Dose Consideration of Lenvatinib’s Anti-Cancer Effect on Hepatocellular Carcinoma and the Potential Benefit of Combined Colchicine Therapy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Drugs
2.2. Anti-Proliferative Experiments
2.3. Quantitative Reverse Transcriptase-Polymerase Chain Reaction (qRT-PCR) Experiments
2.4. Statistical Analysis
3. Results
3.1. Anti-Proliferative Experiments
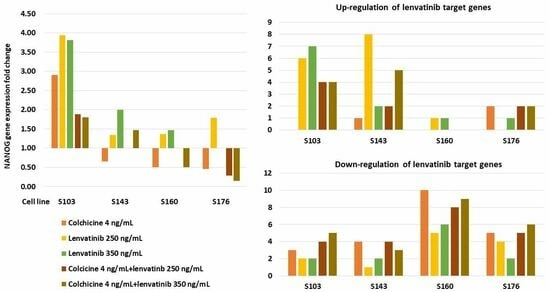
3.2. qRT-PCR Experiments
3.2.1. Influence on Expressions of Lenvatinib Target Genes
3.2.2. Influence on Expression of NANOG
3.3. Combined Analysis the Results of Anti-Proliferative Effects and qRT-PCR Experiments
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meyers, B.M.; Knox, J.J.; Cosby, R.; Beecroft, J.R.; Chan, K.K.; Coburn, N.; Feld, J.J.; Jonker, D.; Mahmud, A.; Ringash, J. Non-surgical management of advanced hepatocellular carcinoma: A systematic review by Cancer Care Ontario. Can. Liver J. 2021, 4, 257–274. [Google Scholar] [CrossRef]
- Tohyama, O.; Matsui, J.; Kodama, K.; Hata-Sugi, N.; Kimura, Y.; Okamoto, K.; Minoshima, Y.; Iwata, M.; Funahashi, Y. Antitumor activity of lenvatinib (e7080): An angiogenesis inhibitor that targets multiple receptor tyrosine kinases in preclinical human thyroid cancer models. J. Thyroid Res. 2014, 2014, 638747. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Matsui, J.; Matsushima, T.; Obaishi, H.; Miyazaki, K.; Nakamura, K.; Tohyama, O.; Semba, T.; Yamaguchi, A.; Hoshi, S.S.; et al. Lenvatinib, an angiogenesis inhibitor targeting VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft models associated with microvessel density and pericyte coverage. Vasc. Cell 2014, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Matsuki, M.; Hoshi, T.; Yamamoto, Y.; Ikemori-Kawada, M.; Minoshima, Y.; Funahashi, Y.; Matsui, J. Lenvatinib inhibits angiogenesis and tumor fibroblast growth factor signaling pathways in human hepatocellular carcinoma models. Cancer Med. 2018, 7, 2641–2653. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, Y.N.; Wang, K.T.; Chen, L. Lenvatinib for hepatocellular carcinoma: From preclinical mechanisms to anti-cancer therapy. Biochim. Biophys. Acta Rev. Cancer 2020, 1874, 188391. [Google Scholar] [CrossRef] [PubMed]
- Oranratnachai, S.; Rattanasiri, S.; Pooprasert, A.; Tansawet, A.; Reungwetwattana, T.; Attia, J.; Thakkinstian, A. Efficacy of First Line Systemic Chemotherapy and Multikinase Inhibitors in Advanced Hepatocellular Carcinoma: A Systematic Review and Network Meta-Analysis. Front. Oncol. 2021, 11, 654020. [Google Scholar] [CrossRef]
- Facciorusso, A.; Tartaglia, N.; Villani, R.; Serviddio, G.; Ramai, D.; Mohan, B.P.; Chandan, S.; Aziz, M.A.A.E.; Evangelista, J.; Cotsoglou, C.; et al. Lenvatinib versus sorafenib as first-line therapy of advanced hepatocellular carcinoma: A systematic review and meta-analysis. Am. J. Transl. Res. 2021, 13, 2379–2387. [Google Scholar] [PubMed]
- Finn, R.S.; Ikeda, M.; Zhu, A.X.; Sung, M.W.; Baron, A.D.; Kudo, M.; Okusaka, T.; Kobayashi, M.; Kumada, H.; Kaneko, S.; et al. Phase Ib Study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J. Clin. Oncol. 2020, 38, 2960–2970. [Google Scholar] [CrossRef]
- Noda, S.; Iida, H.; Fujimoto, T.; Wakasugi, Y.; Yabuta, N.; Sudou, M.; Hira, D.; Tani, M.; Andoh, A.; Morita, S.Y.; et al. Exploratory analysis of target concentration of lenvatinib in the treatment of hepatocellular carcinoma. Cancer Chemother. Pharmacol. 2021, 88, 281–288. [Google Scholar] [CrossRef]
- Endo, M.; Honda, K.; Saito, T.; Shiraiwa, K.; Sueshige, Y.; Tokumaru, T.; Iwao, M.; Tokoro, M.; Arakawa, M.; Tanaka, R.; et al. Maximum plasma concentration of lenvatinib is useful for predicting thrombocytopenia in patients treated for hepatocellular carcinoma. World J. Oncol. 2021, 12, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Taylor, M.H.; Evans, T.R.J.; Okusaka, T.; Glen, H.; Lubiniecki, G.M.; Dutcus, C.; Smith, A.D.; Okpara, C.E.; Hussein, Z.; et al. Lenvatinib dose, efficacy, and safety in the treatment of multiple malignancies. Expert Rev. Anticancer Ther. 2022, 22, 383–400. [Google Scholar] [CrossRef]
- Lin, Z.Y.; Wu, C.C.; Chuang, Y.H.; Chuang, W.L. Anti-cancer mechanisms of clinically acceptable colchicine concentrations on hepatocellular carcinoma. Life Sci. 2013, 93, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.Y.; Yeh, M.L.; Huang, C.I.; Chen, S.C.; Huang, C.F.; Huang, J.F.; Dai, C.Y.; Yu, M.L.; Chuang, W.L. Potential of novel colchicine dosage schedule for the palliative treatment of advanced hepatocellular carcinoma. Kaohsiung J. Med. Sci. 2021, 37, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Zhang, K.; Ge, H.; Li, W.; Li, G.; Wu, J. Prognostic and clinicopathological value of Nanog in hepatocellular carcinoma: A meta-analysis. Clin. Chim. Acta 2018, 477, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.Y.; Yeh, M.L.; Huang, C.I.; Liang, P.C.; Hsu, P.Y.; Chen, S.C.; Huang, C.F.; Huang, J.F.; Dai, C.Y.; Yu, M.Y.; et al. Advantage of clinical colchicine concentration to promote sorafenib or regorafenib anti-cancer effects on hepatocellular carcinoma. Biomed. Pharmacother. 2022, 153, 113540. [Google Scholar] [CrossRef]
- Rochdi, M.; Sabouraud, A.; Girre, C.; Venet, R.; Scherrmann, J.M. Pharmacokinetics and absolute bioavailability of colchicine after i.v. and oral administration in healthy human volunteers and elderly subjects. Eur. J. Clin. Pharmacol. 1994, 46, 351–354. [Google Scholar] [CrossRef]
- Ferron, G.M.; Rochdi, M.; Jusko, W.J.; Scherrmann, J.M. Oral absorption characteristics and pharmacokinetics of colchicine in healthy volunteers after single and multiple doses. J. Clin. Pharmacol. 1996, 36, 874–883. [Google Scholar] [CrossRef]
- Terkeltaub, R.A.; Furst, D.E.; Bennett, K.; Kook, K.A.; Crockett, R.S.; Davis, M.W. High versus low dosing of oral colchicine for early acute gout flare: Twenty-four-hour outcome of the first multicenter, randomized, double-blind, placebo-controlled, parallel-group, dose-comparison colchicine study. Arthritis Rheum. 2010, 62, 1060–1068. [Google Scholar] [CrossRef]
- Ikeda, M.; Okusaka, T.; Mitsunaga, S.; Ueno, H.; Tamai, T.; Suzuki, T.; Hayato, S.; Kadowaki, T.; Okita, K.; Kumada, H. Safety and pharmacokinetics of lenvatinib in patients with advanced hepatocellular carcinoma. Clin. Cancer Res. 2016, 22, 1385–1394. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Pawlik, T.M.; Shindoh, J.; Vauthey, J.N. Liver. In AJCC Cancer Staging Manual, 8th ed.; Amin, M.B., Greene, F.L., Edge, S.B., Compton, C.C., Gershenwald, J.E., Brookland, R.J., Meyer, L., Gress, D.M., Byrd, D.R., Winchester, D.P., Eds.; Springer: New York, NY, USA, 2017; pp. 287–293. [Google Scholar]
- Xu, L.X.; He, M.H.; Dai, Z.H.; Yu, J.; Wang, J.G.; Li, X.C.; Jiang, B.B.; Ke, Z.F.; Su, T.H.; Peng, Z.W.; et al. Genomic and transcriptional heterogeneity of multifocal hepatocellular carcinoma. Ann. Oncol. 2019, 30, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.J.; von Felden, J.; Garcia-Lezana, T.; Sarcognato, S.; Villanueva, A. Tumour evolution in hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 139–152. [Google Scholar] [CrossRef]
- Dong, L.Q.; Peng, L.H.; Ma, L.J.; Liu, D.B.; Zhang, S.; Luo, S.Z.; Rao, J.H.; Zhu, H.W.; Yang, S.X.; Xi, S.J.; et al. Heterogeneous immunogenomic features and distinct escape mechanisms in multifocal hepatocellular carcinoma. J. Hepatol. 2020, 72, 896–908. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; He, M.; Guo, Y.; Li, H.; Shen, S.; Xie, Y.; Li, X.; Xiao, H.; Fang, L.; Li, D.; et al. The influence of immune heterogeneity on the effectiveness of immune checkpoint inhibitors in multifocal hepatocellular carcinomas. Clin. Cancer Res. 2020, 26, 4947–4957. [Google Scholar] [CrossRef]
- Shan, J.; Shen, J.; Liu, L.; Xia, F.; Xu, C.; Duan, G.; Xu, Y.; Ma, Q.; Yang, Z.; Zhang, Q.; et al. Nanog regulates self-renewal of cancer stem cells through the insulin-like growth factor pathway in human hepatocellular carcinoma. Hepatology 2012, 56, 1004–1014. [Google Scholar] [CrossRef]
- Chen, C.L.; Kumar, D.B.U.; Punj, V.; Xu, J.; Sher, L.; Tahara, S.M.; Hess, S.; Machida, K. NANOG metabolically reprograms tumor-initiating stem-like cells through tumorigenic changes in oxidative phosphorylation and fatty acid metabolism. Cell Metab. 2016, 23, 206–219. [Google Scholar] [CrossRef]
- Li, J.; Zhu, Y. Recent Advances in Liver Cancer Stem Cells: Non-coding RNAs, Oncogenes and Oncoproteins. Front Cell Dev. Biol. 2020, 8, 548335. [Google Scholar] [CrossRef] [PubMed]
- Hawash, M. Recent advances of tubulin inhibitors targeting the colchicine binding site for cancer therapy. Biomolecules 2022, 12, 1843. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, B.; Panda, D.; Gupta, S.; Banerjee, M. Anti-mitotic activity of colchicine and the structural basis for its interaction with tubulin. Med. Res. Rev. 2008, 28, 155–183. [Google Scholar] [CrossRef]
- Sivakumar, G. Colchicine semisynthetics: Chemotherapeutics for cancer? Curr. Med. Chem. 2013, 20, 892–898. [Google Scholar]
- Maldonado, E.N.; Patnaik, J.; Mullins, M.R.; Lemasters, J.J. Free tubulin modulates mitochondrial membrane potential in cancer cells. Cancer Res. 2010, 70, 10192–10201. [Google Scholar] [CrossRef] [PubMed]

| Experimental Drug Concentrations | Col 4 ng/mL | L 250 ng/mL | L350 ng/mL | L250 ng/mL + Col 4 ng/mL | L350 ng/mL + Col 4 ng/mL |
|---|---|---|---|---|---|
| S103 | |||||
| Up-regulation | NANOG (2.91) | FGFR1 (1.54), FGFR3 (2.98), FLT4 (2.7), KDR (2.51), KIT (1.88), NANOG (3.94), PDGFRB (1.86) | FGFR1 (1.61), FGFR3 (1.84), FLT4 (2.24), KDR (2.3), KIT (1.9), NANOG (3.82), PDGFRA (1.42), PDGFRB (1.74) | FGFR1 (1.8), FGFR3 (2.41), FLT4 (2.23), KIT (1.59), NANOG (1.88) | FGFR1 (1.59), FGFR3 (1.48), FLT4 (1.63), KIT (1.75), NANOG (1.8) |
| Down-regulation | FLT1 (0.64), KIT (0.62), PDGFRA (0.67) | FGFR4 (0.28), FLT1 (0.08) | FGFR4 (0.27), FLT1 (0.03) | FGFR2 (0.47), FGFR4 (0.16), FLT1 (0.05), RET (0.41) | FGFR2 (0.47), FGFR4 (0.14), FLT1 (0.01), PDGFRA (0.45), RET (0.2) |
| S143 | |||||
| Up-regulation | FGFR1 (1,39) | FGFR1 (1.53), FGFR2 (1.74), FGFR3 (13.52), FGFR4 (2.53), FLT4 (3.06), KIT (1.71), NANOG (1.34), PDGFRA (1.47), PDGFRB (8.36) | FGFR3 (5.71), NANOG (2.0), PDGFRB (2.22) | FGFR1 (1.47), FGFR3 (1.73), | FGFR1 (1.82), FGFR3 (4.75), FGFR4 (2.01), FLT4 (1.39), NANOG (1.47), PDGFRB (1.79) |
| Down-regulation | KDR (0.41), NANOG (0.65), PDGFRA (0.33), PDGFRB (0.69), RET (0.61) | RET (0.59) | FLT1 (0.46), RET (0.17) | FLT1 (0.5), FLT4 (0.58), KDR (0.32), PDGFRA (0.28) | FLT1 (0.25), KDR (0.33), PDGFRA (0.28) |
| S160 | |||||
| Up-regulation | FLT4 (1.32), NANOG (1.37) | FGFR3 (2.88), NANOG (1.47) | |||
| Down-regulation | FLT1 (0.45), FLT4 (0.63), FGFR2 (0.4), FGFR3 (0.29), FGFR4 (0.47), KDR (0.67), KIT (0.03), NANOG (0.5), PDGFRA (0.11), PDGFRB (0.43), RET (0.2) | FGFR1 (0.65), FGFR2 (0.48), FLT1 (0.55), KIT (0.07), PDGFRA (0.18) | FGFR1 (0.66), FGFR2 (0.31), FLT1 (0.46), KIT (0.15), PDGFRA (0.09), RET (0.08) | FGFR2 (0.35), FGFR3 (0.61), FGFR4 (0.48), KDR (0.61), KIT (0.14), PDGFRA (0.02), PDGFRB (0.28), RET (0.08) | FGFR2 (0.35), FGFR3 (0.54), FGFR4 (0.51), FLT1 (0.67), KDR (0.39), KIT (0.08), NANOG (0.50), PDGFRA (0.02), PDGFRB (0.43), RET (0.41) |
| S176 * | |||||
| Up-regulation | FGFR1 (2.49), KIT (2.61) | NANOG (1.79) | FGFR4 (1.49) | FGFR1 (2.58), KIT (2.31) | FGFR1 (2.94), KIT (2.65) |
| Down-regulation | FGFR3 (0.27), FGFR4 (0.55), NANOG (0.46), PDGFRA (0.42), PDGFRB (0.67), RET (0.57) | FLT1 (0.54), KIT (0.54), PDGFRA (0.43), RET (0.62) | FLT1 (0.59), RET (0.52) | FGFR3 (0.58), FGFR4 (0.48), NANOG (0.29), PDGFRA (0.34), PDGFRB (0.59), RET (0.45) | FGFR2 (0.76), FGFR3 (0.65), FGFR4 (0.62), NANOG (0.15), PDGFRA (0.23), PDGFRB (0.33), RET (0.56) |
| Drug Concentrations | Col 4 ng/mL | L 250 ng/mL | L350 ng/mL | L250 ng/mL + Col 4 ng/mL | L350 ng/mL + Col 4 ng/mL |
|---|---|---|---|---|---|
| S103 | |||||
| Up-regulation | 0 | 6 | 7 | 4 | 4 |
| Down-regulation | 3 | 2 | 2 | 4 | 5 |
| S143 | |||||
| Up-regulation | 1 | 8 | 2 | 2 | 5 |
| Down-regulation | 4 | 1 | 2 | 4 | 3 |
| S160 | |||||
| Up-regulation | 0 | 1 | 1 | 0 | 0 |
| Down-regulation | 10 | 5 | 6 | 8 | 9 |
| S176 | |||||
| Up-regulation | 2 | 0 | 1 | 2 | 2 |
| Down-regulation | 5 | 4 | 2 | 5 | 6 |
| Cell Lines | S103 | S143 | S160 | S176 * |
|---|---|---|---|---|
| (a) Strength of significant anti-proliferative effect | L350 + Col > (Col, L250, L250 + Col, L350) | (L250, L250 + Col, L350 + Col) > L350 > Col | L350 + Col > L350 > (Col, L250 + Col) > L250 | L250 > L350 + Col > (Col, L250 + Col) |
| (b) Number of lenvatinib target genes | ||||
| Up-regulation | L350 > L250 > (L250 + Col, L350 + Col) | L250 > L350 + Col > (L250 + Col, L350) > Col | (L250, L350) | (L250 + Col, L350 + Col, Col) > L350 |
| Down-regulation | L350 + Col > L250 + Col > Col > (L250, L350) | (Col, L250 + Col) > L350 + Col > L350 > L250 | Col > L350 + Col > L250 + Col > L350 > L250 | L350 + Col > (L250 + Col, Col) > L250 > L350 |
| (c) Magnitude of NANOG expression | ||||
| Up-regulation | L250 > L350 > Col > (L250 + Col, L350 + Col) | L350 > L350 + Col > L250 | L350 > L250 | L250 |
| Down-regulation | Col | (L350 + Col, Col) | L350 + Col > L250 + Col > Col |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Z.-Y.; Yeh, M.-L.; Liang, P.-C.; Hsu, P.-Y.; Huang, C.-F.; Huang, J.-F.; Dai, C.-Y.; Yu, M.-L.; Chuang, W.-L. Dose Consideration of Lenvatinib’s Anti-Cancer Effect on Hepatocellular Carcinoma and the Potential Benefit of Combined Colchicine Therapy. Cancers 2023, 15, 5097. https://doi.org/10.3390/cancers15205097
Lin Z-Y, Yeh M-L, Liang P-C, Hsu P-Y, Huang C-F, Huang J-F, Dai C-Y, Yu M-L, Chuang W-L. Dose Consideration of Lenvatinib’s Anti-Cancer Effect on Hepatocellular Carcinoma and the Potential Benefit of Combined Colchicine Therapy. Cancers. 2023; 15(20):5097. https://doi.org/10.3390/cancers15205097
Chicago/Turabian StyleLin, Zu-Yau, Ming-Lun Yeh, Po-Cheng Liang, Po-Yao Hsu, Chung-Feng Huang, Jee-Fu Huang, Chia-Yen Dai, Ming-Lung Yu, and Wan-Long Chuang. 2023. "Dose Consideration of Lenvatinib’s Anti-Cancer Effect on Hepatocellular Carcinoma and the Potential Benefit of Combined Colchicine Therapy" Cancers 15, no. 20: 5097. https://doi.org/10.3390/cancers15205097
APA StyleLin, Z. -Y., Yeh, M. -L., Liang, P. -C., Hsu, P. -Y., Huang, C. -F., Huang, J. -F., Dai, C. -Y., Yu, M. -L., & Chuang, W. -L. (2023). Dose Consideration of Lenvatinib’s Anti-Cancer Effect on Hepatocellular Carcinoma and the Potential Benefit of Combined Colchicine Therapy. Cancers, 15(20), 5097. https://doi.org/10.3390/cancers15205097