Heterogeneous Heat Absorption Is Complementary to Radiotherapy
Abstract
:Simple Summary
Abstract
1. Introduction
- External radiation focused on the target, trying to heat the tumor mass as homogeneously as possible without considerably heating surroundings tissues. The heating intention is isothermal, but due to the heterogeneity of the target and the heat distribution dynamics controlled by blood flow, the temperature is not homogeneous (see later). The intensive heating of a larger volume (regional heating) achieves an approximately controllable condition in the tumor at the central position. The treatment evaluation involves the ratio of the isothermal areas. The specific power density (SAR) ranges from 4.6 to 89 W/kg [3], depending on the location and size of the tumor, determining the heated volume and its blood flow.
- Heating good energy absorbers in a localized area by electromagnetic effects, which heats these materials extensively, and in the next step, the absorbers heat up their host tissues. The heating intention is heterogeneous, targets only the dedicated particles (like nanoparticles, seeds, rods, etc.). The dose homogeneity characterizes this method because of the dispersed absorbers. The particles heat up their environment by heat-conduction, realizing more localized heating in the volume. The SAR in nanoparticle methods is surprisingly large because the absorbers have only a tiny mass compared to the surrounding tissue. The small mass (ranging density of 1 specifically absorbs extra-large SAR >> 1 W/g = 1 kW/kg or higher [4], because of the absorption on the tiny target. When it heats the neighboring tissues, the average SAR corresponds to the isothermal heating conditions in the range of about a few W/kg. Targeting various chemical bonds uses even higher SAR because the absorbing mass is lighter than the metallic nanoparticle. These methods focus on molecular changes. The temperature is a possible cofactor.
1.1. Heating Challenge
1.2. Complementary Challenge
1.3. Dosing Challenge
- The invasive temperature sensors available are point detectors. When the point is near the arteries of a highly vascularized area, the temperature is less than in the low vascularization part, so many independent sensors are necessary to attain objective results. However, this induces safety and treatment problems.
- Usually, a near lumen (such as the esophagus, bronchus, colon, or vagina) offers the possibility to approximate the temperature in the distant tumor, but this is again far from the reality in the target.
- The most effective temperature mapping can be done with MRI measurement, using a phantom for reference, usually unionized water. The MRI measurement depends on the temperature, but also strongly depends on the structure of the measured volume. In the temperature measurement, both factors are included in calculating the result, but the calibration does not consider a final element: the changes in the structure, which is the goal of the LRHT treatment.
1.4. Challenge of the Heated Body
1.5. Challenge of Homogeneity
2. Materials and Methods
- The chosen optimal carrier frequency is 13.56 MHz, which belongs to the freely applicable ISM band [114] and does not need shielding.
- The energy is capacitively coupled, but it does not use the plane-wave approach. Plane-wave radiation is devoted to isothermal heating.
- There is precise impedance matching [108] in the mEHT method. Proper impedance matching produces negligible reflected power (order of 1 W), mimicking the galvanic contact with the skin as much as possible.
- It has resonant matching with micro-selection ability, which fits the impedance [109]. It eliminates the imaginary part of the impedance. It differs from the usually applied plane-wave matching.
- The maximum adequate output power of mEHT is limited. The power limit depends on the size of the electrode. In device EHY2000+, the maximal power is 150 W, while in the model of EHY2030, which has optionally larger electrodes too, the limit is 250 W. The applied power in therapy depends on the localization and size of the tumor. The power limitation keeps the SAR less than for isothermal heating, but high enough to select and excite the membrane rafts of the malignant cells [100] and sensitizes to the RT [115,116].
- The modulation spectrum is a low-frequency time-fractal [113], described by fractal physiology [117,118,119,120], which agrees with the homeostatic molecular temporal balance [113]. mEHT extensively uses the modulation technique to identify fractal structures in space and time (dynamics) in spatiotemporal identification [113]. The electric parameters (resistance and capacity) depend on the malignant status [121]. The selection between malignant and healthy cells was measured as a characteristic time-fractal [122]. The modulation delivers temporal information executing enzymatic processes at the cell membranes [123], promoting the consequence of the excitation.
- The correct impedance matching provides an appropriate electric field that ensures the current density (). The is the parameter of the isodose conditions, ensuring the constant current density in the target. A complex value describes the current depending on the phase shift from the applied signal voltage. The dominant dielectric actions (heating and excitation energies) produce thermal and nonthermal effects.
- The modulated -current density actively produces both the thermal and nonthermal effects.
- The patient is interactively connected to the electric circuit, like a discrete element of the RF-net. This solution allows the real-time control of the patient due to the treated tumor being actively sensed and targeted as part of the tuned electric circuit.
3. Results
| No. | Tumor Site | Number of Patients | Treatment Used | Results | Reference |
|---|---|---|---|---|---|
| 1 | Advanced gliomas | 12 | mEHT + RT + ChT | CR = 1, PR = 2, RR = 25%. Median duration of response = 10 m. Median survival = 9 m, 25% survival rate at 1 year. | Fiorentini, et al., 2006 [186] |
| 2 | Various brain-gliomas | 140 | mEHT + RT + ChT | OS = 20.4 m. mEHT was safe and well tolerated. | Sahinbas, et al., 2007 [187] |
| 3 | High-grade gliomas | 179 | mEHT + RT + ChT | Longstanding complete and partial remissions after recurrence in both groups. | Hager, et al., 2008 [188] |
| 4 | Glioblastoma & Astrocytoma | 149 | mEHT + RT + ChT (BSC, palliative range) | 5y-OS = 83% (AST) in mEHT vs. 5y-OS = 25% by BSC. 5y-OS = 3.5% in mEHT vs. 5y-OS = 1.2% by BSC for GBM. Median OS = 14 m of mEHT for GBM and OS = 16.5 m for AST. | Fiorentini, et al., 2019b [189] |
| 5 | Advanced cervical cancer | 236 | Random. Phase III (RT + ChT ± mEHT [preliminary data] | Preliminary data for the first 100 participants. A positive trend in survival and local disease control by mEHT. There were no significant differences in acute adverse events or quality of life between the groups. | Minnaar, et al., 2016 [190] |
| 6 | Advanced cervical cancer | 72 | mEHT + RT + ChT | CR + PR = 73.5%; SD = 14.7%. The addition of mEHT increased the QoL and OS. | Pesti, et al., 2013 [191] |
| 7 | Advanced cervical carcinoma | 20 | mEHT + RT + ChT | mEHT increases the peri-tumor temperature and blood flow in human cervical tumors, promoting the radiotherapy + chemotherapy | Lee, et al., 2018 [132] |
| 8 | Advanced cervical carcinoma | 206 | Random. Phase III (RT + ChT ± mEHT) [abscopal effect] | The abscopal effect grows significantly with mEHT complementary to ChRT. | Minnaar, et al., 2020 [178] |
| 9 | Advanced cervical carcinoma | 206 | Random. Phase III (RT + ChT ± mEHT) [toxicity & Quality of life] | mEHT does not increase the toxicity of ChRT but increases the quality of life | Minnaar, et al., 2020 [178] |
| 10 | Advanced cervical carinoma | 202 | mEHT + RT + ChT | Six-month local disease-free survival (LDFS) = 38.6% for mEHT and LDFS = 19.8% without mEHT (p = 0.003). Local disease control (LDC) = 45.5% with mEHT LDC = 24.1% without mEHT; (p = 0.003). | Minnaar, et al., 2019 [177] |
| 11 | Advanced NSCLC | 97 | mEHT + RT + ChT | Median OS = 9.4 m with mEHT OS = 5.6 m without mEHT; (p < 0.0001). Median PFS = 3 m for mEHT and PFS = 1.85 m without mEHT; p < 0.0001. | Ou, et al., 2020 [192] |
| 12 | Advanced NSCLC | 311 (61 +197 +53) | mEHT + RT + ChT | Two centers PFY (n = 61), HTT (n = 197) control (n = 53). 80% (PFY), 80% (HTT) had distant metastases, conventional therapies failed. Median OS = 16.4 m (PFY), 15.6 m (HTT), 14 m (control); 1st y survival 67.2% (PFY), 64% (HTT), 26.5% (control). | Dani, et al., 2011 + Szasz, 2014 [193] |
| 13 | Advanced rectal cancer | 76 | mEHT + RT + ChT | Downstaging + tumor regression, ypT0, and ypN0 were better with mEHT than without. No statistical significance. | You et al., 2020 [194] |
| 14 | Various types of sarcoma | 13 | mEHT + RT + ChT | Primary, recurrent, and metastatic sarcomas responded to mEHT, the masses regressed. | Jeung, et al., 2015 [195] |
| 15 | Advanced pancreas carcinoma | 106 | mEHT + RT + ChT | After 3 m, PR = 22 (64.7%), SD = 10 (29.4%), PD = 2 (8.3%) with mEHT after 3 m of the therapy. In group without mEHT in the same time: PR = 3 (8.3%), SD = 10 (27.8%), PD = 23 (34.3%). The median OS = 18 m with mEHT and OS = 10.9 m without mEHT. | Fiorentini, et al., 2019 [196] |
| 16 | Advanced pancreas carcinoma | 133 (26 +73 +34) | mEHT + RT + ChT | Two centers PFY (n = 26), HTT (n = 73) control (n = 34). 59% (PFY), 88% (HTT) had distant metastases, conventional therapies failed. Median OS = 12.0 m (PFY), 12.7 m (HTT), 6.5 m (control); 1st y survival 46.2% (PFY), 52.1% (HTT), 26.5% (control) QoL was improved. | Dani, et al., 2008 [197] |
| 17 | Metastatic cancers (colorectal, ovarian, breast) | 23 | mEHT + RT + ChT | OS and time to progression (TTP) were influenced by the number of chemotherapy cycles (p < 0.001) and mEHT sessions (p < 0.001). Bevacizumab-based chemotherapy with mEHT has a favorable tumor response, is feasible, and well-tolerated for metastatic cancer patients. | Ranieri, et al., 2017 [198] |
| 18 | Rectal cancer | 120 | mEHT + RT + surgery | In mEHT group, 80.7% showed down-staging compared with 67.2% in non-mEHT group. | Kim et al., 2021 [199] |
| 19 | Gliomas | 164 | mEHT + RT + ChT | CR + PR is 41.4% for mEHT and 33.4% for conventional therapies. | Fiorentini et al., 2020 [200] |
| 20 | Ovarian, cervical cancer | mEHT + RT + ChT | The feasibility and success of oncothermia is proven. | Wookyeom, et al., 2018 [201], | |
| 21 | Various sites | 784 | mEHT + RT + ChT + surgery | Preliminary results show promising survival trajectories. mEHT is a safe treatment with very few adverse events or side effects, allowing patients to maintain a higher quality of life. | Parmar et al., 2020 [184] |
| 22 | Various sites | mEHT + RT + ChT | Planned trial. | Arrojo et al., 2020 [202] | |
| 23 | Various sites | mEHT + RT + ChT | The feasibility and success of oncothermia are proven. | Szasz AM et al., 2019 [183] | |
| 24 | Advanced glioblastoma | 60 | mEHT + RT + ChT | No added toxicity by immunotherapy. Median progression-free survival (PFS) = 13 m. Median follow-up 17 m, median OS was not reached. The estimated OS at 30 m was 58%. | Van Gool, et al., 2018 [203] |
| 25 | Different types of metastatic/recurrent cancers | 33 | mEHT + RT | CR = 2 (6.1%), Very good PR = 5 (15.2%), PR = 13 (39.4%), SD = 9 (27.3%), PD = 4 (12.1%). Three patients (9.1%) developed autoimmune toxicities. All these three patients had long-lasting abscopal responses outside the irradiated area. | Chi, et al., 2020 [116] |
4. Discussion
4.1. The Electromagnetic Selection
- The thermal effect happens in nanoscopic local “points”, the rafts. These NPs are molecular clusters and sensitive to overheating. When the absorbed energy is too large, it destroys the rafts by overheating. The mEHT loses its most significant advantage, the excitation of signal-transports for apoptosis and immunogenic cell death (ICD).
- The selection mechanisms of mEHT also limit the SAR, which forces temperature development. At high temperatures, the heat spreads extensively, and the microscopic differences vanish on average. A macroscopic average will characterize the target, as in WBH. The limited energy absorption is mandatory for the selection of rafts.
- The appropriate frequency is selected around 10 MHz [94]. When the frequency is larger (>15 MHz), the membrane impedance becomes too small to select the disordered TME accurately. The current will flow through the entire target tissue almost homogeneously, neglecting the selection heterogenic selection factors of malignant cells. When the carrier frequency does not ensure selection, the modulation also activates the healthy cells. The significantly larger amount of membrane rafts between healthy and malignant cells [106] remain selective factors only.
4.2. Nonthermal Processes
4.3. Effect of RF Current Density and the Dynamic Heating
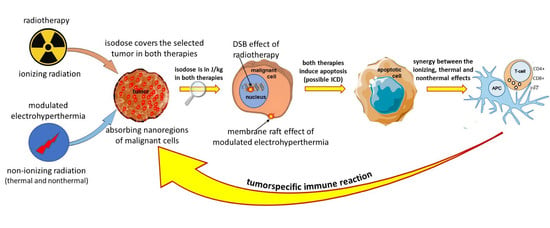
4.4. Complementary to Radiotherapy
4.5. Sequences and Timing of Treatments in Complementary Therapy
- When the oxygenation (blood flow intensity) is high, we expect sensitivity for RT, so apply it first. The maximal frequency of mEHT is every second day.
- When the tumor has hypoxic conditions (low oxygen content), apply the first mEHT to increase it and sensitize the RT.
- When HT is applied first, it sensitizes the RT by oxygenation of the tumor, but there could also be an inhibitory effect when HT induces hypoxic conditions, which may happen at higher temperatures than 43 °C, which usually does not happen with mEHT.
- Both HT and RT produce heat shock proteins (HSPs). The RT-induced stress also produces these chaperone proteins in different amounts and types. For example, HSP70 and HSP27 are involved in regulating the base excision repair (BER) enzymes in response to RT stress [267].
- Developing an antiapoptotic HSP70 chaperone defines the minimal time between the repeated HT treatments. Due to the HSP70 back to the baseline 48 h post-treatment. Consequently, every second day is recommended as the most frequent application. The maximal time between the HT treatments is one week when the possible buildup of the adaptive immune system finishes.
- HT has effects that are not dependent on enzyme activity, such as a variety of irreparable DNA mismatches, heat-activated methylation, hydrolysis, mono- or di-adduct damages, etc. The activity of repairing enzymes grows by temperatures, but at high temperatures (generally 43 °C) it blocks their activity. The enzyme block could be helpful. The high temperature causes intense hypoxia in the tumor and suppresses the RT efficacy, so mild heating of mEHT is optimal.
- HT at lower temperatures is sufficient to enhance perfusion [70] and the formation of numerous reactive oxygen species (ROS), such as hydrogen peroxide, superoxide anions, nitric oxide, hydroxyl radical, etc. Superoxide dismutase (SOD) forms an essential component in the defense against ROS. Heat stress could cause a decrease in SOD levels, which also leads to cell death [268].
- There is a risk that HT could support the activity of DNA repairing enzymes when it is applied after RT, even also when the end temperature is as high to block the enzymatic activity, because the first part of the heating is a “warming up”, presenting a preheating, which could increase the activity of reparation enzymes [269].
4.6. Immunogenetic Effects
5. Summary
6. Conclusions
Funding
Conflicts of Interest
References
- Seegenschmiedt, M.H.; Vernon, C.C. A Historical Perspective on Hyperthermia in Oncology. In Thermoradiotherapy and Thermochemotherapy; Seegenschmiedt, M.H., Fessenden, P., Vernon, C.C., Eds.; Springer: Berlin/Heidelberg, Germany, 1995; Volume 1, pp. 3–44. [Google Scholar]
- Vaupel, P.W.; Kelleher, D.K. Metabolic status and reaction to heat of normal and tumor tissue. In Thermoradiotherapy and Thermochemiotherapy; Seegenschmiedt, M.H., Fessenden, P., Vernon, C.C., Eds.; Springer: Berlin/Heidelberg, Germany, 1995; Volume 1, pp. 157–176. [Google Scholar]
- Griffiths, H.; Ahmed, A.; Smith, C.W.; Moore, J.L.; Kerby, I.J.; Davies, R.M.E. Specific absorption rate and tissue temperature in local hyperthermia. Int. J. Radiat. Oncol. Biol. Phys. 1986, 12, 1997–2002. [Google Scholar] [CrossRef]
- Dutz, S.; Hergt, R. Magnetic nanoparticle heating and heat transfer on a microscale: Basic principles, realities and physical limitations of hyperthermia for tumour therapy. Int. J. Hyperth. 2013, 29, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Datta, N.R.; Puric, E.; Klingbiel, D.; Gomez, S.; Bodis, S. Hyperthermia and Radiation Therapy in Locoregional Recurrent Breast Cancers: A Systematic Review and Meta-analysis. Int. J. Radiat. Oncol. 2016, 94, 1073–1087. [Google Scholar] [CrossRef] [PubMed]
- Datta, N.R.; Rogers, S.; Ordonez, S.G.; Puric, E.; Bodis, S. Hyperthermia and radiotherapy in the management of head and neck cancers: A systematic review and meta-analysis. Int. J. Hyperth. 2016, 32, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Datta, N.R.; Rogers, S.; Klingbiel, D.; Gomez, S.; Puric, E.; Bodis, S. Hyperthermia and radiotherapy with or without chemotherapy in locally advanced cervical cancer: A systematic review with conventional and network meta-analyses. Int. J. Hyperth. 2016, 32, 809–821. [Google Scholar] [CrossRef]
- Rogers, S.J.; Datta, N.R.; Puric, E.; Timm, O.; Marder, D.; Khan, S.; Mamot, C.; Knuchel, J.; Siebenhüner, A.; Pestalozzi, B.; et al. The addition of deep hyperthermia to gemcitabine-based chemoradiation may achieve enhanced survival in unresectable locally advanced adenocarcinoma of the pancreas. Clin. Transl. Radiat. Oncol. 2021, 27, 109–113. [Google Scholar] [CrossRef]
- Issels, R.D.; Lindner, L.H.; Verweij, J.; Wessalowski, R.; Reichardt, P.; Wust, P.; Ghadjar, P.; Hohenberger, P.; Angele, M.; Salat, C.; et al. Effect of Neoadjuvant Chemotherapy Plus Regional Hyperthermia on Long-term Outcomes Among Patients with Localized High-Risk Soft Tissue Sarcoma. JAMA Oncol. 2018, 4, 483–492. [Google Scholar] [CrossRef]
- Falk, M.H.; Issels, R.D. Hyperthermia in oncology: Invited Review. Int. J. Hyperth. 2001, 17, 1–18. [Google Scholar] [CrossRef]
- Streffer, C.; van Beuningen, D.; Dietzel, F.; Röttinger, E.; Robinson, J.E.; Scherer, E.; Seeber, S.; Trott, K.R. Cancer Therapy by Hyperthermia and Radiation; Urban and Schwarzenberg: Baltimore, MD, USA; Munich, Germany, 1978. [Google Scholar]
- Seegenschmiedt, M.H.; Fessenden, P.; Vernon, C.C. Thermoradiotherapy and Thermochemotherapy, Volume 2: Clinical Applications; Springer: Berlin/Heidelberg, Germany, 1996. [Google Scholar]
- Kosaka, M.; Sugahara, T.; Schmidt, K.L.; Simon, E. Thermotherapy for Neoplasia, Inflammation, and Pain; Springer: Tokyo, Japan, 2001. [Google Scholar]
- Matsuda, T. Cancer Treatment by Hyperthermia, Radiation and Drugs; Taylor & Francis: London, UK; Bristol, PA, USA, 1993. [Google Scholar]
- Urano, M.; Douple, E. Hyperthermia and Oncology: Volume 2, Biology of Thermal Potentiation of Radiotherapy; VSP BV: Utrecht, The Netherlands, 1992. [Google Scholar]
- Hehr, T.; Wust, P.; Bamberg, M.; Budach, W. Current and potential role of thermoradiotherapy for solid tumors. Onkologie 2003, 26, 295–302. [Google Scholar]
- van der Zee, J.; Gonzalez, D.G.; van Rhoon, G.C.; van Dijk, J.D.; van Putten, W.L.; Hart, A.A.; The Dutch Deep Hyperthermia Group. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumors: A prospective, randomised, multicentre trial. Lancet 2000, 355, 1119–1125. [Google Scholar] [CrossRef]
- Wust, P.; Hildebrandt, B.; Sreenivasa, G.; Rau, B.; Gellermann, J.; Riess, H.; Felix, R.; Schlag, P.M. Hyperthermia in combined treatment of cancer. Lancet Oncol. 2002, 3, 487–497. [Google Scholar] [CrossRef]
- Overgaard, J.; Gonzalez, D.; Hulshof, M.C.; Arcangeli, G.; Dahl, O.; Mella, O.; Bentzen, S.M. Randomised trial of hyperthermia as adjuvant to radiotherapy for recurrent or metastatic malignant melanoma. Lancet 1995, 345, 540–543. [Google Scholar] [CrossRef]
- van der Zee, J.; Truemiet-Donker, A.D.; The, S.K.; Helle, P.A.; Seldenrath, J.J.; Meerwaldt, J.H.; Wijnmaalen, A.J.; van den Berg, A.P.; van Rhoon, G.C.; Broekmeyer-Reurink, M.P.; et al. Low-dose reirradiation in combination with hyperthermia: A palliative treatment for patients with breast cancer recurring in previously irradiated areas. Int. J. Radiat. Oncol. Biol. Phys. 1988, 15, 1407–1413. [Google Scholar] [CrossRef]
- Vernon, C.C.; Harrison, M. Hyperthermia with low-dose radiotherapy for recurrent breast carcinoma. Lancet 1991, 337, 59. [Google Scholar] [CrossRef]
- Bicher, J.I.; Al-Bussam, N.; Wolfstein, R.S. Thermotherapy with curative intent–breast, head, and neck, and prostate tumours. Dtsch. Z. Fur Oncol. 2006, 38, 116–122. [Google Scholar]
- Peeken, J.C.; Vaupel, P.; Combs, S.E. Integrating Hyperthermia into Modern Radiation Oncology: What Evidence Is Necessary? Front. Oncol. 2017, 7, 132. [Google Scholar] [CrossRef] [Green Version]
- Horsman, M.R.; Overgaard, J. Hyperthermia: A Potent Enhancer of Radiotherapy. Clin. Oncol. 2007, 19, 418–426. [Google Scholar] [CrossRef]
- Molls, M. Hyperthermia—The actual role in radiation oncology and future prospects. Strahlenther. Oncol. 1992, 168, 183–190. [Google Scholar]
- Seegenschmiedt, M.H.; Feldmann, H.J.; Wust, P. Hyperthermia–Its actual role is radiation oncology. Strahlenther. Oncol. 1995, 171, 560–572. [Google Scholar]
- Emami, B.; Scott, C.; Perez, C.A.; Asbell, S.; Swift, P.; Grigsby, P.; Montesano, A.; Rubin, P.; Curran, W.; Delrowe, J.; et al. Phase III study of interstitial thermoradiotherapy compared with interstitial radiotherapy alone in the treatment of recurrent or persistent human tumours: A prospectively controlled randomized study by radiation therapy oncology group. Int. J. Radiat. Oncol. Biol. Phys. 1996, 34, 1097–1104. [Google Scholar] [CrossRef]
- Wust, P.; Rau, B.; Gremmler, M.; Schlag, P.; Jordan, A.; Löffel, J.; Riess, H.; Felix, R. Radio-Thermotherapy in Multimodal Surgical Treatment Concepts. Oncologie 1995, 18, 110–121. [Google Scholar] [CrossRef]
- Overgaard, J. The current and potential role of hyperthermia in radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 1989, 16, 535–549. [Google Scholar] [CrossRef]
- Bauer, K.H. Das Krebsproblem; Springer: Berlin/Heidelberg, Germany, 1964. [Google Scholar]
- Szasz, O.; Szasz, A. Approaching Complexity: Hyperthermia Dose and Its Possible Measurement in Oncology. Open J. Biophys. 2021, 11, 68–132. [Google Scholar] [CrossRef]
- Romanovsky, A.A. Thermoregulation: Some concepts have changed. Functional architecture of the thermoregulatory system. Am. J. Physiol. Integr. Comp. Physiol. 2007, 292, R37–R46. [Google Scholar] [CrossRef]
- Vaupel, P.W.; Hammersen, F. Mikrozirkulation in malignen Tumoren. 6. Jahrestagung der Gesellschaft für Mikrozirkulation e.V., München, November 1982; Karger: Basel, Switzerland, 1983; ISBN 978-3-8055-3762-9. [Google Scholar]
- Charkoudian, N. Skin Blood Flow in Adult Human Thermoregulation: How It Works, When It Does Not, and Why. Mayo Clin. Proc. 2003, 78, 603–612. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Yang, W.Z.; Gao, C.; Fu, X.; Zhang, W.; Zhou, Q.; Chen, W.; Ni, X.; Lin, J.-K.; Yang, J.; et al. A hypothalamic circuit that controls body temperature. Proc. Natl. Acad. Sci. USA 2017, 114, 2042–2047. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, M.; Suzuki, K.; Kodama, S.; Sugahara, T. Normal human cells at confluence get heat resistance by efficient accumulation of HSP72 in nucleus. Carcinogenesis 1995, 16, 2373–2380. [Google Scholar] [CrossRef]
- Jones, E.; Thrall, D.; Dewhirst, M.W.; Vujaskovic, Z. Prospective thermal dosimetry: The key to hyperthermia’s future. Int. J. Hyperth. 2006, 22, 247–253. [Google Scholar] [CrossRef]
- di Lalla, V.; Chaput, G.; Williams, T.; Sultanem, K. Radiotherapy Side Effects: Integrating a Survivorship Clinical Lens to Better Serve Patients. Curr. Oncol. 2020, 27, 107–112. [Google Scholar] [CrossRef]
- Majeed, H.; Gupta, V. Adverse Effects of Radiation Therapy; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://pubmed.ncbi.nlm.nih.gov/33085406/ (accessed on 11 December 2021).
- Kaur, P.; Hurwitz, M.D.; Krishnan, S.; Asea, A. Combined Hyperthermia and Radiotherapy for the Treatment of Cancer. Cancers 2011, 3, 3799–3823. [Google Scholar] [CrossRef]
- Rao, W.; Deng, Z.-S.; Liu, J. A Review of Hyperthermia Combined with Radiotherapy/Chemotherapy on Malignant Tumors. Crit. Rev. Biomed. Eng. 2010, 38, 101–116. [Google Scholar] [CrossRef]
- Elming, P.B.; Sorensen, B.S.; Oei, A.L.; Franken, N.A.P.; Crezee, J.; Overgaard, J.; Horsman, M.R. Hyperthermia: The optimal treatment to overcome radiation resistant hypoxia. Cancers 2019, 11, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kühl, N.M.; Rensing, L. Heat shock effects on cell cycle progression. Cell. Mol. Life Sci. CMLS 2000, 57, 450–463. [Google Scholar] [CrossRef] [PubMed]
- Roti, J.L.; Laszlo, A. The effects of hyperthermia on cellular macromolecules. In Hyperthermia and Oncology Volume 1, Thermal Effects on Cells and Tissues; Urano, M., Douple, E., Eds.; VSP: Utrecht, The Netherlands, 1988; pp. 13–56. [Google Scholar]
- Pandita, T.K.; Pandita, S.; Bhaumik, S.R. Molecular parameters of hyperthermia for radiosensitization. Crit. Rev. Eukaryot. Gene Expr. 2009, 19, 235–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okumura, Y.; Ihara, M.; Shimasaki, T.; Takeshita, S.; Okiachi, K. Heat inactivation of DNA-dependent protein kinase: Possible mechanism of hyperthermic radiosensitization. In Thermotherapy for Neoplasia, Inflammation, and Pain; Kosaka, M., Sugahara, T., Schmidt, K.L., Kosaka, M., Sugahara, T., Simon, E., Eds.; Springer: Tokyo, Japan, 2001; pp. 420–423. [Google Scholar]
- Vujaskovic, Z.; Song, C.W. Physiological mechanisms underlying heat-induced radiosensitization. Int. J. Hyperth. 2004, 20, 163–174. [Google Scholar] [CrossRef]
- Song, C.W.; Shakil, A.; Osborn, J.L.; Iwata, K. Tumour oxygenation is increased by hyperthermia at mild temperatures. Int. J. Hyperth. 2009, 25, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Dudar, T.E.; Jain, R.K. Differential response of normal and tumor microcirculation to hyperthermia. Cancer Res. 1984, 44, 605–612. [Google Scholar] [PubMed]
- Song, C.W.; Lokshina, A.; Rhee, J.G.; Patten, M.; Levitt, S.H. Implication of blood-flow in hyperthermic treatment of tumors. IEEE Trans. Biomed. Eng. 1984, 31, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Pence, D.M.; Song, C.W. Effect of heat on blood-flow. In Hyperthermia in Cancer Treatment; Anghileri, L.J., Robert, J., Eds.; CRC Press Inc.: Boca Raton, FL, USA, 1986; Volume 2, pp. 1–17. [Google Scholar]
- Vaupel, P. Pathophysiological mechanism of hyperthermia in cancer therapy. In Methods of Hyperthermia Control, Clinical Thermology; Gautherie, M., Ed.; Springer: Berlin/Heidelberg, Germany, 1990; pp. 73–134. [Google Scholar]
- Erdmann, B.; Lang, J.; Seebass, M. Optimization of temperature distributions for regional hyperthermia based on a nonlinear heat transfer model. Ann. NYAS 1998, 858, 36–46. [Google Scholar]
- Vaupel, P.; Kallinowski, F.; Okunieff, P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumours: A review. Cancer Res. 1989, 49, 6449–6465. [Google Scholar]
- Takana, Y. Thermal responses of microcirculation and modification of tumour blood flow in treating the tumours. In Theoretical and Experimental Basis of Hyperthermia. Thermotherapy for Neoplasia, Inflammation, and Pain; Kosaka, M., Sugahara, T., Schmidt, K.L., Simon, E., Eds.; Springer: Tokyo, Japan, 2001; pp. 408–419. [Google Scholar]
- Song, C.W.; Choi, I.B.; Nah, B.S.; Sahu, S.K.; Osborn, J.L. Microvasculature and perfusion in normal tissues and tumours. In Thermoradiometry and Thermochemotherapy; Seegenschmiedt, M.H., Fessenden, P., Vernon, C.C., Eds.; Springer: Berlin/Heidelberg, Germany, 1995; Volume 1, pp. 139–156. [Google Scholar]
- Song, C.W.; Park, H.; Griffin, R.J. Theoretical and experimental basis of hyperthermia. In Thermotherapy for Neoplasia, Inflammation, and Pain; Kosaka, M., Sugahara, T., Schmidt, K.L., Simon, E., Eds.; Springer: Tokyo, Japan, 2001; pp. 394–407. [Google Scholar]
- Lindholm, C.E. Hyperthermia and Radiotherapy. Ph.D. Thesis, Lund University, Malmo, Sweden, 1992. [Google Scholar]
- Hafström, L.; Rudenstam, C.M.; Blomquist, E.; Ingvar, C.; Jönsson, P.E.; Lagerlöf, B.; Lindholm, C.; Ringborg, U.; Westman, G.; Ostrup, L. Regional hyperthermic perfusion with melphalan after surgery for recurrent malignant melanoma of the extremities. J. Clin. Oncol. 1991, 9, 2091–2094. [Google Scholar] [CrossRef] [PubMed]
- Dewey, W.C.; Hopwood, L.E.; Sapareto, S.A.; Gerweck, L.E. Cellular Responses to Combinations of Hyperthermia and Radiation. Radiology 1977, 123, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Lee, S.; Seo, D.; Kim, D.; Kim, K.; Kim, E.; Kang, J.; Seong, K.M.; Youn, H.; Youn, B. Cellular Stress Responses in Radiotherapy. Cells 2019, 8, 1105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walleczek, J. Self-Organized Biological Dynamics & Nonlinear Control; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Dewhirst, M.W.; Viglianti, B.L.; Lora-Michiels, M.; Hanson, M.; Hoopes, P.J. Basic principles of thermal dosimetry and thermal thresholds for tissue damage from hyperthermia. Int. J. Hyperth. 2003, 19, 267–294. [Google Scholar] [CrossRef] [PubMed]
- Perez, C.A.; Sapareto, S.A. Thermal dose expression in clinical hyperthermia and correlation with tumor response/control. Cancer Res. 1984, 44, 4818–4825. [Google Scholar]
- Dewey, W.C. Arrhenius relationships from the molecule and cell to the clinic. Int. J. Hyperth. 1994, 10, 457–483. [Google Scholar] [CrossRef]
- Gellerman, J. Nichtinvasive Thermometrie bei lokoregionaler Tiefenhyperthermie, Noninvasive thermometry in loco-regional deep hyperthermia. In Proceedings of the Oncothermia Symphosia, 2016, Cologne, Germany, 22–23 September 2006. [Google Scholar]
- Hegyi, G.; Vincze, G.; Szasz, A. On the Dynamic Equilibrium in Homeostasis. Open J. Biophys. 2012, 2, 60–67. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.-Y.; Szigeti, G.P.; Szasz, A.M. Oncological hyperthermia: The correct dosing in clinical applications. Int. J. Oncol. 2018, 54, 627–643. [Google Scholar] [CrossRef]
- Maguire, P.D.; Samulski, T.V.; Prosnitz, L.R.; Jones, E.L.; Rosner, G.L.; Powers, B.; Layfield, L.W.; Brizel, D.M.; Scully, S.P.; Herrelson, M.; et al. A phase II trial testing the thermal dose parameter CEM43° T90 as a predictor of response in soft tissue sarcomas treated with pre-operative thermorasiotherapy. Int. J. Hyperth. 2001, 17, 283–290. [Google Scholar] [CrossRef]
- Dewhirst, M.W.; Vujaskovic, Z.; Jones, E.; Thrall, D. Re-setting the biologic rationale for thermal therapy. Int. J. Hyperth. 2005, 21, 779–790. [Google Scholar] [CrossRef]
- Thrall, D.E.; Prescott, D.M.; Samulski, T.V.; Rosner, G.L.; Denman, D.L.; Legorreta, R.L.; Dodge, R.K.; Page, R.L.; Cline, J.; Lee, J.; et al. Radiation plus local hyperthermia versus radiation plus the combination of local and whole-body hyperthermia in canine sarcomas. Int. J. Radiat. Oncol. 1996, 34, 1087–1096. [Google Scholar] [CrossRef]
- Hildebrandt, B.; Dräger, J.; Kerner, T.; Deja, M.; Löffel, J.; Stroszczynski, C.; Ahlers, O.; Felix, R.; Riess, H.; Wust, P. Whole-body hyperthermia in the scope of von Ardenne’s systemic cancer multistep therapy (sCMT) combined with chemotherapy in patients with metastatic colorectal cancer: A phase I/II study. Int. J. Hyperth. 2004, 20, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Bakhshandeh, A.; Wiedemann, G.; Zabel, P.; Dalhoff, K.; Kohlmann, T.; Penzel, R.Z.; Wagner, T.; Peters, S. Randomized trial with ICE (ifosfamide, carboplatin, etoposide) plus whole body hyperthermia versus ICE chemotherapy for malignant pleural mesothelioma. J. Clin. Oncol. 2004, 22, 7288. [Google Scholar] [CrossRef]
- de Bruijne, M.; van der Holt, B.; van Rhoon, G.C.; van der Zee, J. Evaluation of CEM43°CT90 thermal dose in superficial hyperthermia: A retrospective analysis. Strahlenther. Onkol. Radiother. Oncol. 2010, 186, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Kraybill, W.G.; Olenki, T.; Evans, S.S.; Ostberg, J.R.; O’Leary, K.A.; Gibbs, J.F.; Repasky, E.A. A phase I study of fever-range whole body hyperthermia (FR-WBH) in patients with advanced solid tumors: Correlation with mouse models. Int. J. Hyperth. 2002, 18, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Toyota, N.; Strebel, F.R.; Stephens, L.C.; Matsuda, H.; Bull, J.M.C. Long-duration, mild whole body hyperthermia with cisplatin: Tumour response and kinetics of apoptosis and necrosis in a metastatic rat mammary adenocarcinoma. Int. J. Hyperth. 1997, 13, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, Y.; Makino, M.; Kaneko, T.; Stephens, L.C.; Strebel, F.R.; Danhauser, L.L.; Jenkins, G.N.; Bull, J.M. Therapeutic efficacy of long duration-low temperature whole body hyperthermia when combined with tumor necrosis factor and carboplatin in rats. Cancer Res. 1994, 54, 2223–2227. [Google Scholar]
- Ostberg, R.; Repasky, E.A. Use of mild, whole body hyperthermia in cancer therapy. Immunol. Investig. 2000, 29, 139–142. [Google Scholar] [CrossRef]
- International Collaborative Hyperthermia Group; Vernon, C.C.; Hand, J.W.; Field, S.B.; Machin, D.; Whaley, J.B.; van der Zee, J.; van Putten, W.L.; van Rhoon, G.C.; van Dijk, J.D.; et al. Radiotherapy with or without hyperthermia in the treatment of superficial localized breast cancer: Results from five randomized controlled trials. Int. J. Radiat. Oncol. Biol. Phys. 1996, 35, 731–744. [Google Scholar] [CrossRef] [Green Version]
- Sherar, M.; Liu, F.-F.; Pintilie, M.; Levin, W.; Hunt, J.; Hill, R.; Hand, J.; Vernon, C.; van Rhoon, G.; van der Zee, J.; et al. Relationship between thermal dose and outcome in thermoradiotherapy treatments for superficial recurrences of breast cancer: Data from a phase III trial. Int. J. Radiat. Oncol. 1997, 39, 371–380. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Patel, F.D.; Sandhu, A.P.S.; Gupta, B.D.; Yadav, N.S. A prospective randomized study of local hyperthermia as a supplement and radiosensitiser in the treatment of carcinoma of the cervix with radiotherapy. Endocurietherapy/Hyperth. Oncol. 1989, 5, 151–159. [Google Scholar]
- Vasanthan, A.; Mitsumori, M.; Park, J.H.; Zhi-Fan, Z.; Yu-Bin, Z.; Oliynychenko, P.; Tatsuzaki, H.; Tanaka, Y.; Hiraoka, M. Regional hyperthermia combined with radiotherapy for uterine cervical cancers: A multi-institutional prospective randomized trial of the international atomic energy agency. Int. J. Radiat. Oncol. 2005, 61, 145–153. [Google Scholar] [CrossRef]
- Harima, Y.; Nagata, K.; Harima, K.; Ostapenko, V.V.; Tanaka, Y.; Sawada, S. A randomized clinical trial of radiation therapy versus thermoradiotherapy in stage IIIB cervical carcinoma. Int. J. Hyperth. 2009, 25, 338–343. [Google Scholar] [CrossRef]
- Roussakow, S.V. A randomized clinical trial of radiation therapy versus thermoradiotherapy in stage IIIB cervical carcinoma of Yoko Harima et al. (2001): Multiple biases and no advantage of hyperthermia. Int. J. Hyperth. 2018, 34, 1400. [Google Scholar] [CrossRef]
- Harima, Y. A randomised clinical trial of radiation therapy versus thermoradiotherapy in stage IIIB cervical carcinoma of Yoko, Harima et al. (2001): A response letter to the editor of comments from Dr. Roussakow. Int. J. Hyperth. 2018, 34, 1401. [Google Scholar] [CrossRef] [Green Version]
- Zolciak-Siwinska, A.; Piotrokowicz, N.; Jonska-Gmyrek, J.; Nicke-Psikuta, M.; Michalski, W.; Kawczyńska, M.; Bijok, M.; Bujko, K. HDR brachytherapy combined with interstitial hyperthermia in locally advanced cervical cancer patients initially treated with concomitant radiochemotherapy–A phase III study. Radiother. Oncol. 2013, 109, 194–199. [Google Scholar] [CrossRef]
- Kay, C.S.; Choi, I.B.; Jang, J.Y.; Choi, B.O.; Kim, I.A.; Shinn, K.S. Thermoradiotherapy in the treatment of locally advanced nonsmall cell lung cancer. Radiat. Oncol. J. 1996, 14, 115–122. [Google Scholar]
- Mitsumori, M.; Zhi-Fan, Z.; Oliynychenko, P.; Park, J.H.; Choi, I.B.; Tatsuzaki, H.; Tanaka, Y.; Hiraoka, M. Regional hyperthermia combined with radiotherapy for locally advanced non-small cell lung cancers: A multi-institutional prospective randomized trial of the International Atomic Energy Agency. Int. J. Clin. Oncol. 2007, 12, 192–198. [Google Scholar] [CrossRef]
- Jones, E.L.; Oleson, J.R.; Prosnith, L.R.; Prosnitz, L.R.; Samulski, T.V.; Vujaskovic, Z.; Yu, D.; Sanders, L.L.; Dewhirst, M.W. Randomized trial of hyperthermia and radiation for superficial tumors. J. Clin. Oncol. 2005, 23, 3079–3085. [Google Scholar] [CrossRef] [Green Version]
- Storm, F.K. What happened to hyperthermia and what is its current status in cancer treatment? J. Surg. Oncol. 1993, 53, 141–143. [Google Scholar] [CrossRef]
- van der Zee, J. Heating the patient: A promising approach? Ann. Oncol. 2002, 13, 1173–1184. [Google Scholar] [CrossRef] [PubMed]
- Datta, N.R.; Kok, H.P.; Crezee, H.; Gaipl, U.S.; Bodis, S. Integrating Loco-Regional Hyperthermia into the Current Oncology Practice: SWOT and TOWS Analyses. Front. Oncol. 2020, 10, 819. [Google Scholar] [CrossRef] [PubMed]
- Wildeboer, R.R.; Southern, P.; Pankhurst, Q.A. On the reliable measurement of specific absorption rates and intrinsic loss parameters in magnetic hyperthermia materials. J. Phys. D Appl. Phys. 2014, 47, 495003. [Google Scholar] [CrossRef]
- Szasz, A.; Szasz, N.; Szasz, O. Oncothermia: Principles and Practices; Springer Science: Heidelberg, Germany, 2010. [Google Scholar] [CrossRef]
- Andocs, G.; Rehman, M.U.; Zhao, Q.L.; Papp, E.; Kondo, T.; Szasz, A. Nanoheating without Artificial Nanoparticles Part II. Experimental support of the nanoheating concept of the modulated electro-hyperthermia method, using U937 cell suspension model. Biol. Med. 2015, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Beachy, S.H.; Repasky, E.A. Toward establishment of temperature thresholds for immunological impact of heat exposure in humans. Int. J. Hyperth. 2011, 27, 344–352. [Google Scholar] [CrossRef]
- Shen, R.-N.; Lu, L.; Young, P.; Shidnia, H.; Hornback, N.B.; Broxmeyer, H.E. Influence of elevated temperature on natural killer cell activity, lymphokine-activated killer cell activity and lectin-dependent cytotoxicity of human umbilical cord blood and adult blood cells. Int. J. Radiat. Oncol. 1994, 29, 821–826. [Google Scholar] [CrossRef]
- Hietanen, T.; Kapanaen, M.; Kellokumpu-Lehtinen, P.-L. Restoring natural killer cell cytotoxicity after hyperthermia alone or combined with radiotherapy. Anticancer. Res. 2016, 36, 555–563. [Google Scholar]
- Repasky, E.; Issels, R. Physiological consequences of hyperthermia: Heat, heat shock proteins and the immune response. Int. J. Hyperth. 2002, 18, 486–489. [Google Scholar] [CrossRef]
- Szasz, A. Thermal and nonthermal effects of radiofrequency on living state and applications as an adjuvant with radiation therapy. J. Radiat. Cancer Res. 2019, 10, 1–17. [Google Scholar] [CrossRef]
- Krenacs, T.; Meggyeshazi, N.; Forika, G.; Kiss, E.; Hamar, P.; Szekely, T.; Vancsik, T. Modulated Electro-Hyperthermia-Induced Tumor Damage Mechanisms Revealed in Cancer Models. Int. J. Mol. Sci. 2020, 21, 6270. [Google Scholar] [CrossRef]
- Vincze, G.; Szasz, A. Similarities of modulation by temperature and by electric field. Open J. Biophys. 2018, 8, 95–103. [Google Scholar] [CrossRef] [Green Version]
- Gowrishankar, T.R.; Weaver, J.C. An approach to electrical modeling of single and multiple cells. Proc. Natl. Acad. Sci. USA 2003, 100, 3203–3208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotnik, T.; Miklavcic, D. Theoretical evaluation of the distributed power dissipation in biological cells exposed to electric fields. Bioelectromagnetics 2000, 21, 385–394. [Google Scholar] [CrossRef]
- Papp, E.; Vancsik, T.; Kiss, E.; Szasz, O. Energy absorption by the membrane rafts in the modulated electro-hyperthermia (mEHT). Open J. Biophys. 2017, 7, 216–229. [Google Scholar] [CrossRef] [Green Version]
- The Physical Sciences-Oncology Centers Network; Agus, D.B.; Alexander, J.F.; Arap, W.; Ashili, S.; Aslan, J.; Austin, R.H.; Backman, V.; Bethel, K.; Bonneau, R.; et al. A physical sciences network characterization of non-tumorigenic and metastatic cells. Sci. Rep. 2013, 3, 01449. [Google Scholar] [CrossRef]
- Arrhenius, S. On the reaction rate of the inversion of non-refined sugar upon souring. Z Phys. Chem. 1889, 4, 226–248. [Google Scholar] [CrossRef] [Green Version]
- Szasz, A. The Capacitive Coupling Modalities for Oncological Hyperthermia. Open J. Biophys. 2021, 11, 252–313. [Google Scholar] [CrossRef]
- Szasz, A.; Vincze, G.; Szasz, O.; Szasz, N. An Energy Analysis of Extracellular Hyperthermia. Electromagn. Biol. Med. 2003, 22, 103–115. [Google Scholar] [CrossRef]
- Fröhlich, H. What are non-thermal electric biological effects? Bioelectromagnetics 1982, 3, 45–46. [Google Scholar] [CrossRef]
- Szasz, A. Therapeutic Basis of Electromagnetic Resonances and Signal-Modulation. Open J. Biophys. 2021, 11, 314–350. [Google Scholar] [CrossRef]
- Neudorfer, C.; Chow, C.T.; Boutet, A.; Loh, A.; Germann, J.; Elias, G.J.; Hutchison, W.D.; Lozano, A.M. Kilohertz-frequency stimulation of the nervous system: A review of underlying mechanisms. Brain Stimul. 2021, 14, 513–530. [Google Scholar] [CrossRef] [PubMed]
- Szasz, A.; Szasz, O. Time-fractal modulation of modulated electro-hyperthermia (mEHT). In Book Challenges and Solutions of Oncological Hyperthermia; Szasz, A., Ed.; Cambridge Scholars: Newcastle upon Tyne, UK, 2020; pp. 377–415. [Google Scholar]
- Rec. ITU-R SM.1056-1. 1 RECOMMENDATION ITU-R SM.1056-1. Limitation of Radiation from Industrial, Scientific and Medical (ISM) Equipment (Question ITU-R 70/1). Available online: https://www.itu.int/dms_pubrec/itu-r/rec/sm/R-REC-SM.1056-1-200704-I!!PDF-E.pdf (accessed on 31 October 2021).
- Yeo, S.-G. Definitive radiotherapy with concurrent oncothermia for stage IIIB non-small-cell lung cancer: A case report. Exp. Ther. Med. 2015, 10, 769–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chi, M.-S.; Mehta, M.P.; Yang, K.-L.; Lai, H.-C.; Lin, Y.-C.; Ko, H.-L.; Wang, Y.-S.; Liao, K.-W.; Chi, K.-H. Putative Abscopal Effect in Three Patients Treated by Combined Radiotherapy and Modulated Electrohyperthermia. Front. Oncol. 2020, 10, 254. [Google Scholar] [CrossRef] [PubMed]
- Deering, W.; West, B.J. Fractal physiology. IEEE Comput. Graph. Appl. 1992, 11, 40–46. [Google Scholar] [CrossRef]
- West, B.J. Fractal Physiology and Chaos in Medicine; World Scientific: Singapore; London, UK, 1990. [Google Scholar]
- Bassingthwaighte, J.B.; Leibovitch, L.S.; West, B.J. Fractal Physiology; Oxford University Press: New York, NY, USA; Oxford, UK, 1994. [Google Scholar]
- Musha, T.; Sawada, Y. Physics of the Living State; IOS Press: Amsterdam, The Netherlands, 1994. [Google Scholar]
- Lovelady, D.C.; Friedman, J.; Patel, S.; Rabson, D.A.; Lo, C.-M. Detecting effects of low levels of cytochalasin B in 3T3 fibroblast cultures by analysis of electrical noise obtained from cellular micromotion. Biosens. Bioelectron. 2009, 24, 2250–2254. [Google Scholar] [CrossRef] [Green Version]
- Lovelady, D.C.; Richmond, T.C.; Maggi, A.N.; Lo, C.-M.; Rabson, D.A. Distinguishing cancerous from noncancerous cells through analysis of electrical noise. Phys. Rev. E 2007, 76, 041908. [Google Scholar] [CrossRef] [Green Version]
- Astumian, R.D.; Chock, P.B.; Tsong, T.Y.; Westerhoff, H.V. Effects of oscillations and energy-driven fluctuations on the dynamics of enzyme catalysis and free-energy transduction. Phys. Rev. A 1989, 39, 6416–6435. [Google Scholar] [CrossRef]
- Astumian, R.D.; Weaver, J.C.; Adair, R.K. Rectification and signal averaging of weak electric fields by biological cells. Proc. Natl. Acad. Sci. USA 1995, 92, 3740–3743. [Google Scholar] [CrossRef] [Green Version]
- Sabah, N.H. Rectification in Biological Membranes. IEEE Eng. Med. Biol. 2000, 19, 106–113. [Google Scholar] [CrossRef]
- Nagy, G.; Meggyeshazi, N.; Szasz, O. Deep temperature measurements in oncothermia processes. In Proceedings of the Conference of the International Clinical Hyperthermia Society 2012, Budapest, Hungary, 12–14 October 2012. [Google Scholar]
- Orczy-Timko, B. Phantom measurements with the EHY-2030 device. In Challenges and Solutions of Oncological Hyperthermia; Szasz, A., Ed.; Cambridge Scholars: Newcastle upon Tyne, UK, 2020; pp. 416–428. [Google Scholar]
- Hossain, M.T.; Prasad, B.; Park, K.S.; Lee, H.J.; Ha, Y.H.; Lee, S.K.; Kim, J.K. Simulation and experimental evaluation of selective heating characteristics of 13.56 MHz radiofrequency hyperthermia in phantom models. Int. J. Precis. Eng. Manuf. 2016, 17, 253–256. [Google Scholar] [CrossRef]
- Prasad, B.; Kim, S.; Cho, W.; Kim, J.K.; Kim, Y.A.; Kim, S.; Wu, H.G. Quantitative estimation of the equivalent radiation dose escalation using radiofrequency hyperthermia in mouse xenograft models of human lung cancer. Sci. Rep. 2019, 9, 3942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balogh, L.; Polyák, A.; Pöstényi, Z.; Kovács-Haász, V.; Gyöngy, M.; Thuróczy, J. Temperature increase induced by modulated electrohyperthermia (onco-thermia®) in the anesthetized pig liver. J. Cancer Res. Ther. 2016, 12, 1153–1159. [Google Scholar] [PubMed]
- Kim, J.K.; Prasad, B.; Kim, S. Temperature mapping and thermal dose calculation in combined radiation therapy and 13.56 MHz radiofrequency hyperthermia for tumor treatment. In SPIE 10047, Optical Methods for Tumor Treatment and Detection: Mechanisms and Techniques in Photodynamic Therapy XXVI, Proceedings of the SPIE Conferences and Exhibitions, San Francisco, CA, USA, 28 January–2 February 2017; SPIE: Bellingham, WA, USA, 2017. [Google Scholar]
- Lee, S.-Y.; Kim, J.-H.; Han, Y.-H.; Cho, D.-H. The effect of modulated electro-hyperthermia on temperature and blood flow in human cervical carcinoma. Int. J. Hyperth. 2018, 34, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.-L.; Huang, C.-C.; Chi, M.-S.; Chiang, H.-C.; Wang, Y.-S.; Hsia, C.-C.; Andocs, G.; Wang, H.-E.; Chi, K.-H. In vitro comparison of conventional hyperthermia and modulated electro-hyperthermia. Oncotarget 2016, 7, 84082–84092. [Google Scholar] [CrossRef] [Green Version]
- Forika, G.; Balogh, A.; Vancsik, T.; Zalatnai, A.; Petovari, G.; Benyo, Z.; Krenacs, T. Modulated Electro-Hyperthermia Resolves Radioresistance of Panc1 Pancreas Adenocarcinoma and Promotes DNA Damage and Apoptosis In Vitro. Int. J. Mol. Sci. 2020, 21, 5100. [Google Scholar] [CrossRef]
- Andocs, G.; Renner, H.; Balogh, L.; Fonyad, L.; Jakab, C.; Szasz, A. Strong synergy of heat and modulated electro- magnetic field in tumor cell killing, Study of HT29 xenograft tumors in a nude mice model. Strahlenther. Onkol. 2009, 185, 120–126. [Google Scholar] [CrossRef]
- Andocs, G.; Rehman, M.U.; Zhao, Q.-L.; Tabuchi, Y.; Kanamori, M.; Kondo, T. Comparison of biological effects of modulated electro-hyperthermia and conventional heat treatment in human lymphoma U937 cells. Cell Death Discov. 2016, 2, 16039. [Google Scholar] [CrossRef] [Green Version]
- Kirson, E.D.; Gurvich, Z.; Schneiderman, R.; Dekel, E.; Itzhaki, A.; Wasserman, Y.; Schatzberger, R.; Palti, Y. Disruption of Cancer Cell Replication by Alternating Electric Fields. Cancer Res. 2004, 64, 3288–3295. [Google Scholar] [CrossRef] [Green Version]
- Vincze, G.; Szasz, A. Reorganization of actin filaments and microtubules by outside electric field. J. Adv. Biol. 2015, 8, 1514–1518. [Google Scholar]
- Dimova, R.; Bezlyepkina, N.; Jordö, M.D.; Knorr, R.L.; Riske, K.A.; Staykova, M.; Vlahovska, P.M.; Yamamoto, T.; Yang, P.; Lipowsky, R. Vesicles in electric fields: Some novel aspects of membrane behavior. Soft Matter 2009, 5, 3201–3212. [Google Scholar] [CrossRef]
- Vincze, G.; Szigeti, G.; Andocs, G.; Szasz, A. Nanoheating without Artificial Nanoparticles. Biol. Med. 2015, 7, 249. [Google Scholar]
- Guo, J.; Lao, Y.; Chang, D.C. Calcium and Apoptosis. In Handbook of Neurochemistry and Molecular Neurobiology; Lajtha, A., Mikoshiba, K., Eds.; Springer: Boston, MA, USA, 2009. [Google Scholar] [CrossRef]
- Cha, J.; Jeon, T.-W.; Lee, C.G.; Oh, S.T.; Yang, H.-B.; Choi, K.-J.; Seo, D.; Yun, I.; Baik, I.H.; Park, K.R.; et al. Electro-hyperthermia inhibits glioma tumorigenicity through the induction of E2F1-mediated apoptosis. Int. J. Hyperth. 2015, 31, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chauve, L.; Phelps, G.; Brielmann, R.M.; Morimoto, R.I. E2F coregulates an essential HSF developmental program that is distinct from the heat-shock response. Genes Dev. 2016, 30, 2062–2075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wust, P.; Kortüm, B.; Strauss, U.; Nadobny, J.; Zschaeck, S.; Beck, M.; Stein, U.; Ghadjar, P. Non-thermal effects of radiofrequency electromagnetic fields. Sci. Rep. 2020, 10, 13488. [Google Scholar] [CrossRef] [PubMed]
- Krenacs, T.; Benyo, Z. Tumor specific stress and immune response induced by modulated electrohyperthermia in relation to tumor metabolic profiles. Oncothermia J. 2017, 20, 264–272. [Google Scholar]
- Forika, G.; Vancsik, T.; Kiss, E.; Hujber, Z.; Sebestyen, A.; Krencz, I.; Benyo, Z.; Hamar, P.; Krenacs, T. The efficiency of modulated electro-hyperthermia may correlate with the tumor metabolic profiles. Oncothermia J. 2017, 20, 228–235. [Google Scholar]
- Vincze, G.; Szasz, N.; Szasz, A. On the thermal noise limit of cellular membranes. Bioelectromagnetics 2004, 26, 28–35. [Google Scholar] [CrossRef]
- Forika, G.; Balogh, A.; Vancsik, T. Elevated apoptosis and tumor stem cell destruction in a radioresistant pancreatic adenocarcinoma cell line when radiotherapy is combined with modulated electrohyperthermia. Oncothermia J. 2019, 26, 90–98. [Google Scholar]
- Meggyeshazi, N.; Andocs, G.; Krenacs, T. Modulated electro-hyperthermia induced programmed cell death in HT29 colorectal carcinoma xenograft. Virchows Arch. 2012, 461 (Suppl. 1), S131–S132. [Google Scholar]
- Danics, L.; Schvarcz, C.A.; Viana, P.; Vancsik, T.; Krenács, T.; Benyó, Z.; Kaucsár, T.; Hamar, P. Exhaustion of Protective Heat Shock Response Induces Significant Tumor Damage by Apoptosis after Modulated Electro-Hyperthermia Treatment of Triple Negative Breast Cancer Isografts in Mice. Cancers 2020, 12, 2581. [Google Scholar] [CrossRef]
- Meggyeshazi, N.; Andocs, G.; Spisak, S.; Krenacs, T. Modulated electrohyperthermia causes caspase independent programmed cell death in HT29 colon cancer xenografts. Virchows Arch. 2013, 463, 329. [Google Scholar]
- Meggyesházi, N.; Andocs, G.; Balogh, L.; Balla, P.; Kiszner, G.; Teleki, I.; Jeney, A.; Krenács, T. DNA fragmentation and caspase-independent programmed cell death by modulated electrohyperthermia. Strahlenther. Onkol. 2014, 190, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Meggyeshazi, N.; Andocs, G.; Spisak, S.; Krenacs, T. Early changes in mRNA and protein expression related to cancer treatment by modulated electro-hyperthermia. Hindawi Publ. Corp. Conf. Pap. Med. 2013, 2013, 249563. [Google Scholar]
- Jeon, T.-W.; Yang, H.; Lee, C.G.; Oh, S.T.; Seo, D.; Baik, I.H.; Lee, E.H.; Yun, I.; Park, K.R.; Lee, Y.-H. Electro-hyperthermia up-regulates tumour suppressor Septin 4 to induce apoptotic cell death in hepatocellular carcinoma. Int. J. Hyperth. 2016, 32, 648–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonald, M.; Corde, S.; Lerch, M.; Rosenfeld, A.; Jackson, M.; Tehei, M. First in vitro evidence of modulated electro-hyperthermia treatment performance in combination with megavoltage radiation by clonogenic assay. Sci. Rep. 2018, 8, 16608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forika, G. Radiotherapy and modulated electro-hyperthermia effect on Panc1 and Capan1 pancreas adenocarcinoma cell lines. Oncothermia J. 2018, 24, 455–463. [Google Scholar]
- Forika, G.; Balogh, A.; Vancsik, T.; Benyo, Z.; Krenacs, T. Apoptotic response and DNA damage of the radioresistant Panc1 pancreas adenocarcinoma to combined modulated electro hyperthermia and radiotherapy. Oncothermia J. 2020, 29, 103–109. [Google Scholar]
- Yoshikata, M.; Junki, H.; Yuta, S. Radiosensitization effect of novel cancer therapy, oncothermia toward overcoming treatment resistance. Oncothermia J. 2019, 25, 68–84. [Google Scholar]
- Prasad, B.; Kim, S.; Cho, W.; Kim, S.; Kim, J.K. Effect of tumor properties on energy absorption, temperature mapping, and thermal dose in 13.56-MHz radiofrequency hyperthermia. J. Therm. Biol. 2018, 74, 281–289. [Google Scholar] [CrossRef]
- Chen, C.-C.; Chen, C.-L.; Li, J.-J.; Chen, Y.-Y.; Wang, C.-Y.; Wang, Y.-S.; Chi, K.-H.; Wang, H.-E. Presence of Gold Nanoparticles in Cells Associated with the Cell-Killing Effect of Modulated Electro-Hyperthermia. ACS Appl. Bio Mater. 2019, 2, 3573–3581. [Google Scholar] [CrossRef]
- Besztercei, B.; Vancsik, T.; Benedek, A.; Major, E.; Thomas, M.J.; Schvarcz, C.A.; Krenács, T.; Benyó, Z.; Balogh, A. Stress-Induced, p53-Mediated Tumor Growth Inhibition of Melanoma by Modulated Electrohyperthermia in Mouse Models without Major Immunogenic Effects. Int. J. Mol. Sci. 2019, 20, 4019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oei, A.L.; Vriend, L.E.M.; Crezee, J.; Franken, N.A.P.; Krawczyk, P.M. Effects of hyperthermia on DNA repair pathways: One treatment to inhibit them all. Radiat. Oncol. 2015, 10, 165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, M.B.; Major, E.; Benedek, A.; Horváth, I.; Máthé, D.; Bergmann, R.; Szasz, A.M.; Krenacs, T.; Benyo, Z. Suppression of metastatic melanoma growth in lung by modulated electro-hyperthermia monitored by a minimally invasive heat stress testing approach in mice. Cancers 2020, 12, 3872. [Google Scholar] [CrossRef] [PubMed]
- Schvarcz, C.; Danics, L.; Krenács, T.; Viana, P.; Béres, R.; Vancsik, T.; Nagy, Á.; Gyenesei, A.; Kun, J.; Fonović, M.; et al. Modulated Electro-Hyperthermia Induces a Prominent Local Stress Response and Growth Inhibition in Mouse Breast Cancer Isografts. Cancers 2021, 13, 1744. [Google Scholar] [CrossRef]
- Andocs, G.; Meggyeshazi, N.; Balogh, L.; Spisak, S.; Maros, M.E.; Balla, P.; Kiszner, G.; Teleki, I.; Kovago, C.; Krenacs, T. Upregulation of heat shock proteins and the promotion of damage-associated molecular pattern signals in a colorectal cancer model by modulated electrohyperthermia. Cell Stress Chaperones 2014, 20, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Daguenet, E.; Louati, S.; Wozny, A.-S.; Vial, N.; Gras, M.; Guy, J.-B.; Vallard, A.; Rodriguez-Lafrasse, C.; Magné, N. Radiation-induced bystander and abscopal effects: Important lessons from preclinical models. Br. J. Cancer 2020, 123, 339–348. [Google Scholar] [CrossRef]
- Piana, R. The Abscopal Effect: A Reemerging Field of Interest. The ASCO Post 2018. Available online: https://ascopost.com/issues/november-25-2018/the-abscopal-effect-a-reemerging-field-of-interest/ (accessed on 12 December 2021).
- Hu, Z.I.; McArthur, H.L.; Ho, A.Y. The Abscopal Effect of Radiation Therapy: What Is It and How Can We Use It in Breast Cancer? Curr. Breast Cancer Rep. 2017, 9, 45–51. [Google Scholar] [CrossRef] [Green Version]
- Vancsik, T.; Máthé, D.; Horváth, I.; Várallyaly, A.A.; Benedek, A.; Bergmann, R.; Krenács, T.; Benyó, Z.; Balogh, A. Modulated Electro-Hyperthermia Facilitates NK-Cell Infiltration and Growth Arrest of Human A2058 Melanoma in a Xenograft Model. Front. Oncol. 2021, 11, 590764. [Google Scholar] [CrossRef]
- Qin, W.; Akutsu, Y.; Andocs, G.; Suganami, A.; Hu, X.; Yusup, G.; Komatsu-Akimoto, A.; Hoshino, I.; Hanari, N.; Mori, M.; et al. Modulated electro-hyperthermia enhances dendritic cell therapy through an abscopal effect in mice. Oncol. Rep. 2014, 32, 2373–2379. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.J.; Kang, S.Y.; Jo, M.S.; Suh, D.S.; Kim, K.H.; Yoon, M.S. S100 expression in dendritic cells is inversely correlated with tumor grade in endometrial carcinoma. Obstet. Gynecol. Sci. 2014, 57, 201–207. [Google Scholar] [CrossRef] [Green Version]
- Tsang, Y.W.; Huang, C.C.; Yang, K.L.; Chi, M.-S.; Chiang, H.-C.; Wang, Y.-S.; Andocs, G.; Szasz, A.; Li, W.-T. Improving immunological tumor microenvironment using electro-hyperthermia followed by dendritic cell immunotherapy. BMC Cancer 2015, 15, 708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vancsik, T.; Kiss, E.; Kovago, C.; Meggyeshazi, N.; Forika, G.; Krenacs, T. Inhibition of proliferation, induction of apoptotic cell death and immune response by modulated electro-hyperthermia in C26 colorectal cancer allografts, thermometry. Oncothermia J. 2017, 20, 277–292. [Google Scholar]
- Vancsik, T.; Kovago, C.; Kiss, E.; Papp, E.; Forika, G.; Benyo, Z.; Meggyeshazi, N.; Krenacs, T. Modulated electro-hyperthermia induced loco-regional and systemic tumor destruction in colorectal cancer allografts. J. Cancer 2018, 9, 41–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vancsik, T.; Forika, G.; Balogh, A.; Kiss, E.; Krenacs, T. Modulated electro-hyperthermia induced p53 driven apoptosis and cell cycle arrest additively support doxorubicin chemotherapy of colorectal cancer in vitro. Cancer Med. 2019, 8, 4292–4303. [Google Scholar] [CrossRef] [Green Version]
- Wismeth, C.; Dudel, C.; Pascher, C.; Ramm, P.; Pietsch, T.; Hirschmann, B.; Reinert, C.; Proescholdt, M.A.; Rümmele, P.; Schuierer, G.; et al. Transcranial electro-hyperthermia combined with alkylating chemotherapy in patients with relapsed high-grade gliomas: Phase I clinical results. J. Neuro-Oncol. 2009, 98, 395–405. [Google Scholar] [CrossRef]
- Minnaar, C.A.; Kotzen, J.A.; Ayeni, O.A.; Naidoo, T.; Tunmer, M.; Sharma, V.; Vangu, M.-D.-T.; Baeyens, A. The effect of modulated electro-hyperthermia on local disease control in HIV-positive and -negative cervical cancer women in South Africa: Early results from a phase III randomized controlled trial. PLoS ONE 2019, 14, e0217894. [Google Scholar] [CrossRef] [Green Version]
- Minnaar, C.A.; Kotzen, J.A.; Naidoo, T.; Tunmer, M.; Sharma, V.; Vangu, M.-D.-T.; Baeyens, A. Analysis of the effects of mEHT on the treatment-related toxicity and quality of life of HIV-positive cervical cancer patients. Int. J. Hyperth. 2020, 37, 263–272. [Google Scholar] [CrossRef] [Green Version]
- Minnaar, C.A.; Kotzen, J.A.; Ayeni, O.A.; Vangu, M.-D.-T.; Baeyens, A. Potentiation of the Abscopal Effect by Modulated Electro-Hyperthermia in Locally Advanced Cervical Cancer Patients. Front. Oncol. 2020, 10, 376. [Google Scholar] [CrossRef] [Green Version]
- Minnaar, C.A.; Kotzen, J.A.; Baeyens, A. Possible potentiation of the abscopal effect of ionising radiation by modulated electro-hyperthermia in locally advanced cervical cancer patients. Oncothermia J. 2018, 24, 122–132. [Google Scholar]
- Minnaar, C.; Kotzen, J. Modulated electro hyperthermia as an immune modulator with checkpoint inhibitors and radiotherapy. Eur. J. Cancer 2019, 110, S19–S20. [Google Scholar] [CrossRef]
- Chi, K.H. Tumor-directed immunotherapy: Combined radiotherapy and oncothermia. Oncothermia J. 2018, 24, 196–235. [Google Scholar]
- Szasz, A.M.; Minnaar, C.A.; Szentmartoni, G.; Szigeti, G.P.; Dank, M. Review of the clinical evidences of modulated electro-hyperthermia (mEHT) method: An update for the practicing oncologist. Front. Oncol. 2019, 9, 1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parmar, G.; Rurak, E.; Elderfield, M.; Li, K.; Soles, S.; Rinas, A. 8-year observational study on naturopathic treatment with modulated electro-hyperthermia (mEHT): A single-centre experience. In Challenges and Solutions of Oncological Hyperthermia; Szasz, A., Ed.; Cambridge Scholars: Newcastle upon Tyne, UK, 2020; pp. 227–266. [Google Scholar]
- Fiorentini, G.; Sarti, D.; Gadaleta, C.D.; Ballerini, M.; Fiorentini, C.; Garfagno, T.; Ranieri, G.; Guadagni, S. A Narrative Review of Regional Hyperthermia: Updates from 2010 to 2019. Integr. Cancer Ther. 2020, 19, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Fiorentini, G.; Giovanis, P.; Rossi, S.; Dentico, P.; Paola, R.; Turrisi, G.; Bernardeschi, P. A phase II clinical study on relapsed malignant gliomas treated with electro-hyperthermia. Vivo 2006, 20, 721–724. [Google Scholar]
- Sahinbas, H.; Groenemeyer, D.H.W.; Boecher, E.; Szasz, A. Retrospective clinical study of adjuvant electro-hyperthermia treatment for advanced brain-gliomas. Dtsch. Z. Für Onkol. 2007, 39, 154–160. [Google Scholar] [CrossRef] [Green Version]
- Hager, E.D.; Sahinbas, H.; Groenemeyer, D.H.; Migeod, F. Prospective phase II trial for recurrent high-grade gliomas with capacitive coupled low radiofrequency (LRF) hyperthermia. J. Clin. Oncol. 2008, 26, 2047. [Google Scholar] [CrossRef]
- Fiorentini, G.; Sarti, D.; Milandri, C.; Dentico, P.; Mambrini, A.; Fiorentini, C.; Mattioli, G.; Casadei, V.; Guadagni, S. Modulated Electrohyperthermia in Integrative Cancer Treatment for Relapsed Malignant Glioblastoma and Astrocytoma: Retrospective Multicenter Controlled Study. Integr. Cancer Ther. 2018, 18, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Minnaar, C.; Baeyens, A.; Kotzen, J. O34. Update on phase III randomized clinical trial investigating the effects of the addition of electro-hyperthermia to chemoradiotherapy for cervical cancer patients in South Africa. Phys. Med. 2016, 32, 151–152. [Google Scholar] [CrossRef]
- Pesti, L.; Dankovics, Z.; Lorencz, P.; Csejtei, A. Treatment of advanced cervical cancer with complex chemoradio–hyperthermia. Hindawi Publ. Corp. Conf. Pap. Med. 2013, 2013, 192435. [Google Scholar] [CrossRef] [Green Version]
- Ou, J.; Zhu, X.; Chen, P.; Du, Y.; Lu, Y.; Peng, X.; Bao, S.; Wang, J.; Zhang, X.; Zhang, T.; et al. A randomized phase II trial of best supportive care with or without hyperthermia and vitamin C for heavily pretreated, advanced, refractory non-small-cell lung cancer. J. Adv. Res. 2020, 24, 175–182. [Google Scholar] [CrossRef]
- Szasz, A. Current Status of Oncothermia Therapy for Lung Cancer. Korean J. Thorac. Cardiovasc. Surg. 2014, 47, 77–93. [Google Scholar] [CrossRef] [PubMed]
- You, S.H.; Kim, S. Feasibility of modulated electro-hyperthermia in preoperative treatment for locally-advanced rectal cancer: Early phase 2 clinical results. Neoplasma 2019, 67, 677–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeung, T.S.; Ma, S.Y.; Choi, J.; Yu, J.; Lee, S.Y.; Lim, S. Results of Oncothermia Combined with Operation, Chemotherapy and Radiation Therapy for Primary, Recurrent and Metastatic Sarcoma. Case Rep. Clin. Med. 2015, 04, 157–168. [Google Scholar] [CrossRef] [Green Version]
- Fiorentini, G.; Sarti, D.; Casadei, V.; Milandri, C.; Dentico, P.; Mambrini, A.; Nani, R.; Fiorentini, C.; Guadagni, S. Modulated electro-hyperthermia as palliative treatment for pancreas cancer: A retrospective observational study on 106 patients. Integr. Cancer Ther. 2019, 18, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Dani, A.; Varkonyi, A.; Magyar, T.; Szasz, A. Clinical study for advanced pancreas cancer treated by oncothermia. Forum Hyperthermie 2008, 1, 13–20. [Google Scholar]
- Ranieri, G.; Ferrari, C.; di Palo, A.; Marech, I.; Porcelli, M.; Falagario, G.; Ritrovato, F.; Ramuni, L.; Fanelli, M.; Rubini, G.; et al. Bevacizumab-Based Chemotherapy Combined with, Regional Deep Capacitive Hyperthermia in Metastatic Cancer Patients: A Pilot Study. Int. J. Mol. Sci. 2017, 18, 1458. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.H.; Cha, J.; You, S.H. Beneficial effects of modulated electro-hyperthermia during neoadjuvant treatment for locally advanced rectal cancer. Int. J. Hyperth. 2021, 38, 144–151. [Google Scholar] [CrossRef]
- Fiorentini, G.; Sarti, D.; Casadei, V.; Milandri, C.; Dentico, P.; Mambrini, A.; Guadagni, S. Modulated electro-hyperthermia for the treatment of relapsed brain gliomas. In Challenges and Solutions of Oncological Hyperthermia; Szasz, A., Ed.; Cambridge Scholars: Newcastle upon Tyne, UK, 2020; pp. 110–125. [Google Scholar]
- Wookyeom, Y.; Han, G.H.; Shin, H.Y.; Lee, E.-J.; Cho, H.; Chay, D.B.; Kim, J.-H. Combined treatment with modulated electro-hyperthermia and an autophagy inhibitor effectively inhibit ovarian and cervical cancer growth. Int. J. Hyperth. 2018, 36, 9–20. [Google Scholar]
- Arrojo, E.E. The position of modulated electro-hyperthermia (oncothermia) in combination with standard chemo- and radiotherapy in clinical practice–Highlights of upcoming phase III clinical studies in hospital Universitario Marqués de Val-decilla (HUMV). In Challenges and Solutions of Oncological Hyperthermia; Szasz, A., Ed.; Cambridge Scholars: Newcastle upon Tyne, UK, 2020; pp. 91–104. [Google Scholar]
- van Gool, S.W.; Makalowski, J.; Feyen, O.; Prix, L.; Schirrmacher, V.; Stuecker, W. The induction of immunogenic cell death (ICD) during maintenance chemotherapy and subsequent multimodal immunotherapy for glioblastoma (GBM). Austin Oncol. Case Rep. 2018, 3, 1–8. [Google Scholar]
- Kurakin, A. The self-organizing fractal theory as a universal discovery method: The phenomenon of life. Theor. Biol. Med Model. 2011, 8, 1–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haken, H. Self-Organization and Information. Phys. Scr. 1987, 35, 247–254. [Google Scholar] [CrossRef]
- Sornette, D. Chaos, Fractals, Self-Organization and Disorder: Concepts and Tools; Springer: Berlin/Heidelberg, Germany; Los Angeles, CA, USA, 2000. [Google Scholar]
- Sha, L.; Ward, E.R.; Stroy, B. A Review of Dielectric Properties of Normal and Malignant Breast Tissue. In Proceedings of the IEEE Southeast Con. 2002, Columbia, SC, USA, 5–7 April 2002; pp. 457–462. [Google Scholar]
- Scholz, B.; Anderson, R. On electrical impedance scanning-principles and simulations. Electromedica 2000, 68, 35–44. [Google Scholar]
- Haemmerich, D.; Staelin, S.T.; Tsai, J.Z.; Tungjitkusolmun, S.; Mahvi, D.M.; Webster, J.G. In vivo electrical conductivity of hepatic tumors. Physiol. Meas. 2003, 24, 251–260. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.R.; Foster, K.R.; Wolf, G.L. Dielectric Properties of VX-2 Carcinoma Versus Normal Liver Tissue. IEEE Trans. Biomed. Eng. 1986, 33, 522–524. [Google Scholar] [CrossRef]
- Gregorie, V.; Chiti, A. PET in radiotherapy planning: Particularly exquisite test or pending and experimental tool? Radiother. Oncol. 2010, 96, 275–276. [Google Scholar] [CrossRef]
- Cope, F.W. A review of the applications of solid state physics concepts to biological systems. J. Biol. Phys. 1975, 3, 1–41. [Google Scholar] [CrossRef]
- Damadian, R. Tumor Detection by Nuclear Magnetic Resonance. Science 1971, 171, 1151–1153. [Google Scholar] [CrossRef]
- Hazlewood, C.F.; Nichols, B.L.; Chamberlain, N.F. Evidence for the Existence of a Minimum of Two Phases of Ordered Water in Skeletal Muscle. Nature 1969, 222, 747–750. [Google Scholar] [CrossRef]
- Durney, C.H.; Johnson, C.C.; Barber, P.W.; Massoudi, H.; Iskander, M.F.; Allen, S.J.; Mitchell, J.C. Descriptive summary: Radiofrequency radiation dosimetry handbook-Second edition. Radio Sci. 1979, 14, 5–7. [Google Scholar] [CrossRef]
- Szent-Györgyi, A. The living state and cancer. Physiol. Chem. Phys. 1980, 12, 99–110. [Google Scholar] [CrossRef]
- Szent-Györgyi, A. Electronic Biology and Cancer; Marcel Dekker: New York, NY, USA, 1998. [Google Scholar]
- Szent-Györgyi, A. Bioelectronics, a Study on Cellular Regulations, Defense and Cancer; Acadamic Press: New York, NY, USA; London, UK, 1968. [Google Scholar]
- Pething, R. Dielectric and Electronic Properties of Biological Materials; John Wiley and Sons: New York, NY, USA, 1979. [Google Scholar]
- Volkov, V.V.; Palmer, D.J.; Righini, R. Distinct Water Species Confined at the Interface of a Phospholipid Membrane. Phys. Rev. Lett. 2007, 99, 078302. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.M.; Cleary, S.F. Absorbed Energy Distribution from Radiofrequency Electromagnetic Radiation in a Mammalian Cell Model: Effect of Membrane-Bound Water. Bioelectromagnetics 1995, 16, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Hendry, B. Membrane Physiology and Membrane Excitation; Croom Helm: London, UK, 1981. [Google Scholar]
- Ma, Y.; Poole, K.; Goyette, J.; Gaus, K. Introducing Membrane Charge and Membrane Potential to T Cell Signaling. Front. Immunol. 2017, 8, 1513. [Google Scholar] [CrossRef] [PubMed]
- Horváth, I.; Multhoff, G.; Sonnleitner, A.; Vígh, L. Membrane-associated stress proteins: More than simply chaperones. Biochim. Biophys. Acta 2008, 1778, 1653–1664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicolau, D.V.; Burrage, K.; Parton, R.G.; Hancock, J.F. Identifying Optimal Lipid Raft Characteristics Required to Promote Nanoscale Protein-Protein Interactions on the Plasma Membrane. Mol. Cell. Biol. 2006, 26, 313–323. [Google Scholar] [CrossRef] [Green Version]
- Nicolson, G.L. The Fluid—Mosaic Model of Membrane Structure: Still relevant to understanding the structure, function and dynamics of biological membranes after more than 40 years. Biochim. Biophys. Acta 2014, 1838, 1451–1466. [Google Scholar] [CrossRef] [Green Version]
- Gramse, G.; Dols-Perez, A.; Edwards, M.A.; Fumagalli, L.; and Gomila, G. Nanoscale Measurement of the Dielectric Constant of Supported Lipid Bilayers in Aqueous Solutions with Electrostatic Force Microscopy. J. Biophys. 2013, 104, 1257–1262. [Google Scholar] [CrossRef] [Green Version]
- Dharia, S. Spatially and Temporally Resolving Radio-Frequency Changes in Effective Cell Membrane Capacitance. Ph.D. Thesis, University of Utah, Salt Lake City, UT, USA, 2011. [Google Scholar]
- Pike, L.J. Lipid rafts: Bringing order to chaos. J. Lipid Res. 2003, 44, 655–667. [Google Scholar] [CrossRef] [Green Version]
- Andersen, O.S.; Koeppe, I.I.; and Roger, E. Bilayer Thickness and Membrane Protein Function: An Energetic Perspective. Annu. Rev. Biophys. Biomol. Struct. 2007, 36, 107–130. [Google Scholar] [CrossRef] [Green Version]
- Govorov, A.O.; Richardson, H.H. Generating heat with metal nanoparticles. Nano Today 2007, 2, 30–38. [Google Scholar] [CrossRef]
- Potoyan, D.A.; Wolynes, P.G. On the dephasing of genetic oscillators. Proc. Natl. Acad. Sci. USA 2014, 111, 2391–2396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ptitsyn, A.A.; Zvonic, S.; Gimble, J.M. Digital Signal Processing Reveals Circadian Baseline Oscillation in Majority of Mammalian Genes. PLOS Comput. Biol. 2007, 3, e120. [Google Scholar] [CrossRef] [PubMed]
- Carey, S.P.; Kraning-Rush, C.M.; Williams, R.M.; Reinhart-King, C.A. Biophysical control of invasive tumor cell behavior by extracellular matrix microarchitecture. Biomaterials 2012, 33, 4157–4165. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wang, X.; Wohland, T.; Sampath, K. Extracellular interactions and ligand degradation shape the nodal morphogen gradient. Elife 2016, 5, e13879. [Google Scholar] [CrossRef]
- Szendro, P.; Vincze, G.; Szasz, A. Pink noise behaviour of the biosystems. Eur. Biophys. J. 2001, 30, 227–231. [Google Scholar] [CrossRef]
- Szendro, P.; Vincze, G.; Szasz, A. Bio-response to white noise excitation. Electro- Magn. 2001, 20, 215–229. [Google Scholar] [CrossRef]
- Nasir, N.; Al Ahmad, M. Cells Electrical Characterization: Dielectric Properties, Mixture, and Modeling Theories. J. Eng. 2020, 2020, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Cole, K.S. Membranes, Ions and Impulses; University of California Press: Berkeley, CA, USA, 1968. [Google Scholar]
- Schwan, H.P. Determination of biological impedances. In Physical Techniques in Biological Research; Academic Press: New York, NY, USA, 1963; pp. 323–406. [Google Scholar]
- Schwan, H.P.; Takashima, S. Dielectric behavior of biological cells and membranes. Bull. Inst. Chem. Res. 1991, 69, 459–475. [Google Scholar]
- Anderson, J.C. Dielectrics; Chapman & Hall: London, UK, 1964. [Google Scholar]
- Grant, E.H.; Sheppard, R.J.; South, S.P. Dielectric Behavior of Biological Molecules in Solution; Clarendon Press: Oxford, UK, 1978. [Google Scholar] [CrossRef]
- Pennock, B.E.; Schwan, H.P. Further observations on the electrical properties of hemoglobin-bound water. J. Phys. Chem. 1969, 73, 2600–2610. [Google Scholar] [CrossRef]
- Pethig, R.R. Dielectrophoresis: Theory, Methodology and Biological Applications; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Asami, K. Characterization of biological cells by dielectric spectroscopy. J. Non-Cryst. Solids 2002, 305, 268–277. [Google Scholar] [CrossRef]
- Pauly, H.; Schwan, H.P. Über die Impedanz einer Suspension von kugelförmigen Teilchen mit einer Schale. Z. Für Nat. B 1959, 14, 125–131. [Google Scholar] [CrossRef]
- Stubbe, M.; Gimsa, J. Maxwell’s Mixing Equation Revisited: Characteristic Impedance Equations for Ellipsoidal Cells. Biophys. J. 2015, 109, 194–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pliquett, F.; Pliquett, U. Tissue impedance, measured by pulse deformation. In Proceedings of the 8th International Conference on Electrical Bio-impedance, University of Kuopio, Kuopio, Finland, 28–31 July 1992; pp. 179–181. [Google Scholar]
- Loft, S.M.; Conway, J.; Brown, B.H. Bioimpedance and cancer therapy. In Proceedings of the 8th International Conference on Electrical Bio-impedance, University of Kuopio, Kuopio, Finland, 28–31 July 1992; pp. 119–121. [Google Scholar]
- Tan, L.T.-H.; Chan, K.-G.; Pusparajah, P.; Lee, W.-L.; Chuah, L.-H.; Khan, T.M.; Lee, L.-H.; Goh, B.-H. Targeting membrane lipid a potential cancer cure? Front. Pharmacol. 2017, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Chidambaram, R.; Ramanadham, M. Hydrogen bonding in biological molecules—An update. Phys. B Condens. Matter 1991, 174, 300–305. [Google Scholar] [CrossRef]
- Meggyeshazi, N. Studies on Modulated Electrohyperthermia Induced Tumor Cell Death in a Colorectal Carcinoma Model. Ph.D. Thesis, Pathological Sciences Doctoral School, Semmelweis University, Budapest, Hungary, 2015. [Google Scholar]
- Wust, P.; Ghadjar, P.; Nadobny, J.; Beck, M.; Kaul, D.; Winter, L.; Zschaeck, S. Physical analysis of temperature-dependent effects of amplitude-modulated electromagnetic hyperthermia. Int. J. Hyperth. 2019, 36, 1245–1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wust, P.; Nadobny, J.; Zschaeck, S.; Ghadjar, P. Physics of hyperthermia–Is physics really against us? In Challenges and Solutions of Oncological Hyperthermia; Szasz, A., Ed.; Cambridge Scholars: Newcastle upon Tyne, UK, 2020; pp. 346–376. [Google Scholar]
- Romanenko, S.; Begley, R.; Harvey, A.R.; Hool, L.; Wallace, V. The interaction between electromagnetic fields at megahertz, gigahertz and terahertz frequencies with cells, tissues and organisms: Risks and potential. J. R. Soc. Interface 2017, 14, 20170585. [Google Scholar] [CrossRef] [Green Version]
- Szasz, O.; Szasz, A. Heating, Efficacy and Dose of Local Hyperthermia. Open J. Biophys. 2016, 6, 10–18. [Google Scholar] [CrossRef] [Green Version]
- Szasz, O. Bioelectromagnetic Paradigm of Cancer Treatment—Modulated Electro-Hyperthermia (mEHT). Open J. Biophys. 2019, 9, 98–109. [Google Scholar] [CrossRef] [Green Version]
- Kao, P.H.J.; Chen, C.H.; Chang, Y.W.; Lin, C.-S.; Chiang, H.-C.; Huang, C.-C.; Chi, M.-S.; Yang, K.-L.; Li, W.-T.; Kao, S.-J.; et al. Relationship between energy dosage and apoptotic cell death by modulated electro-hyperthermia. Sci. Rep. 2020, 10, 8936. [Google Scholar] [CrossRef]
- Almeida, V.M.; Marana, S.R. Optimum temperature may be a misleading parameter in enzyme characterization and application. PLoS ONE 2019, 14, e0212977. [Google Scholar] [CrossRef] [Green Version]
- Eppink, B.; Krawczyk, P.M.; Stap, J.; Kanaar, R. Hyperthermia induced DNA repair deficiency suggests novel therapeutic anti-cancer strategies. Int. J. Hyperth. 2012, 28, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Datta, N.R.; Bodis, S. Hyperthermia with radiotherapy reduces tumor alpha/beta: Insights from trials of thermoradiotherapy vs radiotherapy alone. Radiother. Oncol. 2019, 138, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fowler, J.F. The linear-quadratic formula and progress in fractionated radiotherapy. Br. J. Radiol. 1989, 62, 679–694. [Google Scholar] [CrossRef] [PubMed]
- Kok, H.P.; Crezee, J.; Franken, N.; Stalpers, L.J.; Barendsen, G.W.; Bel, A. Quantifying the Combined Effect of Radiation Therapy and Hyperthermia in Terms of Equivalent Dose Distributions. Int. J. Radiat. Oncol. 2014, 88, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-S.; Wang, H.-J.; Qian, H.-L. Biological effects of radiation on cancer cells. Mil. Med. Res. 2018, 5, 1–10. [Google Scholar] [CrossRef]
- Zalba, S.; Ten Hagen, T.L.M. Cell membrane modulation as adjuvant in cancer therapy. Cancer Treat. Rev. 2016, 52, 48–57. [Google Scholar] [CrossRef] [Green Version]
- Mendez, F.; Sandigursky, M.; Franklin, W.A.; Kenny, M.K.; Kureekattil, R.; Bases, R. Heat-Shock Proteins Associated with Base Excision Repair Enzymes in HeLa Cells. Radiat. Res. 2000, 153, 186–195. [Google Scholar] [CrossRef]
- Gaitanaki, C.; Mastri, M.; Aggeli, I.-K.S.; Beis, I. Differential roles of p38-MAPK and JNKs in mediating early protection or apoptosis in the hyperthermic perfused amphibian heart. J. Exp. Biol. 2008, 211, 2524–2532. [Google Scholar] [CrossRef] [Green Version]
- Daniel, R.M.; Danson, M.J. Temperature and the catalytic activity of enzymes: A fresh understanding. FEBS Lett. 2013, 587, 2738–2743. [Google Scholar] [CrossRef]
- Vashum, S.; Singh, I.R.R.; Das, S.; Azharuddin, M.; Vasudevan, P. Quantification of DNA double-strand break induced by radiation in cervix cancer cells: In vitro study. J. Radiother. Pract. 2019, 18, 55–62. [Google Scholar] [CrossRef]
- Macphail, S.H.; Banáth, J.P.; Chu, E.H.M.; Lambur, H.; Olive, P.L. Expression of phosphorylated histone H2AX in cultured cell lines following exposure to X-rays. Int. J. Radiat. Biol. 2003, 79, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Banáth, J.P.; MacPhail, S.H.; Olive, P.L. Radiation sensitivity, H2AX phosphorylation, and kinetics of repair of DNA strand breaks in irradiated cervical cancer cell lines. Cancer Res. 2004, 64, 7144–7149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mei, X.; Ten Cate, R.; van Leeuwen, C.M.; Rodermond, H.M.; de Leeuw, L.; Dimitrakopoulou, D.; Stalpers, L.J.A.; Crezee, J.; Kok, H.P.; Franken, N.A.P.; et al. Radiosensitization by hyperthermia: The effects of temperature, sequence, and time interval in cervical cell lines. Cancers 2020, 12, 582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Leuwen, C.M.; Oei, A.L.; Chin, K.W.T.K.; Crezee, J.; Bel, A.; Westerman, A.M.; Buist, M.R.; Franken, N.A.P.; Stalpers, L.J.A.; Kok, H.P. A short time interval between radiotherapy and hyperthermia reduces in-filed recurrence and mortality in women with advanced cervical cancer. Radiat. Oncol. 2017, 12, 1–8. [Google Scholar] [CrossRef]
- van Leuwen, C.M.; Oei, A.L.; Ten Cate, R.; Franken, N.A.P.; Bel, A.; Stalpers, L.J.A.; Crezee, J.; Kok, H.P. Measurement and analysis of the impact of time interval temperature and radiation dose on tumor cell survival and its application in thermoradiotherapy plan evaluation. Int. J. Hyperth. 2018, 34, 30–38. [Google Scholar] [CrossRef] [Green Version]
- Raaphorst, G.P. Thermal radiosensitization in vitro. In Hyperthermia and Oncology; Urano, M., Douple, E., Eds.; VSP: Utrecht, The Netherlands, 1994; Volume 2, pp. 17–51. [Google Scholar]
- Overgaard, J. Simultaneous and sequential hyperthermia and radiation treatment of an experimental tumor and its surrounding normal tissue in vivo. Int. J. Radiat. Oncol. 1980, 6, 1507–1517. [Google Scholar] [CrossRef]
- Kroesen, M.; Mulder, H.T.; van Holthe, J.M.L.; Aangeenbrug, A.A.; Mens, J.W.M.; van Doorn, H.C.; Paulides, M.M.; Oomen-de Hoop, E.; Vernhout, R.M.; Lutgens, L.C.; et al. The Effect of the Time Interval Between Radiation and Hyperthermia on Clinical Outcome in 400 Locally Advanced Cervical Carcinoma Patients. Front. Oncol. 2019, 9, 134. [Google Scholar] [CrossRef]
- Crezee, H.; Kok, H.P.; Oei, A.L.; Franken, N.A.P.; Stalpers, L.J.A. The Impact of the Time Interval Between Radiation and Hyperthermia on Clinical Outcome in Patients with Locally Advanced Cervical Cancer. Front. Oncol. 2019, 9, 412. [Google Scholar] [CrossRef] [Green Version]
- Kroesen, M.; Mulder, H.T.; van Rhoon, G.C.; Franckena, M. Commentary: The Impact of the Time Interval Between Radiation and Hyperthermia on Clinical Outcome in Patients with Locally Advanced Cervical Cancer. Front. Oncol. 2019, 9, 1387. [Google Scholar] [CrossRef] [Green Version]
- Horsman, M.R.; Overgaard, J. Thermal radiosensitization in animal tumors: The potential for therapeutic gain. In Hyperthermia and Oncology; Urano, M., Douple, E., Eds.; VSP: Utrecht, The Netherlands, 1989; Volume 2, pp. 113–145. [Google Scholar]
- Aguilar, A.; Ho, M.; Chang, E.; Carlson, K.; Natarajan, A.; Marciano, T.; Bomzon, Z.; Patel, C. Permeabilizing Cell Membranes with Electric Fields. Cancers 2021, 13, 2283. [Google Scholar] [CrossRef]
- Okamura, Y.; Kawanabe, A.; Kawai, T. Voltage-Sensing Phosphatases: Biophysics, Physiology, and Molecular Engineering. Physiol. Rev. 2018, 98, 2097–2131. [Google Scholar] [CrossRef] [PubMed]
- Szasz, A.M.; Arkosy, P.; Arrojo, E.E.; Bakacs, T.; Balogh, A.; Barich, A.; Borbenyi, E.; Chi, K.H.; Csoszi, T.; Daniilidis, L.; et al. Guidelines for local hyperthermia treatment in oncology. In Challenges and Solutions of Oncological Hyperthermia; Szasz, A., Ed.; Cambridge Scholars: Newcastle upon Tyne, UK, 2020; pp. 32–71. [Google Scholar]
- Griffin, R.J.; Dings, R.P.M.; Jamshidi-Parsian, A.; Song, C.W. Mild temperature hyperthermia and radiation therapy: Role of tumor vascular thermotolerance and relevant physiological factors. Int. J. Hyperth. 2010, 26, 256–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, W.; Kim, M.S.; Kim, H.J.; Lee, E.; Jeong, J.-H.; Park, I.; Jeong, Y.K.; Jang, W.I. Role of HIF-1α in response of tumors to a combination of hyperthermia and radiation in vivo. Int. J. Hyperth. 2017, 34, 276–283. [Google Scholar] [CrossRef] [Green Version]
- Hance, M.W.; Nolan, K.D.; Isaacs, J.S. The Double-Edged Sword: Conserved Functions of Extracellular Hsp90 in Wound Healing and Cancer. Cancers 2014, 6, 1065–1097. [Google Scholar] [CrossRef]
- Tittelmeier, J.; Nachman, E.; Nussbaum-Krammer, C. Molecular Chaperones: A Double-Edged Sword in Neurodegenerative Diseases. Front. Aging Neurosci. 2020, 12, 581374. [Google Scholar] [CrossRef]
- Giri, B.; Sethi, V.; Modi, S.; Garg, B.; Banerjee, S.; Saluja, A.; Dudeja, V. Heat shock protein 70 in pancreatic diseases: Friend or foe. J. Surg. Oncol. 2017, 116, 114–122. [Google Scholar] [CrossRef]
- Pockley, A.G.; Multhoff, G. Cell Stress Proteins in Extracellular Fluids: Friend or Foe? Ciba Found. Symp. Nat. Sleep 2008, 291, 86–100. [Google Scholar] [CrossRef]
- Wu, T.; Tanguay, R. Antibodies against heat shock proteins in environmental stresses and diseases: Frend or foe? Cell Stress Chaper. 2006, 11, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Taha, E.A.; Ono, K.; Eguchi, T. Roles of Extracellular HSPs as Biomarkers in Immune Surveillance and Immune Evasion. Int. J. Mol. Sci. 2019, 20, 4588. [Google Scholar] [CrossRef] [Green Version]
- Derer, A.; Deloch, L.; Rubner, Y.; Fietkau, R.; Frey, B.; Gaipl, U.S. Radio-Immunotherapy-Induced Immunogenic Cancer Cells as Basis for Induction of Systemic Anti-Tumor Immune Responses–Pre-Clinical Evidence and Ongoing Clinical Applications. Front. Immunol. 2015, 6, 505. [Google Scholar] [CrossRef] [Green Version]
- Stagg, A.J.; Knight, S.C. Antigen-Presenting Cells, Nature. 2001. Available online: http://labs.icb.ufmg.br/lbcd/pages2/bernardo/Bernardo/Artigos/Antigen-presenting%20Cells.pdf (accessed on 7 October 2020).
- Rapoport, B.L.; Anderson, R. Realizing the Clinical Potential of Immunogenic Cell Death in Cancer Chemotherapy and Radiotherapy. Int. J. Mol. Sci. 2019, 20, 959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szasz, O. Local treatment with systemic effect: Abscopal outcome. In Challenges and Solutions of Oncological Hyperthermia; Szasz, A., Ed.; Cambridge Scholars: Newcastle upon Tyne, UK, 2020; pp. 192–205. [Google Scholar]
- Mole, R.H. Whole body irradiation-radiology or medicine? Br. J. Radiol. 1953, 26, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.T.; Elmali, A.; Yazici, G. Abscopal Effect, From Myth to Reality: From Radiation Oncologists’ Perspective. Cureus 2019, 11, e3860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Craig, D.J.; Nanavay, N.S.; Devanaboyina, M.; Stanbery, L.; Hamouda, D.; Edelman, G.; Dworkin, L.; Nemunaitis, J.J. The abscopal effect of radiation therapy. Future Oncol. 2021, 17, 1683–1694. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dong, Y.; Kong, L.; Shi, F.; Zhu, H.; Yu, J. Abscopal effect of radiotherapy combined with immune checkpoint inhibitors. J. Hematol. Oncol. 2018, 11, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Reynders, K.; Illidge, T.; Siva, S.; Chang, J.Y.; de Ruysscher, D. The abscopal effect of local radiotherapy: Using immunotherapy to make a rare event clinically relevant. Cancer Treat. Rev. 2015, 41, 503–510. [Google Scholar] [CrossRef] [Green Version]
- Dewan, M.Z.; Galloway, A.E.; Kawashima, N.; Dewyngaert, J.K.; Babb, J.S.; Formenti, S.C.; Demaria, S. Fractionated but not single dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin. Cancer Res. 2009, 15, 5379–5388. [Google Scholar] [CrossRef] [Green Version]
- Chi, K.H. Tumour-directed immunotherapy: Clinical results of radiotherapy with modulated electro-hyperthermia. In Challenges and Solutions of Oncological Hyperthermia; Szasz, A., Ed.; Cambridge Scholars: Newcastle upon Tyne, UK, 2020; pp. 206–226. [Google Scholar]
- Dank, M.; Meggyeshazi, N.; Szigeti, G.; Andocs, G. Immune effects by selective heating of membrane rafts of cancer-cells. J. Clin. Oncol. 2016, 34, e14571. [Google Scholar] [CrossRef]
- Andocs, G.; Meggyeshazi, N.; Okamoto, Y.; Balogh, L.; Kovago, C.; Szasz, O. Oncothermia treatment induced immunogenic cancer cell death. Oncothermia J. 2013, 9, 28–37. [Google Scholar]















| Tumor Characteristics | Oncological Hyperthermia Including All Technical Solutions | Synergy with Radiotherapy |
|---|---|---|
| Cell cycle | Arrests the cycle of cells at the S stage, activates the malignant cell from its dormant (G0) phase making attack possible for chemo- and radio-therapies | Radiotherapy arrests the M/G2 stages of the cell cycle well completes the arrest |
| pH dependence | Kills cancer cells in an acidic environment (Hippocrates’ original idea) | It kills cancer cells in an alkaline environment, completes the cell desertion in all environmental conditions |
| Oxygenation | Acts in the hypoxic state | Acts in an oxygenated state |
| Increased temperature | Heated tumor mass increases the oxygen delivery | Makes strand breaks on DNA, the fixing of which means oxygen blocks the reparation |
| Synergistic Addition of Modulated Electrohyperthermia | |
|---|---|
| Nanoscopic action | Selects malignant cells and nonthermally excites, marginal heating of the healthy cells renders less vulnerable to ionizing radiation |
| Apoptotic effect | Mostly natural apoptosis, no inflammation, no large cytokine liberation, no extra injury current, no extra pH hypoxia |
| Immune effect | Immunogenic processes, abscopal effect. Both the innate and adaptive immune system are activated, vaccination facility (patented) |
| Homeostatic effect | Harmonized with homeostatic controls, the temperature increase in the nuclei is moderate, does not make an additional enzymatic activity for reparation |
| Side effects | Lower incident power puts less load on the skin, which is anyway irritated by radiotherapy, so the synergy has fewer adverse effects |
| Quality of life | Improves quality of life by reducing side effects |
| The broad range of application | Possible to combine with radiotherapy in localizations which were not possible with radiative hyperthermia (like the brain) |
| Applicable for palliative conditions | Resensitizes to radiotherapy in highly metastatic advanced refractory cases, when conventional therapies are ineffective |
| Long-time application | mEHT is applicable as a chronic treatment for as long as is necessary with radiotherapy complementation |
| Applicability | mEHT is applicable with most comorbidities as well as in combination with any other oncotherapies |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szasz, A. Heterogeneous Heat Absorption Is Complementary to Radiotherapy. Cancers 2022, 14, 901. https://doi.org/10.3390/cancers14040901
Szasz A. Heterogeneous Heat Absorption Is Complementary to Radiotherapy. Cancers. 2022; 14(4):901. https://doi.org/10.3390/cancers14040901
Chicago/Turabian StyleSzasz, Andras. 2022. "Heterogeneous Heat Absorption Is Complementary to Radiotherapy" Cancers 14, no. 4: 901. https://doi.org/10.3390/cancers14040901
APA StyleSzasz, A. (2022). Heterogeneous Heat Absorption Is Complementary to Radiotherapy. Cancers, 14(4), 901. https://doi.org/10.3390/cancers14040901