Z.; Gershenwald, J.E. The Eighth Edition American Joint Committee on Cancer (AJCC) Melanoma Staging System: Implications for Melanoma Treatment and Care. Expert Rev. Anticancer Ther. 2018, 18, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, A.S.; Hagan, S.; Rath, O.; Kolch, W. MAP Kinase Signalling Pathways in Cancer. Oncogene 2007, 26, 3279–3290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raman, M.; Chen, W.; Cobb, M.H. Differential Regulation and Properties of MAPKs. Oncogene 2007, 26, 3100–3112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, H.; Bignell, G.R.; Cox, C.; Stephens, P.; Edkins, S.; Clegg, S.; Teague, J.; Woffendin, H.; Garnett, M.J.; Bottomley, W.; et al. Mutations of the BRAF Gene in Human Cancer. Nature 2002, 417, 949–954. [Google Scholar] [CrossRef]
- Yang, K.; Oak, A.S.W.; Slominski, R.M.; Brożyna, A.A.; Slominski, A.T. Current Molecular Markers of Melanoma and Treatment Targets. Int. J. Mol. Sci. 2020, 21, 3535. [Google Scholar] [CrossRef] [PubMed]
- Melis, C.; Rogiers, A.; Bechter, O.; van den Oord, J.J. Molecular Genetic and Immunotherapeutic Targets in Metastatic Melanoma. Virchows Arch. 2017, 471, 281–293. [Google Scholar] [CrossRef]
- Menzies, A.M.; Haydu, L.E.; Visintin, L.; Carlino, M.S.; Howle, J.R.; Thompson, J.F.; Kefford, R.F.; Scolyer, R.A.; Long, G.V. Distinguishing Clinicopathologic Features of Patients with V600E and V600K BRAF-Mutant Metastatic Melanoma. Clin. Cancer Res. 2012, 18, 3242–3249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, G.V.; Menzies, A.M.; Nagrial, A.M.; Haydu, L.E.; Hamilton, A.L.; Mann, G.J.; Hughes, T.M.; Thompson, J.F.; Scolyer, R.A.; Kefford, R.F. Prognostic and Clinicopathologic Associations of Oncogenic BRAF in Metastatic Melanoma. J. Clin. Oncol. 2011, 29, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Choi, J.-W.; Kim, Y.-S. Frequencies of BRAF and NRAS Mutations Are Different in Histological Types and Sites of Origin of Cutaneous Melanoma: A Meta-Analysis. Br. J. Dermatol. 2011, 164, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Beadling, C.; Jacobson-Dunlop, E.; Hodi, F.S.; Le, C.; Warrick, A.; Patterson, J.; Town, A.; Harlow, A.; Cruz, F.; Azar, S.; et al. KIT Gene Mutations and Copy Number in Melanoma Subtypes. Clin. Cancer Res. 2008, 14, 6821–6828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabbah, M.; Najem, A.; Krayem, M.; Awada, A.; Journe, F.; Ghanem, G.E. RTK Inhibitors in Melanoma: From Bench to Bedside. Cancers 2021, 13, 1685. [Google Scholar] [CrossRef] [PubMed]
- Curtin, J.A.; Busam, K.; Pinkel, D.; Bastian, B.C. Somatic Activation of KIT in Distinct Subtypes of Melanoma. J. Clin. Oncol. 2006, 24, 4340–4346. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, A.M.; Chan, M.; Harland, M.; Hayward, N.K.; Demenais, F.; Bishop, D.T.; Azizi, E.; Bergman, W.; Bianchi-Scarra, G.; Bruno, W.; et al. Features Associated with Germline CDKN2A Mutations: A GenoMEL Study of Melanoma-Prone Families from Three Continents. J. Med. Genet. 2007, 44, 99–106. [Google Scholar] [CrossRef]
- Vasilovici, A.F.; Grigore, L.E.; Ungureanu, L.; Fechete, O.; Candrea, E.; Trifa, A.P.; Vișan, S.; Șenilă, S.; Cosgarea, R. Vitamin D Receptor Polymorphisms and Melanoma (Review). Oncol. Lett. 2019, 17, 4162–4169. [Google Scholar] [CrossRef] [Green Version]
- Tagliabue, E.; Gandini, S.; Bellocco, R.; Maisonneuve, P.; Newton-Bishop, J.; Polsky, D.; Lazovich, D.; Kanetsky, P.A.; Ghiorzo, P.; Gruis, N.A.; et al. MC1R Variants as Melanoma Risk Factors Independent of at-Risk Phenotypic Characteristics: A Pooled Analysis from the M-SKIP Project. Cancer Manag. Res. 2018, 10, 1143–1154. [Google Scholar] [CrossRef] [Green Version]
- Yokoyama, S.; Woods, S.L.; Boyle, G.M.; Aoude, L.G.; MacGregor, S.; Zismann, V.; Gartside, M.; Cust, A.E.; Haq, R.; Harland, M.; et al. A Novel Recurrent Mutation in MITF Predisposes to Familial and Sporadic Melanoma. Nature 2011, 480, 99–103. [Google Scholar] [CrossRef]
- Wolf Horrell, E.M.; Boulanger, M.C.; D’Orazio, J.A. Melanocortin 1 Receptor: Structure, Function, and Regulation. Front. Genet. 2016, 7, 95. [Google Scholar] [CrossRef] [Green Version]
- Song, X.; Mosby, N.; Yang, J.; Xu, A.; Abdel-Malek, Z.; Kadekaro, A.L. α-MSH Activates Immediate Defense Responses to UV-Induced Oxidative Stress in Human Melanocytes. Pigment Cell Melanoma Res. 2009, 22, 809–818. [Google Scholar] [CrossRef]
- Jarrett, S.G.; Horrell, E.M.W.; Christian, P.A.; Vanover, J.C.; Boulanger, M.C.; Zou, Y.; D’Orazio, J.A. PKA-Mediated Phosphorylation of ATR Promotes Recruitment of XPA to UV-Induced DNA Damage. Mol. Cell 2014, 54, 999–1011. [Google Scholar] [CrossRef] [Green Version]
- Fargnoli, M.C.; Gandini, S.; Peris, K.; Maisonneuve, P.; Raimondi, S. MC1R Variants Increase Melanoma Risk in Families with CDKN2A Mutations: A Meta-Analysis. Eur. J. Cancer Oxf. Engl. 2010, 46, 1413–1420. [Google Scholar] [CrossRef]
- Yasumoto, K.; Yokoyama, K.; Shibata, K.; Tomita, Y.; Shibahara, S. Microphthalmia-Associated Transcription Factor as a Regulator for Melanocyte-Specific Transcription of the Human Tyrosinase Gene. Mol. Cell. Biol. 1994, 14, 8058–8070. [Google Scholar] [CrossRef]
- Bertolotto, C.; Lesueur, F.; Giuliano, S.; Strub, T.; de Lichy, M.; Bille, K.; Dessen, P.; d’Hayer, B.; Mohamdi, H.; Remenieras, A.; et al. A SUMOylation-Defective MITF Germline Mutation Predisposes to Melanoma and Renal Carcinoma. Nature 2011, 480, 94–98. [Google Scholar] [CrossRef]
- McGill, G.G.; Horstmann, M.; Widlund, H.R.; Du, J.; Motyckova, G.; Nishimura, E.K.; Lin, Y.-L.; Ramaswamy, S.; Avery, W.; Ding, H.-F.; et al. Bcl2 Regulation by the Melanocyte Master Regulator Mitf Modulates Lineage Survival and Melanoma Cell Viability. Cell 2002, 109, 707–718. [Google Scholar] [CrossRef] [Green Version]
- Du, J.; Widlund, H.R.; Horstmann, M.A.; Ramaswamy, S.; Ross, K.; Huber, W.E.; Nishimura, E.K.; Golub, T.R.; Fisher, D.E. Critical Role of CDK2 for Melanoma Growth Linked to Its Melanocyte-Specific Transcriptional Regulation by MITF. Cancer Cell 2004, 6, 565–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Joyce, K.M. Surgical Management of Melanoma. Exon Publ. 2017, 91–100. [Google Scholar] [CrossRef]
- Moreira, A.; Heinzerling, L.; Bhardwaj, N.; Friedlander, P. Current Melanoma Treatments: Where Do We Stand? Cancers 2021, 13, 221. [Google Scholar] [CrossRef]
- Prasad, S.; Tyagi, A.K.; Aggarwal, B.B. Chapter 5-Chemosensitization by Ursolic Acid: A New Avenue for Cancer Therapy. In Role of Nutraceuticals in Cancer Chemosensitization; Bharti, A.C., Aggarwal, B.B., Eds.; Cancer Sensitizing Agents for Chemotherapy; Academic Press: New York, NY, USA, 2018; Volume 2, pp. 99–109. [Google Scholar] [CrossRef]
- Fellner, C. Ipilimumab (Yervoy) Prolongs Survival in Advanced Melanoma. Pharm. Ther. 2012, 37, 503–530. [Google Scholar]
- Dummer, R.; Hauschild, A.; Santinami, M.; Atkinson, V.; Mandalà, M.; Kirkwood, J.M.; Sileni, V.C.; Larkin, J.; Nyakas, M.; Dutriaux, C.; et al. Five-Year Analysis of Adjuvant Dabrafenib plus Trametinib in Stage III Melanoma. N. Engl. J. Med. 2020, 383, 1139–1148. [Google Scholar] [CrossRef]
- Larkin, J.; Ascierto, P.A.; Dréno, B.; Atkinson, V.; Liszkay, G.; Maio, M.; Mandalà, M.; Demidov, L.; Stroyakovskiy, D.; Thomas, L.; et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N. Engl. J. Med. 2014, 371, 1867–1876. [Google Scholar] [CrossRef] [Green Version]
- Houten, R.; Greenhalgh, J.; Mahon, J.; Nevitt, S.; Beale, S.; Boland, A.; Lambe, T.; Dundar, Y.; Kotas, E.; McEntee, J. Encorafenib with Binimetinib for the Treatment of Patients with BRAF V600 Mutation-Positive Unresectable or Metastatic Melanoma: An Evidence Review Group Perspective of a NICE Single Technology Appraisal. Pharm. Econ.-Open 2021, 5, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Rehman, H.; Silk, A.W.; Kane, M.P.; Kaufman, H.L. Into the clinic: Talimogene laherparepvec (T-VEC), a first-in-class intratumoral oncolytic viral therapy. J. Immunother. Cancer 2016, 4, 53. [Google Scholar] [CrossRef] [Green Version]
- Lardone, R.D.; Chan, A.A.; Lee, A.F.; Foshag, L.J.; Faries, M.B.; Sieling, P.A.; Lee, D.J. Mycobacterium Bovis Bacillus Calmette–Guérin Alters Melanoma Microenvironment Favoring Antitumor T Cell Responses and Improving M2 Macrophage Function. Front. Immunol. 2017, 8, 965. [Google Scholar] [CrossRef] [Green Version]
- Domingues, B.; Lopes, J.M.; Soares, P.; Pópulo, H. Melanoma Treatment in Review. ImmunoTargets Ther. 2018, 7, 35–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belgrano, V.; Pettersson, J.; Nilsson, J.A.; Mattsson, J.; Katsarelias, D.; Olofsson Bagge, R. Response and Toxicity of Repeated Isolated Limb Perfusion (Re-ILP) for Patients with In-Transit Metastases of Malignant Melanoma. Ann. Surg. Oncol. 2019, 26, 1055–1062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, S.J.; Puric, E.; Eberle, B.; Datta, N.R.; Bodis, S.B. Radiotherapy for Melanoma: More than DNA Damage. Dermatol. Res. Pract. 2019, 2019, e9435389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.; Chen, X.; Li, Y.; Guo, S.; Wang, Z.; Yu, X. Advances in Pharmacological Activities of Terpenoids. Nat. Prod. Commun. 2020, 15, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Cox-Georgian, D.; Ramadoss, N.; Dona, C.; Basu, C. Therapeutic and Medicinal Uses of Terpenes. In Medicinal Plants: From Farm to Pharmacy; Joshee, N., Dhekney, S.A., Parajuli, P., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 333–359. [Google Scholar] [CrossRef]
- Gershenzon, J.; Dudareva, N. The Function of Terpene Natural Products in the Natural World. Nat. Chem. Biol. 2007, 3, 408–414. [Google Scholar] [CrossRef]
- Bergman, M.E.; Davis, B.; Phillips, M.A. Medically Useful Plant Terpenoids: Biosynthesis, Occurrence, and Mechanism of Action. Molecules 2019, 24, 3961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markman, M.; Mekhail, T.M. Paclitaxel in cancer therapy. Expert Opin. Pharmacother. 2005, 3, 755–766. [Google Scholar] [CrossRef]
- Ahmad, A.; Mishra, R.K.; Vyawahare, A.; Kumar, A.; Rehman, M.U.; Qamar, W.; Khan, A.Q.; Khan, R. Thymoquinone (2-isopropyl-5-methyl-1,4-benzoquinone) as a chemopreventive/anticancer agent: Chemistry and biological effects. Saudi Pharm. J. 2019, 27, 1113–1126. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.R.; Rajpal, V.; Singh, P.; Saraf, S.A. Recent findings on thymoquinone and its applications as a nanocarrier for the treatment of cancer and rheumatoid arthritis. Pharmaceutics 2021, 13, 775. [Google Scholar] [CrossRef]
- Elmowafy, M.; Samy, A.; Raslan, M.A.; Salama, A.; Said, R.A.; Abdelaziz, A.E.; El-Eraky, W.; El Awdan, A.; Viitala, T. Enhancement of bioavailability and pharmacodynamic effects of thymoquinone via nanostructured lipid carrier (NLC) formulation. AAPS PharmSciTech 2016, 17, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Goyal, S.N.; Prajapati, C.P.; Gore, P.R.; Patil, C.R.; Mahajan, U.B.; Sharma, C.; Talla, S.P.; Ojha, S.K. Therapeutic Potential and Pharmaceutical Development of Thymoquinone: A Multitargeted Molecule of Natural Origin. Front. Pharmacol. 2017, 8, 656. [Google Scholar] [CrossRef] [PubMed]
- Taha, M.M.E.; Sheikh, B.Y.; Salim, L.Z.A.; Mohan, S.; Khan, A.; Kamalidehghan, B.; Ahmadipour, F.; Abdelwahab, S.I. Thymoquinone Induces Apoptosis and Increase ROS in Ovarian Cancer Cell Line. Cell. Mol. Biol. 2016, 62, 97–101. [Google Scholar]
- Badary, O.A.; Hamza, M.S.; Tikamadas, R. Thymoquinone: A Promising Natural Compound with Potential Benefits for COVID-19 Prevention and Cure. Drug Des. Devel. Ther. 2021, 15, 1819–1833. [Google Scholar] [CrossRef]
- Almajali, B.; Al-Jamal, H.A.N.; Taib, W.R.W.; Ismail, I.; Johan, M.F.; Doolaanea, A.A.; Ibrahim, W.N. Thymoquinone, as a Novel Therapeutic Candidate of Cancers. Pharmaceuticals 2021, 14, 369. [Google Scholar] [CrossRef] [PubMed]
- Hatiboglu, M.A.; Kocyigit, A.; Guler, E.M.; Akdur, K.; Nalli, A.; Karatas, E.; Tuzgen, S. Thymoquinone Induces Apoptosis in B16-F10 Melanoma Cell Through Inhibition of p-STAT3 and Inhibits Tumor Growth in a Murine Intracerebral Melanoma Model. World Neurosurg. 2018, 114, e182–e190. [Google Scholar] [CrossRef] [PubMed]
- Hatiboglu, M.A.; Kocyigit, A.; Guler, E.M.; Akdur, K.; Khan, I.; Nalli, A.; Karatas, E.; Tuzgen, S. Thymoquinone Enhances the Effect of Gamma Knife in B16-F10 Melanoma Through Inhibition of Phosphorylated STAT3. World Neurosurg. 2019, 128, e570–e581. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Yu, S.-M.; Kim, S.J. Inhibitory Effects on Melanogenesis by Thymoquinone Are Mediated through the Β-catenin Pathway in B16F10 Mouse Melanoma Cells. Int. J. Oncol. 2020, 56, 379–389. [Google Scholar] [CrossRef]
- Slominski, A.; Kim, T.-K.; Brożyna, A.A.; Janjetovic, Z.; Brooks, D.L.P.; Schwab, L.P.; Skobowiat, C.; Jóźwicki, W.; Seagroves, T.N. The role of melanogenesis in regulation of melanoma behavior: Melanogenesis leads to stimulation of HIF-1a expression and HIF-dependent attendant pathways. Arch. Biochem. Biophys. 2014, 563, 79–93. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, W.N.; Rosli, L.M.B.M.; Doolaanea, A.A. Formulation, Cellular Uptake and Cytotoxicity of Thymoquinone-Loaded PLGA Nanoparticles in Malignant Melanoma Cancer Cells. Int. J. Nanomed. 2020, 15, 8059–8074. [Google Scholar] [CrossRef]
- Eo, S.-H.; Yu, S.-M.; Han, Y.; Han, J.; Kim, S.M.; Kim, D.-B.; Jeon, B.K.; Lee, W.K.; Kim, S.J. Effects of Thymoquinone and Iksan 526 callus Extract on B16F10 and A375 Cell Lines. Int. J. Pharmacol. 2020, 16, 479–491. [Google Scholar] [CrossRef]
- Khaleel, C.; Tabanca, N.; Buchbauer, G. α-Terpineol, a Natural Monoterpene: A Review of Its Biological Properties. Open Chem. 2018, 16, 349–361. [Google Scholar] [CrossRef]
- Bisht, A.S.; Bahukhandi, A.; Rana, M.; Rana, A.J.; Kumar, A. Chapter 3.2.12-Acorus Calamus. In Naturally Occurring Chemicals Against Alzheimer’s Disease; Belwal, T., Nabavi, S.M., Nabavi, S.F., Dehpour, A.R., Shirooie, S., Eds.; Academic Press: New York, NY, USA, 2021; pp. 337–349. [Google Scholar] [CrossRef]
- Aluyor, E.O.; Oboh, I.O. PRESERVATIVES | Traditional Preservatives–Vegetable Oils. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Academic Press: Oxford, UK, 2014; pp. 137–140. [Google Scholar] [CrossRef]
- Negreiros, H.A.; de Moura, K.G.; Barreto do Nascimento, M.L.L.; do Nascimento Rodrigues, D.C.; Ferreir, P.M.P.; Braz, D.C.; de Farias, M.G.; de Sousa Corrêia, L.; Pereira, A.R.S.; Santos, L.K.B. Alpha-Terpineol as Antitumor Candidate in Pre-Clinical Studies. Anticancer Agents Med. Chem. 2021, 21, 2023–2031. [Google Scholar] [CrossRef]
- Shapira, S.; Pleban, S.; Kazanov, D.; Tirosh, P.; Arber, N. Terpinen-4-Ol: A Novel and Promising Therapeutic Agent for Human Gastrointestinal Cancers. PLoS ONE 2016, 11, e0156540. [Google Scholar] [CrossRef] [Green Version]
- Batista, F.A.; Fontele, S.B.C.; Santos, L.K.B.; Filgueiras, L.A.; Nascimento, S.Q.; e Sousa, J.M.D.C.; Gonçalves, J.C.R.; Mendes, A.N. Synthesis, Characterization of α-Terpineol-Loaded PMMA Nanoparticles as Proposed of Therapy for Melanoma. Mater. Today Commun. 2020, 22, 100762. [Google Scholar] [CrossRef]
- Di Martile, M.; Garzoli, S.; Sabatino, M.; Valentini, E.; D’Aguanno, S.; Ragno, R.; Del Bufalo, D. Antitumor Effect of Melaleuca Alternifolia Essential Oil and Its Main Component Terpinen-4-Ol in Combination with Target Therapy in Melanoma Models. Cell Death Discov. 2021, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, Q.; Xia, L.; Li, X.; Sun, C.; Wang, Q.; Cai, X.; Yang, G. Borneol Promotes Apoptosis of Human Glioma Cells through Regulating HIF-1a Expression via MTORC1/EIF4E Pathway. J. Cancer 2020, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zhao, X.; Hu, K. 9-Physically Open BBB. In Brain Targeted Drug Delivery System; Gao, H., Gao, X., Eds.; Academic Press: New York, NY, USA, 2019; pp. 197–217. [Google Scholar] [CrossRef]
- Meng, X.; Dong, X.; Wang, W.; Yang, L.; Zhang, X.; Li, Y.; Chen, T.; Ma, H.; Qi, D.; Su, J. Natural Borneol Enhances Paclitaxel-Induced Apoptosis of ESCC Cells by Inactivation of the PI3K/AKT. J. Food Sci. 2018, 83, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Pei, W.; Zhao, J.; Cheng, Y.; Zheng, X.; Rong, J. Bornyl Caffeate Induces Apoptosis in Human Breast Cancer MCF-7 Cells via the ROS- and JNK-Mediated Pathways. Acta Pharmacol. Sin. 2014, 35, 113–123. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Li, L.; Su, J.; Li, B.; Chen, T.; Wong, J.-S. Synergistic Apoptosis-Inducing Effects on A375 Human Melanoma Cells of Natural Borneol and Curcumin. PLoS ONE 2014, 9, e101277. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Xie, Q.; Ma, R.; Li, Y.; Yuan, J.; Ren, M.; Li, H.; Wang, J.; Lu, D.; Xu, Z.; et al. Recent progress on the synergistic antitumor effect of a borneol-modified nanocarrier drug delivery system. Front Med. 2021, 8, 750170. [Google Scholar] [CrossRef]
- Lu, Y.; Du, S.Y.; Chen, X.L.; Wu, Q.; Song, X.; Xu, B.; Zhai, Y.S. Enhancing effect of natural borneol on the absorption of geniposide in rat via intranasal administration. J. Zhejiang Univ. Sci. B 2011, 12, 143–148. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.-Y.; Wu, Y.-J.; Chang, C.-I.; Chiu, C.-C.; Wu, M.-L. The Effect of Bornyl Cis-4-Hydroxycinnamate on Melanoma Cell Apoptosis Is Associated with Mitochondrial Dysfunction and Endoplasmic Reticulum Stress. Int. J. Mol. Sci. 2018, 19, 1370. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.-Y.; Wu, M.-L.; Chang, C.-I.; Liu, C.-I.; Cheng, T.-C.; Wu, Y.-J. Bornyl Cis-4-Hydroxycinnamate Suppresses Cell Metastasis of Melanoma through FAK/PI3K/Akt/MTOR and MAPK Signaling Pathways and Inhibition of the Epithelial-to-Mesenchymal Transition. Int. J. Mol. Sci. 2018, 19, 2152. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.-J.; Su, T.-R.; Chang, C.-I.; Chen, C.-R.; Hung, K.-F.; Liu, C. (+)-Bornyl p-Coumarate Extracted from Stem of Piper betle Induced Apoptosis and Autophagy in Melanoma Cells. Int. J. Mol. Sci. 2020, 21, 3737. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.B.; Marcu, J. Chapter Three-Cannabis Pharmacology: The Usual Suspects and a Few Promising Leads. In Advances in Pharmacology; Kendall, D., Alexander, S.P.H., Eds.; Cannabinoid Pharmacology; Academic Press: New York, NY, USA, 2017; Volume 80, pp. 67–134. [Google Scholar] [CrossRef]
- Upadhyay, S.; Bisht, K.; Bahukhandi, A.; Bisht, M.; Mehta, P.; Bisht, A. Chapter 3.2.6-Rosmarinus officinalis L. In Naturally Occurring Chemicals Against Alzheimer’s Disease; Belwal, T., Nabavi, S.M., Nabavi, S.F., Dehpour, A.R., Shirooie, S., Eds.; Academic Press: New York, NY, USA, 2021; pp. 271–281. [Google Scholar] [CrossRef]
- Kuete, V. Chapter 23-Myristica Fragrans: A Review. In Medicinal Spices and Vegetables from Africa; Kuete, V., Ed.; Academic Press: New York, NY, USA, 2017; pp. 497–512. [Google Scholar] [CrossRef]
- Girola, N.; Figueiredo, C.R.; Farias, C.F.; Azevedo, R.A.; Ferreira, A.K.; Teixeira, S.F.; Capello, T.M.; Martins, E.G.A.; Matsuo, A.L.; Travassos, L.R.; et al. Camphene Isolated from Essential Oil of Piper Cernuum (Piperaceae) Induces Intrinsic Apoptosis in Melanoma Cells and Displays Antitumor Activity in Vivo. Biochem. Biophys. Res. Commun. 2015, 467, 928–934. [Google Scholar] [CrossRef]
- Skaria, B.P.; Joy, P.P.; Mathew, S.; Mathew, G. 24-Lemongrass. In Handbook of Herbs and Spices; Peter, K.V., Ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2006; pp. 400–419. [Google Scholar] [CrossRef]
- Kittler, J.; Krüger, H.; Ulrich, D.; Zeiger, B.; Schütze, W.; Böttcher, C.; Krähmer, A.; Gudi, G.; Kästner, U.; Heuberger, H.; et al. Content and Composition of Essential Oil and Content of Rosmarinic Acid in Lemon Balm and Balm Genotypes (Melissa Officinalis). Genet. Resour. Crop. Evol. 2018, 65, 1517–1527. [Google Scholar] [CrossRef] [Green Version]
- De Martino, L.; D’Arena, G.; Minervini, M.M.; Deaglio, S.; Fusco, B.M.; Cascavilla, N.; De Feo, V. Verbena Officinalis Essential Oil and its Component Citral as Apoptotic-Inducing Agent in Chronic Lymphocytic Leukemia. Int. J. Immunopathol. Pharmacol. 2009, 22, 1097–1104. [Google Scholar] [CrossRef]
- Shi, C.; Song, K.; Zhang, X.; Sun, Y.; Sui, Y.; Chen, Y.; Jia, Z.; Sun, H.; Sun, Z.; Xia, X. Antimicrobial Activity and Possible Mechanism of Action of Citral against Cronobacter Sakazakii. PLoS ONE 2016, 11, e0159006. [Google Scholar] [CrossRef] [Green Version]
- Santoro, G.F.; Cardoso, M.G.; Guimarães, L.G.L.; Freire, J.M.; Soares, M.J. Anti-Proliferative Effect of the Essential Oil of Cymbopogon Citratus (DC) Stapf (Lemongrass) on Intracellular Amastigotes, Bloodstream Trypomastigotes and Culture Epimastigotes of Trypanosoma Cruzi (Protozoa: Kinetoplastida). Parasitology 2007, 134 Pt 11, 1649–1656. [Google Scholar] [CrossRef]
- Nordin, N.; Yeap, S.K.; Rahman, H.S.; Zamberi, N.R.; Abu, N.; Mohamad, N.E.; How, C.W.; Masarudin, M.J.; Abdullah, R.; Alitheen, N.B. In Vitro Cytotoxicity and Anticancer Effects of Citral Nanostructured Lipid Carrier on MDA MBA-231 Human Breast Cancer Cells. Sci. Rep. 2019, 9, 1614. [Google Scholar] [CrossRef] [Green Version]
- White, B.; Evison, A.; Dombi, E.; Townley, H.E. Improved delivery of the anticancer agent citral using BSA nanoparticles and polymeric wafers. Nanotechnol. Sci. Appl. 2017, 10, 163–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanches, L.J.; Marinello, P.C.; Panis, C.; Fagundes, T.R.; Morgado-Díaz, J.A.; de-Freitas-Junior, J.C.M.; Cecchini, R.; Cecchini, A.L.; Luiz, R.C. Cytotoxicity of citral against melanoma cells: The involvement of oxidative stress generation and cell growth protein reduction. Tumor Biol. 2017, 39, 1010428317695914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agus, H.H. Chapter 4-Terpene Toxicity and Oxidative Stress. In Toxicology; Patel, V.B., Preedy, V.R., Eds.; Academic Press: New York, NY, USA, 2021; pp. 33–42. [Google Scholar] [CrossRef]
- Richter, G.; Hazzah, T.; Hartsel, J.A.; Eades, J.; Hickory, B.; Makriyannis, A. Chapter 38-Cannabis Sativa: An Overview. In Nutraceuticals, 2nd ed.; Gupta, R.C., Lall, R., Srivastava, A., Eds.; Academic Press: New York, NY, USA, 2021; pp. 603–624. [Google Scholar] [CrossRef]
- Alipanah, H.; Farjam, M.; Zarenezhad, E.; Roozitalab, G.; Osanloo, M. Chitosan Nanoparticles Containing Limonene and Limonene-Rich Essential Oils: Potential Phytotherapy Agents for the Treatment of Melanoma and Breast Cancers. BMC Complement. Med. Ther. 2021, 21, 186. [Google Scholar] [CrossRef]
- Ahmed, A.; Choudhary, M.I.; Farooq, A.; Demirci, B.; Demirci, F.; Can Başer, K.H. Essential Oil Constituents of the Spice Cinnamomum Tamala (Ham.) Nees & Eberm. Flavour Fragr. J. 2000, 15, 388–390. [Google Scholar] [CrossRef]
- Han, H.D.; Cho, Y.-J.; Cho, S.K.; Byeon, Y.; Jeon, H.N.; Kim, H.-S.; Kim, B.-G.; Bae, D.-S.; Lopez-Berestein, G.; Sood, A.K.; et al. Linalool-Incorporated Nanoparticles as a Novel Anticancer Agent for Epithelial Ovarian Carcinoma. Mol. Cancer Ther. 2016, 15, 618–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Usta, J.; Kreydiyyeh, S.; Knio, K.; Barnabe, P.; Bou-Moughlabay, Y.; Dagher, S. Linalool Decreases HepG2 Viability by Inhibiting Mitochondrial Complexes I and II, Increasing Reactive Oxygen Species and Decreasing ATP and GSH Levels. Chem. Biol. Interact. 2009, 180, 39–46. [Google Scholar] [CrossRef]
- Cerchiara, T.; Straface, S.V.; Brunelli, E.; Tripepi, S.; Gallucci, M.C.; Chidichimo, G. Antiproliferative Effect of Linalool on RPMI 7932 Human Melanoma Cell Line: Ultrastructural Studies. Nat. Prod. Commun. 2015, 10, 547–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, Z.; Yao, C.; Zhu, J.; Xie, Y.; Ye, X.-Y.; Bai, R.; Xie, T. Anti-tumor drug discovery based on natural product b-elemene: Anti-tumor mechanisms and structural modification. Molecules 2021, 26, 1499. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, R.; Yang, Q.; Zhang, J.; Zhao, Y.; Zheng, Y.; Yang, J. Chapter One-Recent Advances in the Biosynthesis of Isoprenoids in Engineered Saccharomyces Cerevisiae. In Advances in Applied Microbiology; Gadd, G.M., Sariaslani, S., Eds.; Academic Press: New York, NY, USA, 2021; Volume 114, pp. 1–35. [Google Scholar] [CrossRef]
- Wiart, C. Chapter 2-Terpenes. In Lead Compounds from Medicinal Plants for the Treatment of Cancer; Wiart, C., Ed.; Academic Press: New York, NY, USA, 2013; pp. 97–265. [Google Scholar] [CrossRef]
- Balavandi, Z.; Neshasteh-Riz, A.; Koosha, F.; Eynali, S.; Hoormand, M.; Shahidi, M. The Use of β-Elemene to Enhance Radio Sensitization of A375 Human Melanoma Cells. Cell J. 2019, 21, 419. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Liu, L.; Liu, L.; Geng, J.; Zhou, Y.; Chen, L. β-Elemene inhibits the metastasis of B16F10 melanoma cells by downregulation of the expression of uPA, uPAR, MMP-2, and MMP-9. Melanoma Res. 2014, 24, 99–107. [Google Scholar] [CrossRef]
- Chaturvedi, D.; Dwivedi, P.K. Chapter 6-Recent Developments on the Antidiabetic Sesquiterpene Lactones and Their Semisynthetic Analogues. In Discovery and Development of Antidiabetic Agents from Natural Products; Brahmachari, G., Ed.; Natural Product Drug Discovery; Elsevier: Amsterdam, The Netherlands, 2017; pp. 185–207. [Google Scholar] [CrossRef]
- Hartsel, J.A.; Eades, J.; Hickory, B.; Makriyannis, A. Chapter 53-Cannabis Sativa and Hemp. In Nutraceuticals; Gupta, R.C., Ed.; Academic Press: Boston, MA, USA, 2016; pp. 735–754. [Google Scholar] [CrossRef]
- Jung, J.I.; Kim, E.J.; Kwon, G.T.; Jung, Y.J.; Park, T.; Kim, Y.; Yu, R.; Choi, M.-S.; Chun, H.S.; Kwon, S.-H.; et al. β-Caryophyllene Potently Inhibits Solid Tumor Growth and Lymph Node Metastasis of B16F10 Melanoma Cells in High-Fat Diet–Induced Obese C57BL/6N Mice. Carcinogenesis 2015, 36, 1028–1039. [Google Scholar] [CrossRef] [Green Version]
- Merfort, I. Perspectives on Sesquiterpene Lactones in Inflammation and Cancer. Curr. Drug Targets 2011, 12, 1560–1573. [Google Scholar] [CrossRef]
- Estévez-Sarmiento, F.; Saavedra, E.; Ruiz-Estévez, M.; León, F.; Quintana, J.; Brouard, I.; Estévez, F. Chlorinated Guaiane-Type Sesquiterpene Lactones as Cytotoxic Agents against Human Tumor Cells. Int. J. Mol. Sci. 2020, 21, 9767. [Google Scholar] [CrossRef]
- Ita, K. Chapter 9-Iontophoresis, Magnetophoresis, and Electroporation. In Transdermal Drug Delivery; Ita, K., Ed.; Academic Press: New York, NY, USA, 2020; pp. 183–229. [Google Scholar] [CrossRef]
- Brahmachari, G. Andrographolide: A Molecule of Antidiabetic Promise. In Natural Product Drug Discovery, Discovery and Development of Antidiabetic Agents from Natural Products; Brahmachari, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–27. [Google Scholar] [CrossRef]
- Jada, S.R.; Hamzah, A.S.; Lajis, N.H.; Saad, M.S.; Stevens, M.F.G.; Stanslas, J. Semisynthesis and cytotoxic activities of andrographolide analogues. J. Enzyme Inhib. Med. Chem. 2006, 21, 145–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.-Q.; Zhou, D.-L.; Ding, Y.; Liu, H.-Y.; Lei, Y.; Fang, H.-Y.; Gu, Q.-L.; He, X.-D.; Qi, C.-L.; Yang, Y.; et al. Andrographolide Inhibits Melanoma Tumor Growth by Inactivating the TLR4/NF-ΚB Signaling Pathway. Melanoma Res. 2014, 24, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Chu, H. Andrographolide Inhibits Proliferation and Induces Cell Cycle Arrest and Apoptosis in Human Melanoma Cells. Oncol. Lett. 2018, 15, 5301–5305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birtić, S.; Dussort, P.; Pierre, F.-X.; Bily, A.C.; Roller, M. Carnosic acid. Phytochemistry 2015, 115, 9–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.Y.; Song, H.; Sung, M.-K.; Kang, Y.-H.; Lee, K.W.; Park, J.H.Y. Carnosic Acid Inhibits the Epithelial-Mesenchymal Transition in B16F10 Melanoma Cells: A Possible Mechanism for the Inhibition of Cell Migration. Int. J. Mol. Sci. 2014, 15, 12698–12713. [Google Scholar] [CrossRef] [Green Version]
- Shuttleworth, S.; Townsend, P.; Silva, F.; Cecil, A.; Hill, T.; Tomassi, C.; Rogers, H.; Harrison, R. Progress in the Development of Small Molecule Therapeutics Targeting Th17 Cell Function for the Treatment of Immune-Inflammatory Diseases. In Progress in Medicinal Chemistry; Lawton, G., Witty, D.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 50, pp. 109–133. [Google Scholar] [CrossRef]
- Deng, Y.; Li, F.; He, P.; Yang, Y.; Yang, J.; Zhang, Y.; Liu, J.; Tong, Y.; Li, Q.; Mei, X.; et al. Triptolide Sensitizes Breast Cancer Cells to Doxorubicin through the DNA Damage Response Inhibition. Mol. Carcinog. 2018, 57, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.; Beyer, G.; Chugh, R.; Skube, S.J.; Majumder, K.; Banerjee, S.; Sangwan, V.; Li, L.; Dawra, R.; Subramanian, S.; et al. Triptolide Abrogates Growth of Colon Cancer and Induces Cell Cycle Arrest by Inhibiting Transcriptional Activation of E2F. Lab. Investig. J. Tech. Methods Pathol. 2015, 95, 648–659. [Google Scholar] [CrossRef] [Green Version]
- Tao, Y.; Zhang, M.-L.; Ma, P.-C.; Sun, J.-F.; Zhou, W.-Q.; Cao, Y.-P.; Li, L.-J. Triptolide Inhibits Proliferation and Induces Apoptosis of Human Melanoma A375 Cells. Asian Pac. J. Cancer Prev. 2012, 13, 1611–1615. [Google Scholar] [CrossRef] [Green Version]
- Jao, H.-Y.; Yu, F.-S.; Yu, C.-S.; Chang, S.-J.; Liu, K.-C.; Liao, C.-L.; Ji, B.-C.; Bau, D.-T.; Chung, J.-G. Suppression of the Migration and Invasion Is Mediated by Triptolide in B16F10 Mouse Melanoma Cells through the NF-KappaB-Dependent Pathway. Environ. Toxicol. 2016, 31, 1974–1984. [Google Scholar] [CrossRef]
- Zarei, S.M.; Ayatollahi, A.M.; Ghanadian, M.; Kobarfard, F.; Aghaei, M.; Choudhary, M.I.; Fallahian, F. Unusual Ingenoids from Euphorbia Erythradenia Bioss. with pro-Apoptotic Effects. Fitoterapia 2013, 91, 87–94. [Google Scholar] [CrossRef]
- Fallahian, F.; Ghanadian, M.; Aghaei, M.; Zarei, S.M. Induction of G2/M Phase Arrest and Apoptosis by a New Tetrahydroingenol Diterpenoid from Euphorbia Erythradenia Bioss. in Melanoma Cancer Cells. Biomed. Pharmacother. 2017, 86, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Shivashankara, A.R.; Venkatesh, S.; Bhat, H.P.; Palatty, P.L.; Baliga, M.S. Chapter 17-Can Phytochemicals Be Effective in Preventing Ethanol-Induced Hepatotoxicity in the Geriatric Population? An Evidence-Based Revisit. In Foods and Dietary Supplements in the Prevention and Treatment of Disease in Older Adults; Watson, R.R., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 163–170. [Google Scholar] [CrossRef]
- Baliga, M.S.; Shivashankara, A.R.; Venkatesh, S.; Bhat, H.P.; Palatty, P.L.; Bhandari, G.; Rao, S. Chapter 7-Phytochemicals in the Prevention of Ethanol-Induced Hepatotoxicity: A Revisit. In Dietary Interventions in Liver Disease; Watson, R.R., Preedy, V.R., Eds.; Academic Press: New York, NY, USA, 2019; pp. 79–89. [Google Scholar] [CrossRef]
- Calvani, R.; Landi, F.; Collamati, A.; Serafini, E.; Bernabei, R.; Marzetti, E. Chapter 22-Nutritional Strategies Against Sarcopenia of Aging: Current Evidence and Future Directions. In Foods and Dietary Supplements in the Prevention and Treatment of Disease in Older Adults; Watson, R.R., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 231–238. [Google Scholar] [CrossRef]
- Madkour, L.H. Chapter 12-Endoplasmic Reticulum Stress-Induced Cell Death Mechanism. In Reactive Oxygen Species (ROS), Nanoparticles, and Endoplasmic Reticulum (ER) Stress-Induced Cell Death Mechanisms; Madkour, L.H., Ed.; Academic Press: New York, NY, USA, 2020; pp. 299–342. [Google Scholar] [CrossRef]
- Mazumder, K.; Tanaka, K.; Fukase, K. Cytotoxic activity of ursolic acid derivatives obtained by isolation and oxidative derivatization. Molecules 2013, 18, 8929–8944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, N.C.; Demuner, A.J.; dos Santos, M.H.; Maltha, C.R.A.; de Alvarenga, E.A.; Komarnytsky, S. Metals complexes formed with oleanolic acid. Int. J. Org. Chem. 2018, 8, 160. [Google Scholar] [CrossRef] [Green Version]
- Khusnutdinova, E.; Petrova, A.; Zileeva, Z.; Kuzmina, U.; Zainullina, L.; Vakhitova, Y.; Babkov, D.; Kazakova, O. Novel a-ring chalcone derivatives of oleanolic and ursolic amides with anti-proliferative effect mediated through ROS-triggered apoptosis. Int. J. Mol. Sci. 2021, 22, 9796. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-H.; Wang, E.; Kumar, N.; Glickman, R.D. Ursolic Acid Differentially Modulates Apoptosis in Skin Melanoma and Retinal Pigment Epithelial Cells Exposed to UV–VIS Broadband Radiation. Apoptosis 2014, 19, 816–828. [Google Scholar] [CrossRef]
- Caunii, A.; Oprean, C.; Cristea, M.; Ivan, A.; Danciu, C.; Tatu, C.; Paunescu, V.; Marti, D.; Tzanakakis, G.; Spandidos, D.A.; et al. Effects of Ursolic and Oleanolic on SK-MEL-2 Melanoma Cells: In Vitro and in Vivo Assays. Int. J. Oncol. 2017, 51, 1651–1660. [Google Scholar] [CrossRef] [Green Version]
- Díaz, M.T.B.; Font, R.; Gómez, P.; Río Celestino, M.D. Chapter 14-Summer Squash. In Nutritional Composition and Antioxidant Properties of Fruits and Vegetables; Jaiswal, A.K., Ed.; Academic Press: New York, NY, USA, 2020; pp. 239–254. [Google Scholar] [CrossRef]
- Jing, S.; Zou, H.; Wu, Z.; Ren, L.; Zhang, T.; Zhang, J.; Wei, Z. Cucurbitacins: Bioactivities and synergistic effect with small-molecule drugs. J. Funct. Foods 2020, 72, 104042. [Google Scholar] [CrossRef]
- Wu, D.; Wang, Z.; Lin, M.; Shang, Y.; Wang, F.; Zhou, J.; Wang, F.; Zhang, X.; Luo, X.; Huang, W. In Vitro and In Vivo Antitumor Activity of Cucurbitacin C, a Novel Natural Product from Cucumber. Front. Pharmacol. 2019, 10, 1287. [Google Scholar] [CrossRef] [Green Version]
- Sikander, M.; Hafeez, B.B.; Malik, S.; Alsayari, A.; Halaweish, F.T.; Yallapu, M.M.; Chauhan, S.C.; Jaggi, M. Cucurbitacin D Exhibits Potent Anti-Cancer Activity in Cervical Cancer. Sci. Rep. 2016, 6, 36594. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Halaweish, F.T. Cucurbitacins: Potential Candidates Targeting Mitogen-Activated Protein Kinase Pathway for Treatment of Melanoma. J. Enzyme Inhib. Med. Chem. 2014, 29, 162–167. [Google Scholar] [CrossRef]
- Fulda, S. Betulinic Acid for Cancer Treatment and Prevention. Int. J. Mol. Sci. 2008, 9, 1096–1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coricovac, D.; Dehelean, C.A.; Pinzaru, I.; Mioc, A.; Aburel, O.-M.; Macasoi, I.; Draghici, G.A.; Petean, C.; Soica, C.; Boruga, M.; et al. Assessment of Betulinic Acid Cytotoxicity and Mitochondrial Metabolism Impairment in a Human Melanoma Cell Line. Int. J. Mol. Sci. 2021, 22, 4870. [Google Scholar] [CrossRef] [PubMed]



| Studied Effect | Terpenoid/Cell Line or Melanoma In Vivo Model | Reference |
|---|---|---|
| Dose-dependent cytotoxicity | TQ (B16F10); TQ-PLGA NPs (A375); TQ + Iksan526 (A375, B16F10); α-terpineol in PMMA nanoparticles (B16F10, SK-MEL-28); terpinene-4-ol alone, or in combination with dabrafenib, or trametinib (M14, A375); NB + curcumin (A375); bornyl cis-4-hydroxycinnamate (A2058, A375); bornyl p-coumarate (A2058. A375); camphene (B16F10-Nex2); citral (B16F10, SK-MEL-147, UACC-257); limonene, limonene-containing nanoparticles, limonene-enriched EOs, and their NFs (A375); linalool (RPMI 7932); β-elemene alone, or in combination with X-ray (A375); β-elemene (B16F10); chlorinated guaianolides (SK-MEL-1); Andro (B16, A375, C8161); triptolide (B16F10); DANPT (A375, HMCB); UA alone, or in combination with UVR (CRL-11147); UA and OA (SK-MEL-2); Cucs (SK-MEL-28, A375); BA (A375) | [57,59,61,62,68,69,74,77,78,79,83,91,94,98,102,103,108,113,120,122,130,131,136,138] |
| DNA damage | TQ (B16F10) | [57] |
| morphological features of apoptosis/phosphatidylserine translocation | TQ (B16F10); NB + curcumin (A375); bornyl cis-4-hydroxycinnamate (A2058, A375); bornyl p-coumarate (A2058. A375); camphene (B16F10-Nex2), citral (B16F10); linalool (RPMI 7932); β-elemene (A375); Andro (B16); DANPT (A375, HMCB); UA alone, or in combination with UVR (CRL-11147); BA (A375) | [57,58,74,77,78,79,83,91,98,102,112,122,130,138] |
| increased ROS generation/oxidative stress | TQ (B16F10); NB + curcumin (A375); citral (B16F10), DANPT (A375, HMCB); UA + UVR (CRL-11147) | [57,74,91,122,130] |
| mitochondria loss of function/loss of ∆ψ m | bornyl cis-4-hydroxycinnamate (A2058, A375); bornyl p-coumarate (A2058, A375); camphene (B16F10-Nex2); UA + UVR (CRL-11147); BA (A375) | [77,79,83,130,138] |
| decrease in OCR | BA (A375) | [138] |
| decrease in ECAR | BA (A375) | [138] |
| decrease in OXPHOS | BA (A375) | [138] |
| decrease in maximal respiratory capacity of ETS | BA (A375) | [138] |
| ER stress | bornyl cis-4-hydroxycinnamate (A2058, A375); bornyl p-coumarate (A2058, A375); camphene (B16F10-Nex2) | [77,79,83] |
| cell cycle arrest | BCP {HFD-induced obese C57BL/6J mice with B16F10}; Andro (C8161, A375, B16); Andro (A375); DANPT (A375, HMCB); UA + UVR (CRL-11147); UA (SK-MEL-2) | [106,112,113,122,130,131] |
| necrosis | citral (B16F10) | [91] |
| autophagy | bornyl p-coumarate (A2058, A375); citral (B16F10) | [79,91] |
| increased caspases/cleaved caspases 3/8/9 | TQ (B16F10); terpinene-4-ol alone, or in combination with dabrafenib, or trametinib (M14, A375); NB + curcumin (A375); bornyl cis-4-hydroxycinnamate (A2058, A375); bornyl p-coumarate (A2058, A375); camphene (B16F10-Nex2); linalool (RPMI 7932); Andro (A375) | [57,69,74,77,79,83,98,113] |
| increased PARP/cleaved PARP | terpinene-4-ol alone, or in combination with dabrafenib, or trametinib (M14, A375); NB + curcumin (A375); bornyl cis-4-hydroxycinnamate (A2058, A375); bornyl p-coumarate (A2058, A375); Andro (A375) | [56,74,77,79,113] |
| decreased anti-apoptotic proteins/genes (Blc-2, Bcl-xl, Mcl-1) | TQ (B16F10); bornyl cis-4-hydroxycinnamate (A2058, A375) | [57,77] |
| increased proapoptotic proteins/genes (Bax, Bad, Bak) | TQ (B16F10); bornyl cis-4-hydroxycinnamate (A2058, A375); BA (A375) | [57,77,138] |
| decreased survivin | TQ (B16F10) | [57] |
| increased cytosolic cytochrome c | bornyl cis-4-hydroxycinnamate (A2058, A375) | [77] |
| increased p53 | NB + curcumin (A375); DANPT (A375, HMCB) | [74,122] |
| decreased regulators and participants of melanogenesis (MITF, tyrosinase) | TQ + Iksan526 (A375, B16F10) | [62] |
| decreased tyrosinase activity | TQ (B16F10) | [59] |
| decreased COX-2 | TQ + Iksan526 (A375, B16F10) | [62] |
| decreased expression of NF-κB/lack of its nuclear translocation and DNA binding | citral (B16F10); triptolide (B16F10) | [91,120] |
| blocking of JAK2/STAT3 | TQ (B16F10) | [57] |
| ERK 1/2 pathway inhibition | NB + curcumin (A375); citral (B16F10); triptolide (B16F10); Cucs (A375) | [74,82,120,136] |
| decreased expression of FAK/PI3K/Akt/mTOR pathway proteins, or their phosphorylated forms | NB + curcumin (A375); bornyl cis-4-hydroxycinnamate (A2058, A375); citral (B16F10); CA (B16F10); triptolide (B16F10) | [74,78,91,115,120] |
| increased p-JNK | NB + curcumin (A375); Andro (A375) | [74,113] |
| reduced p-JNK | triptolide (B16F10) | [120] |
| increased p-p38 | Andro (A375) | [113] |
| decreased p-Src | CA (B16F10) | [115] |
| decreased β-catenin | TQ (B16F10); CA (B16F10) | [59,115] |
| decreased GRB2 pathway members | bornyl cis-4-hydroxycinnamate (A2058, A375); triptolide (B16F10) | [78,120] |
| decreased CXCR4 | triptolide (B16F10) | [120] |
| decreased SOS 1 | triptolide (B16F10) | [120] |
| decreased Rho A | triptolide (B16F10) | [120] |
| decreased Rock-1 | triptolide (B16F10) | [120] |
| decreased NO | citral (B16F10) | [91] |
| increased p-Brca1 and p-ATM | NB + curcumin (A375) | [74] |
| decreased expression of angiogenesis-related proteins (MCP-1, TGF-β1, RANTES) | TQ (B16F10); BCP (B16F10) | [59,106] |
| inhibition of angiogenesis | BCP {HFD-induced obese C57BL/6J mice with B16F10}; OA {chicken CAM} | [106,131] |
| decreased lymphangiogenesis | BCP {HFD-induced obese C57BL/6J mice with B16F10} | [106] |
| decreased M-CSF | BCP (B16F10) | [106] |
| decreased TLR 4 pathway components | Andro (B16); Andro {C57BL/6J mice with B16 melanoma subcutaneous model} | [112] |
| decreased cell-matrix adhesion | triptolide (B16F10); UA (SK-MEL-2) | [120,131] |
| decreased expression/secretion of uPA and uPA receptor | β-elemene (B16F10); CA (B16F10) | [103,115] |
| reduced CCL19 and CCL21 in LN | BCP {HFD-induced obese C57BL/6J mice with B16F10} | [106] |
| reduced CCR7 in tumour | BCP {HFD-induced obese C57BL/6J mice with B16F10} | [106] |
| increased TIMP-1 | CA (B16F10) | [115] |
| decreased EMT/EMT-associated proteins (vimentin, N-cadherin, Snail, Slug) | bornyl cis-4-hydroxycinnamate (A2058, A375); CA (B16F10) | [78,115] |
| increased E-cadherin | bornyl cis-4-hydroxycinnamate (A2058, A375) | [78] |
| decreased activity/expression/secretion of MMP-2/MMP-9 | bornyl cis-4-hydroxycinnamate (A2058, A375); β-elemene (B16F10); CA (B16F10); triptolide (B16F10) | [78,103,115,120] |
| decreased cell migration/invasion | bornyl cis-4-hydroxycinnamate (A2058, A375); β-elemene (B16F10); CA (B16F10); triptolide (B16F10); OA (SK-MEL-2) | [78,103,115,120,131] |
| reduced LN metastasis | BCP {HFD-induced obese C57BL/6J mice with B16F10} | [106] |
| increased median overall survival time of tumour-bearing mice | TQ {C57BL/6J mice with B16F10}; TQ + gamma knife {C57BL/6J mice with B16F10} | [58,59] |
| reduction in tumour growth | camphene {C57BL/6J mice with B16F10}; BCP {HFD-induced obese C57BL/6J mice with B16F10}; Andro {C57BL/6J mice with B16 melanoma subcutaneous model} | [83,106,112] |
| changes in tumour microenvironment and LN-surrounding adipose tissue | BCP {HFD-induced obese C57BL/6J mice with B16F10} | [106] |
| decreased number and size of metastatic foci | Andro {C57BL/6J mice with B16 melanoma lung metastasis model} | [112] |
| radio-sensitization | β-elemene (B16F10) | [102] |
| phototoxicity | UA + UVR (CRL-11147) | [130] |
| synergistic action with chemotherapeutics | terpinene-4-ol in combination with dabrafenib, or trametinib (M14, A375) | [69] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kłos, P.; Chlubek, D. Plant-Derived Terpenoids: A Promising Tool in the Fight against Melanoma. Cancers 2022, 14, 502. https://doi.org/10.3390/cancers14030502
Kłos P, Chlubek D. Plant-Derived Terpenoids: A Promising Tool in the Fight against Melanoma. Cancers. 2022; 14(3):502. https://doi.org/10.3390/cancers14030502
Chicago/Turabian StyleKłos, Patrycja, and Dariusz Chlubek. 2022. "Plant-Derived Terpenoids: A Promising Tool in the Fight against Melanoma" Cancers 14, no. 3: 502. https://doi.org/10.3390/cancers14030502
APA StyleKłos, P., & Chlubek, D. (2022). Plant-Derived Terpenoids: A Promising Tool in the Fight against Melanoma. Cancers, 14(3), 502. https://doi.org/10.3390/cancers14030502