Biological Role of Tumor/Stromal CXCR4-CXCL12-CXCR7 in MITO16A/MaNGO-OV2 Advanced Ovarian Cancer Patients
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
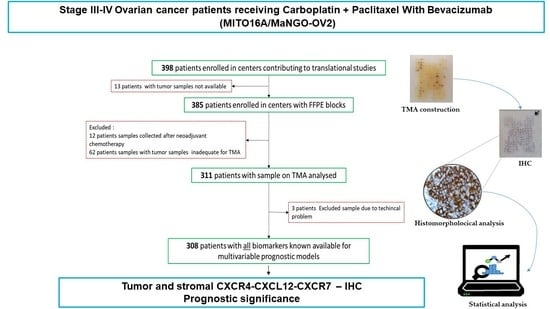
2.1. Description of the Study
2.2. Sample Collection and Review
2.3. Preparation of Tissue Microarray
2.4. Immunohistochemistry (IHC)
2.5. Statistical Analyses
3. Results
3.1. Patients Clinical and Pathological Data
3.2. CXCR4-CXCL12-CXCR7 Axis Is Highly Expressed in Advanced EOC
3.3. Prognostic Meaning for CXCR4-CXCL12-CXCR7 in Advanced EOC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arend, R.; Martinez, A.; Szul, T.; Birrer, M.J. Biomarkers in ovarian cancer: To be or not to be. Cancer 2019, 125 (Suppl. S24), 4563–4572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaitskell, K.; Green, J.; Pirie, K.; Barnes, I.; Hermon, C.; Reeves, G.K.; Beral, V. Histological subtypes of ovarian cancer associated with parity and breastfeeding in the prospective Million Women Study. Int. J. Cancer 2018, 142, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Tropé, C.; Kaern, J. Adjuvant Chemotherapy for Early-Stage Ovarian Cancer: Review of the Literature. J. Clin. Oncol. 2007, 25, 2909–2920. [Google Scholar] [CrossRef] [PubMed]
- Bast, R.C., Jr.; Hennessy, B.; Mills, G.B. The biology of ovarian cancer: New opportunities for translation. Nat. Rev. Cancer 2009, 9, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Colombo, N.; Sessa, C.; du Bois, A.; Ledermann, J.; McCluggage, W.G.; McNeish, I.; Morice, P.; Pignata, S.; Ray-Coquard, I.; Vergote, I.; et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: Pathology and molecular biology, early and advanced stages, borderline tumours and recurrent diseasedagger. Ann. Oncol. 2019, 30, 672–705. [Google Scholar] [CrossRef] [Green Version]
- Ledermann, J.A.; Raja, F.A.; Fotopoulou, C.; Gonzalez-Martin, A.; Colombo, N.; Sessa, C. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013, 24, vi24–vi32. [Google Scholar] [CrossRef]
- Moore, K.; Colombo, N.; Scambia, G.; Kim, B.G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2018, 379, 2495–2505. [Google Scholar] [CrossRef]
- Poveda, A.; Floquet, A.; Ledermann, J.A.; Asher, R.; Penson, R.T.; Oza, A.M.; Korach, J.; Huzarski, T.; Pignata, S.; Friedlander, M.; et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): A final analysis of a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2021, 22, 620–631. [Google Scholar] [CrossRef]
- Buys, S.S.; Partridge, E.; Black, A.; Johnson, C.C.; Lamerato, L.; Isaacs, C.; Reding, D.J.; Greenlee, R.T.; Yokochi, L.A.; Kessel, B.; et al. Effect of screening on ovarian cancer mortality: The Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial. JAMA 2011, 305, 2295–2303. [Google Scholar] [CrossRef]
- Jiang, Y.P.; Wu, X.H.; Shi, B.; Wu, W.X.; Yin, G.R. Expression of chemokine CXCL12 and its receptor CXCR4 in human epithelial ovarian cancer: An independent prognostic factor for tumor progression. Gynecol. Oncol. 2006, 103, 226–233. [Google Scholar] [CrossRef]
- Mao, T.L.; Fan, K.F.; Liu, C.L. Targeting the CXCR4/CXCL12 axis in treating epithelial ovarian cancer. Gene Ther. 2017, 24, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Figueras, A.; Alsina-Sanchis, E.; Lahiguera, A.; Abreu, M.; Muinelo-Romay, L.; Moreno-Bueno, G.; Casanovas, O.; Graupera, M.; Matias-Guiu, X.; Vidal, A.; et al. A Role for CXCR4 in Peritoneal and Hematogenous Ovarian Cancer Dissemination. Mol. Cancer Ther. 2018, 17, 532–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turut, F.A.; Acidereli, H.; Cevik, O. Suppression of MicroRNA-144 Promotes CXCR4 and CXCL12 Expression and Downregulates Apoptosis in Ovarian Cancer Cells. bioRxiv 2020, bioRxiv2020.2004.2017.042382. [Google Scholar] [CrossRef]
- De Clercq, E. Mozobil® (Plerixafor, AMD3100), 10 years after its approval by the US Food and Drug Administration. Antivir. Chem. Chemother. 2019, 27, 2040206619829382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, J.; Li, R.; Gao, D.; Chen, F.; Xie, H. CXCL12/CXCR4 Axis-Targeted Dual-Functional Nano-Drug Delivery System against Ovarian Cancer. Int. J. Nanomed. 2020, 15, 5701–5718. [Google Scholar] [CrossRef]
- Zheng, N.; Liu, W.; Chen, J.; Li, B.; Liu, J.; Wang, J.; Gao, Y.; Shao, J.; Jia, L. CXCR7 is not obligatory for CXCL12-CXCR4-induced epithelial-mesenchymal transition in human ovarian cancer. Mol. Carcinog. 2019, 58, 144–155. [Google Scholar] [CrossRef]
- Zhang, J.; Quan, L.-N.; Meng, Q.; Wang, H.-Y.; Wang, J.; Yu, P.; Fu, J.-T.; Li, Y.-J.; Chen, J.; Cheng, H.; et al. miR-548e Sponged by ZFAS1 Regulates Metastasis and Cisplatin Resistance of OC by Targeting CXCR4 and let-7a/BCL-XL/S Signaling Axis. Mol. Ther.-Nucleic Acids 2020, 20, 621–638. [Google Scholar] [CrossRef]
- Scotton, C.J.; Wilson, J.L.; Milliken, D.; Stamp, G.; Balkwill, F.R. Epithelial Cancer Cell Migration. Cancer Res. 2001, 61, 4961. [Google Scholar]
- Kryczek, I.; Lange, A.; Mottram, P.; Alvarez, X.; Cheng, P.; Hogan, M.; Moons, L.; Wei, S.; Zou, L.; Machelon, V.; et al. CXCL12 and vascular endothelial growth factor synergistically induce neoangiogenesis in human ovarian cancers. Cancer Res. 2005, 65, 465–472. [Google Scholar]
- Wang, F.Q.; So, J.; Reierstad, S.; Fishman, D.A. Vascular endothelial growth factor-regulated ovarian cancer invasion and migration involves expression and activation of matrix metalloproteinases. Int. J. Cancer 2006, 118, 879–888. [Google Scholar] [CrossRef]
- Kajiyama, H.; Shibata, K.; Terauchi, M.; Ino, K.; Nawa, A.; Kikkawa, F. Involvement of SDF-1alpha/CXCR4 axis in the enhanced peritoneal metastasis of epithelial ovarian carcinoma. Int. J. Cancer 2008, 122, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Popple, A.; Durrant, L.G.; Spendlove, I.; Rolland, P.; Scott, I.V.; Deen, S.; Ramage, J.M. The chemokine, CXCL12, is an independent predictor of poor survival in ovarian cancer. Br. J. Cancer 2012, 106, 1306–1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balabanian, K.; Lagane, B.; Infantino, S.; Chow, K.Y.; Harriague, J.; Moepps, B.; Arenzana-Seisdedos, F.; Thelen, M.; Bachelerie, F. The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J. Biol. Chem. 2005, 280, 35760–35766. [Google Scholar] [CrossRef] [Green Version]
- Furuya, M.; Suyama, T.; Usui, H.; Kasuya, Y.; Nishiyama, M.; Tanaka, N.; Ishiwata, I.; Nagai, Y.; Shozu, M.; Kimura, S. Up-regulation of CXC chemokines and their receptors: Implications for proinflammatory microenvironments of ovarian carcinomas and endometriosis. Hum. Pathol. 2007, 38, 1676–1687. [Google Scholar] [CrossRef] [PubMed]
- Daniele, G.; Raspagliesi, F.; Scambia, G.; Pisano, C.; Colombo, N.; Frezzini, S.; Tognon, G.; Artioli, G.; Gadducci, A.; Lauria, R.; et al. Bevacizumab, carboplatin, and paclitaxel in the first line treatment of advanced ovarian cancer patients: The phase IV MITO-16A/MaNGO-OV2A study. Int. J. Gynecol. Cancer 2021, 31, 875–882. [Google Scholar] [CrossRef]
- Calzolari, A.; Napolitano, M.; Bravo, E. Review of the Italian current legislation on research biobanking activities on the eve of the participation of national biobanks’ network in the legal consortium BBMRI-ERIC. Biopreserv. Biobank. 2013, 11, 124–128. [Google Scholar] [CrossRef]
- Califano, D.; Russo, D.; Scognamiglio, G.; Losito, S.N.; Spina, A.; Bello, M.A.; Capiluongo, A.; Galdiero, F.; De Cecio, R.; Bevilacqua, S.; et al. Ovarian Cancer Translational Activity of the Multicenter Italian Trial in Ovarian Cancer (MITO) Group: Lessons Learned in 10 Years of Experience. Cells 2020, 9, 903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pimentel, R.S.; Niewiadomska-Bugaj, M.; Wang, J.-C. Association of zero-inflated continuous variables. Stat. Probab. Lett. 2015, 96, 61–67. [Google Scholar] [CrossRef]
- Holländer, N.; Sauerbrei, W.; Schumacher, M. Confidence intervals for the effect of a prognostic factor after selection of an ‘optimal’ cutpoint. Stat. Med. 2004, 23, 1701–1713. [Google Scholar] [CrossRef]
- Yu, P.F.; Huang, Y.; Xu, C.L.; Lin, L.Y.; Han, Y.Y.; Sun, W.H.; Hu, G.H.; Rabson, A.B.; Wang, Y.; Shi, Y.F. Downregulation of CXCL12 in mesenchymal stromal cells by TGFβ promotes breast cancer metastasis. Oncogene 2017, 36, 840–849. [Google Scholar] [CrossRef] [Green Version]
- Machelon, V.; Gaudin, F.; Camilleri-Broët, S.; Nasreddine, S.; Bouchet-Delbos, L.; Pujade-Lauraine, E.; Alexandre, J.; Gladieff, L.; Arenzana-Seisdedos, F.; Emilie, D.; et al. CXCL12 expression by healthy and malignant ovarian epithelial cells. BMC Cancer 2011, 11, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pils, D.; Pinter, A.; Reibenwein, J.; Alfanz, A.; Horak, P.; Schmid, B.C.; Hefler, L.; Horvat, R.; Reinthaller, A.; Zeillinger, R.; et al. In ovarian cancer the prognostic influence of HER2/neu is not dependent on the CXCR4/SDF-1 signalling pathway. Br. J. Cancer 2007, 96, 485–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daniele, G.; Lorusso, D.; Scambia, G.; Cecere, S.C.; Nicoletto, M.O.; Breda, E.; Colombo, N.; Artioli, G.; Cannella, L.; Lo Re, G.; et al. Feasibility and outcome of interval debulking surgery (IDS) after carboplatin-paclitaxel-bevacizumab (CPB): A subgroup analysis of the MITO-16A-MaNGO OV2A phase 4 trial. Gynecol. Oncol. 2017, 144, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Di Liello, R.; Arenare, L.; Raspagliesi, F.; Scambia, G.; Pisano, C.; Colombo, N.; Frezzini, S.; Tognon, G.; Artioli, G.; Gadducci, A.; et al. Thromboembolic events and antithrombotic prophylaxis in advanced ovarian cancer patients treated with bevacizumab: Secondary analysis of the phase IV MITO-16A/MaNGO-OV2A trial. Int. J. Gynecol. Cancer 2021, 31, 1348. [Google Scholar] [CrossRef] [PubMed]
- Califano, D.; Gallo, D.; Rampioni Vinciguerra, G.L.; De Cecio, R.; Arenare, L.; Signoriello, S.; Russo, D.; Ferrandina, G.; Citron, F.; Losito, N.S.; et al. Evaluation of Angiogenesis-Related Genes as Prognostic Biomarkers of Bevacizumab Treated Ovarian Cancer Patients: Results from the Phase IV MITO16A/ManGO OV-2 Translational Study. Cancers 2021, 13, 5152. [Google Scholar] [CrossRef]
- Ovarian Tumor Tissue Analysis (OTTA) Consortium; Goode, E.L.; Block, M.S.; Kalli, K.R.; Vierkant, R.A.; Chen, W.; Fogarty, Z.C.; Gentry-Maharaj, A.; Tołoczko, A.; Hein, A.; et al. Dose-Response Association of CD8+ Tumor-Infiltrating Lymphocytes and Survival Time in High-Grade Serous Ovarian Cancer. JAMA Oncol. 2017, 3, e173290. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Sánchez, A.; Cybulska, P.; Mager, K.L.; Koplev, S.; Cast, O.; Couturier, D.-L.; Memon, D.; Selenica, P.; Nikolovski, I.; Mazaheri, Y.; et al. Unraveling tumor–immune heterogeneity in advanced ovarian cancer uncovers immunogenic effect of chemotherapy. Nat. Genet. 2020, 52, 582–593. [Google Scholar] [CrossRef]
- Böhm, S.; Montfort, A.; Pearce, O.M.; Topping, J.; Chakravarty, P.; Everitt, G.L.; Clear, A.; McDermott, J.R.; Ennis, D.; Dowe, T.; et al. Neoadjuvant Chemotherapy Modulates the Immune Microenvironment in Metastases of Tubo-Ovarian High-Grade Serous Carcinoma. Clin. Cancer Res. 2016, 22, 3025–3036. [Google Scholar] [CrossRef] [Green Version]
- Friese, C.; Harbst, K.; Borch, T.H.; Westergaard, M.C.W.; Pedersen, M.; Kverneland, A.; Jönsson, G.; Donia, M.; Svane, I.M.; Met, Ö. CTLA-4 blockade boosts the expansion of tumor-reactive CD8+ tumor-infiltrating lymphocytes in ovarian cancer. Sci. Rep. 2020, 10, 3914. [Google Scholar] [CrossRef] [Green Version]
- Hamanishi, J.; Mandai, M.; Iwasaki, M.; Okazaki, T.; Tanaka, Y.; Yamaguchi, K.; Higuchi, T.; Yagi, H.; Takakura, K.; Minato, N.; et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc. Natl. Acad. Sci. USA 2007, 104, 3360–3365. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Y.; Li, B.; Liang, Y.; Reeves, P.M.; Qu, X.; Ran, C.; Liu, Q.; Callahan, M.V.; Sluder, A.E.; Gelfand, J.A.; et al. Dual blockade of CXCL12-CXCR4 and PD-1-PD-L1 pathways prolongs survival of ovarian tumor-bearing mice by prevention of immunosuppression in the tumor microenvironment. FASEB J. 2019, 33, 6596–6608. [Google Scholar] [CrossRef] [PubMed]
- Givel, A.-M.; Kieffer, Y.; Scholer-Dahirel, A.; Sirven, P.; Cardon, M.; Pelon, F.; Magagna, I.; Gentric, G.; Costa, A.; Bonneau, C.; et al. miR200-regulated CXCL12β promotes fibroblast heterogeneity and immunosuppression in ovarian cancers. Nat. Commun. 2018, 9, 1056. [Google Scholar] [CrossRef]
- Zhang, F.; Cui, J.-Y.; Gao, H.-F.; Yu, H.; Gao, F.-F.; Chen, J.-L.; Chen, L. Cancer-associated fibroblasts induce epithelial-mesenchymal transition and cisplatin resistance in ovarian cancer via CXCL12/CXCR4 axis. Future Oncol. 2020, 16, 2619–2633. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mistarz, A.; Komorowski, M.P.; Graczyk, M.A.; Gil, M.; Jiang, A.; Opyrchal, M.; Rokita, H.; Odunsi, K.O.; Kozbor, D. Recruitment of Intratumoral CD103(+) Dendritic Cells by a CXCR4 Antagonist-Armed Virotherapy Enhances Antitumor Immunity. Mol. Ther. Oncolytics 2019, 14, 233–245. [Google Scholar] [CrossRef] [Green Version]



| CXCL12 | Biomarker CXCR4 | CXCR7 | Epithelial Cells N (%) | Stromal Cells N (%) |
|---|---|---|---|---|
| + | + | + | 174 (56.5) | 22 (7.1) |
| + | + | - | 19 (6.2) | 2 (0.6) |
| + | - | + | 5 (1.6) | 10 (3.2) |
| - | + | + | 57 (18.5) | 125 (40.6) |
| + | - | - | 5 (1.6) | 1 (0.3) |
| - | + | - | 27 (8.8) | 57 (18.5) |
| - | - | + | 7 (2.3) | 45 (14.6) |
| - | - | - | 14 (4.5) | 6 (1.9) |
| Progression Free Survival | Overall Survival | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Original Coefficient | Shrunken Coefficients | Original Coefficient | Shrunken Coefficients | |||||||||
| HR | CI(95%) | P | HR | CI(95%) | P | HR | CI(95%) | P | HR | CI(95%) | P | |
| CXCR7 Epithelial | ||||||||||||
| >36.7 | 0.79 | (0.6–1.04) | 0.093 | 0.86 | (0.4–1.85) | 0.695 | 0.84 | (0.56–1.28) | 0.423 | 1.10 | (0.19–6.21) | 0.916 |
| CXCR7 Stromal | ||||||||||||
| >20.0 | 1.16 | (0.88–1.54) | 0.297 | 1.01 | (0.49–2.09) | 0.974 | 1.23 | (0.81–1.86) | 0.335 | 0.98 | (0.2–4.9) | 0.985 |
| CXCL12 Epithelial | ||||||||||||
| >21.7 | 1.39 | (1.06–1.81) | 0.016 | 1.31 | (0.67–2.58) | 0.430 | 1.64 | (1.11–2.42) | 0.014 | 1.51 | (0.66–3.48) | 0.334 |
| CXCL12 Stromal | ||||||||||||
| >6.7 | 0.67 | (0.4–1.11) | 0.117 | 0.79 | (0.4–1.56) | 0.490 | 0.54 | (0.24–1.24) | 0.149 | 0.73 | (0.12–4.47) | 0.732 |
| CXCR4 Epithelial | ||||||||||||
| >130.0 | 0.69 | (0.43–1.08) | 0.106 | 0.79 | (0.34–1.83) | 0.585 | 0.56 | (0.27–1.18) | 0.130 | 0.72 | (0.13–4.02) | 0.710 |
| CXCR4 Stromal | ||||||||||||
| >65.0 | 0.77 | (0.51–1.16) | 0.209 | 0.91 | (0.42–1.96) | 0.807 | 0.68 | (0.37–1.24) | 0.210 | 0.87 | (0.21–3.68) | 0.849 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Alterio, C.; Spina, A.; Arenare, L.; Chiodini, P.; Napolitano, M.; Galdiero, F.; Portella, L.; Simeon, V.; Signoriello, S.; Raspagliesi, F.; et al. Biological Role of Tumor/Stromal CXCR4-CXCL12-CXCR7 in MITO16A/MaNGO-OV2 Advanced Ovarian Cancer Patients. Cancers 2022, 14, 1849. https://doi.org/10.3390/cancers14071849
D’Alterio C, Spina A, Arenare L, Chiodini P, Napolitano M, Galdiero F, Portella L, Simeon V, Signoriello S, Raspagliesi F, et al. Biological Role of Tumor/Stromal CXCR4-CXCL12-CXCR7 in MITO16A/MaNGO-OV2 Advanced Ovarian Cancer Patients. Cancers. 2022; 14(7):1849. https://doi.org/10.3390/cancers14071849
Chicago/Turabian StyleD’Alterio, Crescenzo, Anna Spina, Laura Arenare, Paolo Chiodini, Maria Napolitano, Francesca Galdiero, Luigi Portella, Vittorio Simeon, Simona Signoriello, Francesco Raspagliesi, and et al. 2022. "Biological Role of Tumor/Stromal CXCR4-CXCL12-CXCR7 in MITO16A/MaNGO-OV2 Advanced Ovarian Cancer Patients" Cancers 14, no. 7: 1849. https://doi.org/10.3390/cancers14071849
APA StyleD’Alterio, C., Spina, A., Arenare, L., Chiodini, P., Napolitano, M., Galdiero, F., Portella, L., Simeon, V., Signoriello, S., Raspagliesi, F., Lorusso, D., Pisano, C., Colombo, N., Zannoni, G. F., Losito, N. S., De Cecio, R., Scognamiglio, G., Califano, D., Russo, D., ... Scala, S. (2022). Biological Role of Tumor/Stromal CXCR4-CXCL12-CXCR7 in MITO16A/MaNGO-OV2 Advanced Ovarian Cancer Patients. Cancers, 14(7), 1849. https://doi.org/10.3390/cancers14071849