The Ying and Yang of Ganglioside Function in Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
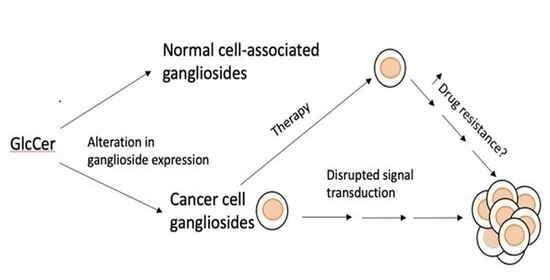
2. Ganglioside Synthesis
3. Gangliosides and Signal Transduction
4. Altered Expression of Proteins Required for Ganglioside Synthesis in Cancer Cells
| Enzyme a | Problem | Ganglioside b | Correlation with Cancer Prognosis c | Reference d |
|---|---|---|---|---|
| UGCG | ↑ Expression | Glc-Cer | + metastasis and chemotherapy resistance | [83] |
| B4GALT5 e | ↑ mRNA & expression | Lac-Cer | + survival | [84] |
| B4GALNT1 | ↑ Expression | ↑ GM2 | Depends on tumor type | [85] |
| B3GALT4 | ↓ Activity | ↑ GD2 | + survival from neuroblastoma | [86] |
| ST3GAL2 | ↑ mRNA | ↑ GD1a? | + progression | [87,88,89] |
| ST3GAL5 | ↑ Expression | ↑ GM3 | + poor prognosis for ccRCC f | [90] |
| ST8SIA1 | ↑ Expression | ↑ GD3 | + poor outcome neuro-ectodermal cancers + tumor growth and metastasis in breast cancer | [28] |
| [91,92,93] | ||||
| ST8SIA5 | ↓ Expression | ↓ GT3, GD1c,GT1a GQ1b | + poor survival colon cancer | [94] |
| ST6GALNT5 | ↑ Expression | ↑ GD1α | + decreased adhesion of human BrM2 cells to an in vitro BBB model | [82] |
| NEU 3 | ↑ Expression | ↑ GM1, GM2, GM3 | + renal cell carcinoma | [95] |
5. Effects of Circulating Gangliosides Shed from Tumor Cells
6. Use of a Cancer-Associated Ganglioside in the Development of an Anti-Ganglioside Antibody Cancer Therapy
7. Additional Questions That Need to Be Further Interrogated Relative to Potential Treatment of Patients with a Ganglioside-Characterized Cancer
7.1. Expression of Proteins That Might Cause Aberrant Glycosylation
7.2. Gangliosides and Immune Checkpoint Inhibitors
7.3. Gangliosides and Angiogenesis
7.4. Potential Uses of Cancer Data Bank Information to Guide Research/Treatment
7.5. Use and Possible Advantages of Multivalency When Targeting Binding to Saccharides
8. Summary
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martínez-Palomo, A. The Surface Coats of Animal Cells. Part of the Personal Work Mentioned in This Review Was Performed at the Institut de Recherches Scientifiques Sur Le Cancer, Villejuif, France. In International Review of Cytology; Bourne, G.H., Danielli, J.F., Jeon, K.W., Eds.; Academic Press: Cambridge, MA, USA, 1970; Volume 29, pp. 29–75. ISBN 0074-7696. [Google Scholar] [CrossRef]
- Luft, J.H. Fine Structures of Capillary and Endocapillary Layer as Revealed by Ruthenium Red. FASEB J. 1966, 25, 1773–1783. [Google Scholar]
- Sipione, S.; Monyror, J.; Galleguillos, D.; Steinberg, N.; Kadam, V. Gangliosides in the Brain: Physiology, Pathophysiology and Therapeutic Applications. Front. Neurosci. 2020, 14, 572965. [Google Scholar] [CrossRef] [PubMed]
- Thudichum, J.L.W. A Treatise on the Chemical Constitution of the Brain: Based throughout upon Original Researches; Bailliere, Tindall, and Cox: London, UK, 1884. [Google Scholar]
- Sonnino, S.; Chigorno, V. Ganglioside Molecular Species Containing C18- and C20-Sphingosine in Mammalian Nervous Tissues and Neuronal Cell Cultures. Biochim. Biophys. Acta 2000, 1469, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and Their Metabolism in Physiology and Disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 175–191. [Google Scholar] [CrossRef]
- Hamamura, K.; Furukawa, K.; Hayashi, T.; Hattori, T.; Nakano, J.; Nakashima, H.; Okuda, T.; Mizutani, H.; Hattori, H.; Ueda, M.; et al. Ganglioside GD3 Promotes Cell Growth and Invasion through P130Cas and Paxillin in Malignant Melanoma Cells. Proc. Natl. Acad. Sci. USA 2005, 102, 11041–11046. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.; Faber, A.C.; Shelton, L.M.; Baek, R.C.; Chiles, T.C.; Seyfried, T.N. Thematic Review Series: Sphingolipids. Ganglioside GM3 Suppresses the Proangiogenic Effects of Vascular Endothelial Growth Factor and Ganglioside GD1a. J. Lipid Res. 2008, 49, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.W.; Kim, S.J.; Choi, H.J.; Kim, K.J.; Kim, M.J.; Kim, S.H.; Lee, H.J.; Ko, J.H.; Lee, Y.C.; Suzuki, A.; et al. Ganglioside GM3 Inhibits VEGF/VEGFR-2-Mediated Angiogenesis: Direct Interaction of GM3 with VEGFR-2. Glycobiology 2009, 19, 229–239. [Google Scholar] [CrossRef]
- Groux-Degroote, S.; Delannoy, P. Cancer-Associated Glycosphingolipids as Tumor Markers and Targets for Cancer Immunotherapy. Int. J. Mol. Sci. 2021, 22, 6145. [Google Scholar] [CrossRef]
- Grassi, S.; Giussani, P.; Mauri, L.; Prioni, S.; Sonnino, S.; Prinetti, A. Lipid Rafts and Neurodegeneration: Structural and Functional Roles in Physiologic Aging and Neurodegenerative Diseases. J. Lipid Res. 2020, 61, 636–654. [Google Scholar] [CrossRef]
- Simons, K.; Ikonen, E. Functional Rafts in Cell Membranes. Nature 1997, 387, 569–572. [Google Scholar] [CrossRef]
- Sonnino, S.; Chiricozzi, E.; Grassi, S.; Mauri, L.; Prioni, S.; Prinetti, A. Chapter Three—Gangliosides in Membrane Organization. In Progress in Molecular Biology and Translational Science; Schnaar, R.L., Lopez, P.H.H., Eds.; Academic Press: Cambridge, MA, USA, 2018; Volume 156, pp. 83–120. [Google Scholar] [CrossRef]
- Julien, S.; Bobowski, M.; Steenackers, A.; Le Bourhis, X.; Delannoy, P. How Do Gangliosides Regulate RTKs Signaling? Cells 2013, 2, 751–767. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, N.; Toyoda, M.; Ishiwata, T. Gangliosides as Signaling Regulators in Cancer. Int. J. Mol. Sci. 2021, 22, 5076. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, M.; Kholodenko, I.; Kalinovsky, D.; Shamanskaya, T.; Doronin, I.; Konovalov, D.; Mironov, A.; Kuzmin, D.; Nikitin, D.; Deyev, S.; et al. RNA Sequencing-Based Identification of Ganglioside GD2-Positive Cancer Phenotype. Biomedicines 2020, 8, 142. [Google Scholar] [CrossRef] [PubMed]
- Todeschini, R.A.; Hakomori, S.I. Functional Role of Glycosphingolipids and Gangliosides in Control of Cell Adhesion, Motility, and Growth, through Glycosynaptic Microdomains. Biochim. Biophys. Acta 2008, 1780, 421–433. [Google Scholar] [CrossRef]
- Bremer, E.G.; Schlessinger, J.; Hakomori, S.-I. Ganglioside-Mediated Modulation of Cell Growth. Specific Effects of GM3 on Tyrosine Phosphorylation of the Epidermal Growth Factor Receptor. J. Biol. Chem. 1986, 261, 2434–2440. [Google Scholar] [CrossRef]
- Mirkin, B.L.; Clark, S.H.; Zhang, C. Inhibition of Human Neuroblastoma Cell Proliferation and EGF Receptor Phosphorylation by Gangliosides GM1, GM3, GD1A and GT1B. Cell Prolif. 2002, 35, 105–115. [Google Scholar] [CrossRef]
- Birklé, S.; Zeng, G.; Gao, L.; Yu, R.K.; Aubry, J. Role of Tumor-Associated Gangliosides in Cancer Progression. Biochimie 2003, 85, 455–463. [Google Scholar] [CrossRef]
- Liu, Y.; Wondimu, A.; Yan, S.; Bobb, D.; Ladisch, S. Tumor Gangliosides Accelerate Murine Tumor Angiogenesis. Angiogenesis 2014, 17, 563–571. [Google Scholar] [CrossRef]
- Arumugam, S.; Schmieder, S.; Pezeshkian, W.; Becken, U.; Wunder, C.; Chinnapen, D.; Ipsen, J.H.; Kenworthy, A.K.; Lencer, W.; Mayor, S.; et al. Ceramide Structure Dictates Glycosphingolipid Nanodomain Assembly and Function. Nat. Commun. 2021, 12, 3675. [Google Scholar] [CrossRef]
- Kawano, T.; Koyama, S.; Takematsu, H.; Kozutsumi, Y.; Kawasaki, H.; Kawashima, S.; Kawasaki, T.; Suzuki, A. Molecular Cloning of Cytidine Monophospho-N-Acetylneuraminic Acid Hydroxylase. Regulation of Species- and Tissue-Specific Expression of N-Glycolylneuraminic Acid. J. Biol. Chem. 1995, 270, 16458–16463. [Google Scholar] [CrossRef]
- Suzuki, A. Genetic Basis for the Lack of N-Glycolylneuraminic Acid Expression in Human Tissues and Its Implication to Human Evolution. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2006, 82, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Samraj, A.; Läubli, H.; Varki, N.; Varki, A. Involvement of a Non-Human Sialic Acid in Human Cancer. Front. Oncol. 2014, 4, 33. [Google Scholar] [CrossRef] [PubMed]
- Sandhoff, R.; Sandhoff, K. Neuronal Ganglioside and Glycosphingolipid (GSL) Metabolism and Disease: Cascades of Secondary Metabolic Errors Can Generate Complex Pathologies (in LSDs). Adv. Neurobiol. 2023, 29, 330–390. [Google Scholar] [CrossRef]
- Miyagi, T.; Wada, T.; Iwamatsu, A.; Hata, K.; Yoshikawa, Y.; Tokuyama, S.; Sawada, M. Molecular Cloning and Characterization of a Plasma Membrane-Associated Sialidase Specific for Gangliosides. J. Biol. Chem. 1999, 274, 5004–5011. [Google Scholar] [CrossRef] [PubMed]
- Kasprowicz, A.; Sophie, G.D.; Lagadec, C.; Delannoy, P. Role of GD3 Synthase ST8Sia I in Cancers. Cancers 2022, 14, 1299. [Google Scholar] [CrossRef]
- Svennerholm, L. Gangliosides and Synaptic Transmission. In Structure and Function of Gangliosides; Springer: Berlin/Heidelberg, Germany, 1980; pp. 533–544. [Google Scholar] [CrossRef]
- Yoshihara, T.; Satake, H.; Nishie, T.; Okino, N.; Hatta, T.; Otani, H.; Naruse, C.; Suzuki, H.; Sugihara, K.; Kamimura, E.; et al. Lactosylceramide Synthases Encoded by B4galt5 and 6 Genes Are Pivotal for Neuronal Generation and Myelin Formation in Mice. PLoS Genet. 2018, 14, e1007545. [Google Scholar] [CrossRef]
- Kono, M.; Yoshida, Y.; Kojima, N.; Tsuji, S. Molecular Cloning and Expression of a Fifth Type of Alpha2,8-Sialyltransferase (ST8Sia V). Its Substrate Specificity Is Similar to That of SAT-V/III, Which Synthesize GD1c, GT1a, GQ1b and GT3. J. Biol. Chem. 1996, 271, 29366–29371. [Google Scholar] [CrossRef]
- Bremer, E.G.; Hakomori, S.; Bowen-Pope, D.F.; Raines, E.; Ross, R. Ganglioside-Mediated Modulation of Cell Growth, Growth Factor Binding, and Receptor Phosphorylation. J. Biol. Chem. 1984, 259, 6818–6825. [Google Scholar] [CrossRef]
- Kundu, M.; Mahata, B.; Banerjee, A.; Chakraborty, S.; Debnath, S.; Ray, S.S.; Ghosh, Z.; Biswas, K. Ganglioside GM2 Mediates Migration of Tumor Cells by Interacting with Integrin and Modulating the Downstream Signaling Pathway. Biochim. Biophys. Acta (BBA)–Mol. Cell Res. 2016, 1863, 1472–1489. [Google Scholar] [CrossRef]
- Russo, D.; Parashuraman, S.; D’Angelo, G. Glycosphingolipid–Protein Interaction in Signal Transduction. Int. J. Mol. Sci. 2016, 17, 1732. [Google Scholar] [CrossRef]
- Kolter, T. Ganglioside Biochemistry. ISRN Biochem. 2012, 2012, 506160. [Google Scholar] [CrossRef] [PubMed]
- Tettamanti, G.; Bonali, F.; Marchesini, S.; Zambotti, V. A New Procedure for the Extraction, Purification and Fractionation of Brain Gangliosides. Biochim. Biophys. Acta (BBA)–Lipids Lipid Metab. 1973, 296, 160–170. [Google Scholar] [CrossRef]
- Zhao, Z.; Nelson, A.R.; Betsholtz, C.; Zlokovic, B.V. Establishment and Dysfunction of the Blood-Brain Barrier. Cell 2015, 163, 1064–1078. [Google Scholar] [CrossRef] [PubMed]
- Tokuda, N.; Zhang, Q.; Yoshida, S.; Kusunoki, S.; Urano, T.; Furukawa, K.; Furukawa, K. Genetic Mechanisms for the Synthesis of Fucosyl GM1 in Small Cell Lung Cancer Cell Lines. Glycobiology 2006, 16, 916–925. [Google Scholar] [CrossRef]
- Cheever, M.A.; Allison, J.P.; Ferris, A.S.; Finn, O.J.; Hastings, B.M.; Hecht, T.T.; Mellman, I.; Prindiville, S.A.; Viner, J.L.; Weiner, L.M.; et al. The Prioritization of Cancer Antigens: A National Cancer Institute Pilot Project for the Acceleration of Translational Research. Clin. Cancer Res. 2009, 15, 5323–5337. [Google Scholar] [CrossRef]
- Cao, S.; Hu, X.; Ren, S.; Wang, Y.; Shao, Y.; Wu, K.; Yang, Z.; Yang, W.; He, G.; Li, X. The biological role and immunotherapy of gangliosides and GD3 synthase in cancers. Front. Cell Dev. Biol. 2023, 11, 1076862. [Google Scholar] [CrossRef]
- Wegner, M.-S.; Schömel, N.; Gruber, L.; Örtel, S.B.; Kjellberg, M.A.; Mattjus, P.; Kurz, J.; Trautmann, S.; Peng, B.; Wegner, M.; et al. UDP-Glucose Ceramide Glucosyltransferase Activates AKT, Promoted Proliferation, and Doxorubicin Resistance in Breast Cancer Cells. Cell. Mol. Life Sci. 2018, 75, 3393–3410. [Google Scholar] [CrossRef]
- Bremer, E.G.; Hakomori, S. GM3 Ganglioside Induces Hamster Fibroblast Growth Inhibition in Chemically-Defined Medium: Ganglioside May Regulate Growth Factor Receptor Function. Biophys. Biochem. Res. Commun. 1982, 106, 711–718. [Google Scholar] [CrossRef]
- Slevin, M.; Kumar, S.; He, Z.; Gaffney, J.; Gaffney, J. Physiological Concentrations of Gangliosides Gm1, Gm2 and Gm3 Differentially Modify Basic-Fibroblast-Growth-Factor-Induced Mitogenesis and the Associated Signalling Pathway in Endothelial Cells. Int. J. Cancer 1999, 82, 412–423. [Google Scholar] [CrossRef]
- Van Brocklyn, J.; Bremer, E.G.; Yates, A.J. Gangliosides Inhibit Platelet-Derived Growth Factor-Stimulated Receptor Dimerization in Human Glioma U-1242MG and Swiss 3T3 Cells. J. Neurochem. 1993, 61, 371–374. [Google Scholar] [CrossRef]
- Wang, X.-Q.; Sun, P.; Go, L.; Koti, V.; Fliman, M.; Paller, A.S. Ganglioside GM3 Promotes Carcinoma Cell Proliferation via Urokinase Plasminogen Activator-Induced Extracellular Signal-Regulated Kinase-Independent P70S6 Kinase Signaling. J. Investig. Dermatol. 2006, 126, 2687–2696. [Google Scholar] [CrossRef] [PubMed]
- Hanai, N.; Dohi, T.; Nores, G.A.; Hakomori, S. A Novel Ganglioside, de-N-Acetyl-GM3 (II3NeuNH2LacCer), Acting as a Strong Promoter for Epidermal Growth Factor Receptor Kinase and as a Stimulator for Cell Growth. J. Biol. Chem. 1988, 263, 6296–6301. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, N.; Hirabayashi, K.; Michishita, M.; Takahashi, K.; Hasegawa, F.; Gomi, F.; Itakura, Y.; Nakamura, N.; Toyoda, M.; Ishiwata, T. Abstract 135: Increased Expression of Ganglioside GM2 Correlates with Aggressiveness of Human Pancreatic Ductal Adenocarcinoma. Cancer Res. 2020, 80, 135. [Google Scholar] [CrossRef]
- Mutoh, T.; Tokuda, A.; Miyadai, T.; Hamaguchi, M.; Fujiki, N. Ganglioside GM1 Binds to the Trk Protein and Regulates Receptor Function. Proc. Natl. Acad. Sci. USA 1995, 92, 5087–5091. [Google Scholar] [CrossRef] [PubMed]
- Lange, A.M.; Lo, H.-W. Inhibiting TRK Proteins in Clinical Cancer Therapy. Cancers 2018, 10, 105. [Google Scholar] [CrossRef]
- Rusnati, M.; Urbinati, C.; Tanghetti, E.; Dell’Era, P.; Lortat-Jacob, H.; Presta, M. Cell Membrane GM1 Ganglioside Is a Functional Coreceptor for Fibroblast Growth Factor 2. Proc. Natl. Acad. Sci. USA 2002, 99, 4367–4372. [Google Scholar] [CrossRef]
- Zhuo, D.; Guan, F. Ganglioside GM1 Promotes Contact Inhibition of Growth by Regulating the Localization of Epidermal Growth Factor Receptor from Glycosphingolipid-Enriched Microdomain to Caveolae. Cell Prolif. 2019, 52, e12639. [Google Scholar] [CrossRef]
- Chiricozzi, E.; Biase, E.D.; Maggioni, M.; Lunghi, G.; Fazzari, M.; Pomè, D.Y.; Casellato, R.; Loberto, N.; Mauri, L.; Sonnino, S. GM1 Promotes TrkA-Mediated Neuroblastoma Cell Differentiation by Occupying a Plasma Membrane Domain Different from TrkA. J. Neurochem. 2019, 149, 231–241. [Google Scholar] [CrossRef]
- Furukawa, K.; Kambe, M.; Miyata, M.; Ohkawa, Y.; Tajima, O.; Furukawa, K. Ganglioside GD3 Induces Convergence and Synergism of Adhesion and Hepatocyte Growth Factor/Met Signals in Melanomas. Cancer Sci. 2014, 105, 52–63. [Google Scholar] [CrossRef]
- De Maria, R.; Lenti, L.; Malisan, F.; d’Agostino, F.; Tomassini, B.; Zeuner, A.; Rippo, M.R.; Testi, R. Requirement for GD3 Ganglioside in CD95- and Ceramide-Induced Apoptosis. Science 1997, 277, 1652–1655. [Google Scholar] [CrossRef]
- van der Haar Àvila, I.; Windhouwer, B.; van Vliet, S.J. Current State-of-the-Art on Ganglioside-Mediated Immune Modulation in the Tumor Microenvironment. Cancer Metastasis Rev. 2023, 42, 941–958. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, Y.; Momota, H.; Kato, A.; Hashimoto, N.; Tsuda, Y.; Kotani, N.; Honke, K.; Suzumura, A.; Furukawa, K.; Ohmi, Y.; et al. Ganglioside GD3 Enhances Invasiveness of Gliomas by Forming a Complex with Platelet-Derived Growth Factor Receptor α and Yes Kinase. J. Biol. Chem. 2015, 290, 16043–16058. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.; Maira, M.; Gagnon, M.; Saragovi, H.U. Ligands Binding to Cell Surface Ganglioside GD2 Cause Src-Dependent Activation of N-Methyl-D-Aspartate Receptor Signaling and Changes in Cellular Morphology. PLoS ONE 2015, 10, e0134255. [Google Scholar] [CrossRef] [PubMed]
- Durbas, M.; Horwacik, I.; Boratyn, E.; Kamycka, E.; Rokita, H. GD2 Ganglioside Specific Antibody Treatment Downregulates PI3K/Akt/MTOR Signaling Network in Human 449 Neuroblastoma Cell Lines. Int. J. Oncol. 2015, 47, 1143–1159. [Google Scholar] [CrossRef]
- Hyuga, S.; Kawasaki, N.; Hyuga, M.; Ohta, M.; Shibayama, R.; Kawanishi, T.; Yamagata, S.; Yamagata, T.; Hayakawa, T. Ganglioside GD1a Inhibits HGF-Induced Motility and Scattering of Cancer Cells through Suppression of Tyrosine Phosphorylation of c-Met. Int. J. Cancer 2001, 94, 328–334. [Google Scholar] [CrossRef]
- Ha, S.-H.; Lee, J.-M.; Kwon, K.-M.; Kwak, C.-H.; Abekura, F.; Park, J.-Y.; Cho, S.-H.; Lee, K.; Chang, Y.-C.; Lee, Y.-C.; et al. Exogenous and Endogeneous Disialosyl Ganglioside GD1b Induces Apoptosis of MCF-7 Human Breast Cancer Cells. Int. J. Mol. Sci. 2016, 17, 652. [Google Scholar] [CrossRef]
- Hwang, J.-H.; Sung, J.-S.; Kim, J.M.; Chung, Y.-H.; Park, J.S.; Lee, S.-H.; Jang, I.-S. Caveolin-1-Dependent and -Independent UPAR Signaling Pathways Contribute to Ganglioside GT1b Induced Early Apoptosis in A549 Lung Cancer Cells. Am. J. Cancer Res. 2014, 4, 801–810. [Google Scholar]
- Kanda, N.; Nakai, K.; Watanabe, S. Gangliosides GD1b, GT1b, and GQ1b Suppress the Growth of Human Melanoma by Inhibiting Interleukin-8 Production: The Inhibition of Adenylate Cyclase. J. Investig. Dermatol. 2001, 117, 284–293. [Google Scholar] [CrossRef]
- Ornitz, D.M.; Itoh, N. Fibroblast Growth Factors. Genome Biol. 2001, 2, reviews3005.1. [Google Scholar] [CrossRef]
- Peach, C.J.; Mignone, V.W.; Arruda, M.A.; Alcobia, D.C.; Hill, S.J.; Kilpatrick, L.E.; Woolard, J. Molecular Pharmacology of VEGF-A Isoforms: Binding and Signalling at VEGFR2. Int. J. Mol. Sci. 2018, 19, 1264. [Google Scholar] [CrossRef]
- Wieduwilt, M.J.; Moasser, M.M. The Epidermal Growth Factor Receptor Family: Biology Driving Targeted Therapeutics. Cell. Mol. Life Sci. 2008, 65, 1566–1584. [Google Scholar] [CrossRef]
- Fayazzadeh, E.; Yavarifar, H.; Rafie, S.R.; Motamed, S.; Sotoudeh Anvari, M.; Boroumand, M.A. Fibroblast Growth Factor-1 vs. Fibroblast Growth Factor-2 in Ischemic Skin Flap Survival in a Rat Animal Model. World J. Plast Surg. 2016, 5, 274–279. [Google Scholar]
- Brennan, C.W.; Verhaak, R.G.; McKenna, A.; Campos, B.; Noushmehr, H.; Salama, S.R.; Zheng, S.; Chakravarty, D.; Sanborn, J.Z.; Berman, S.H.; et al. The Somatic Genomic Landscape of Glioblastoma. Cell 2013, 155, 466–477. [Google Scholar] [CrossRef]
- Uribe, M.L.; Marrocco, I.; Yarden, Y. EGFR in Cancer: Signaling Mechanisms, Drugs, and Acquired Resistance. Cancers 2021, 13, 2748. [Google Scholar] [CrossRef]
- Calvo, E.; Baselga, J. Ethnic Differences in Response to Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors. J. Clin. Oncol. 2006, 24, 2158–2163. [Google Scholar] [CrossRef]
- Zhou, W.; Christiani, D.C. East Meets West: Ethnic Differences in Epidemiology and Clinical Behaviors of Lung Cancer between East Asians and Caucasians. Chin. J. Cancer 2011, 30, 287. [Google Scholar] [CrossRef] [PubMed]
- Nomura, M.; Shigematsu, H.; Li, L.; Suzuki, M.; Takahashi, T.; Estess, P.; Siegelman, M.; Feng, Z.; Kato, H.; Marchetti, A.; et al. Polymorphisms, Mutations, and Amplification of the EGFR Gene in Non-Small Cell Lung Cancers. PLoS Med. 2007, 4, e125. [Google Scholar] [CrossRef] [PubMed]
- Miyagi, T.; Yamamoto, K. Sialidase NEU3 and Its Pathological Significance. Glycoconj. J. 2022, 39, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Schengrund, C.-L.; Rosenberg, A.; Repman, M.A. Ecto-Ganglioside-Sialidase Activity of Herpes Simplex Virus Transformed Hamster Embryo Fibroblasts. J. Cell Biol. 1976, 70, 555–561. [Google Scholar] [CrossRef]
- Miyagi, T.; Yamaguchi, K. Mammalian sialidases: Physiological and pathological roles in cellular functions. Glycobiology 2012, 22, 880–896. [Google Scholar] [CrossRef]
- Kawamura, S.; Sato, I.; Wada, T.; Yamaguchi, K.; Li, Y.; Li, D.; Zhao, X.; Ueno, S.; Aoki, H.; Tochigi, T. Plasma Membrane-Associated Sialidase (NEU3) Regulates Progression of Prostate Cancer to Androgen-Independent Growth through Modulation of Androgen Receptor Signaling. Cell Death Differ. 2012, 19, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Mandal, C.; Tringali, C.; Mondal, S.; Anastasia, L.; Chandra, S.; Venerando, B.; Mandal, C. Down Regulation of Membrane-bound Neu3 Constitutes a New Potential Marker for Childhood Acute Lymphoblastic Leukemia and Induces Apoptosis Suppression of Neoplastic Cells. Int. J. Cancer 2010, 126, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Proshin, S.; Yamaguchi, K.; Yamashita, Y.; Katakura, R.; Yamamoto, K.; Shima, H.; Hosono, M.; Miyagi, T. Sialidase NEU3 Defines Invasive Potential of Human Glioblastoma Cells by Regulating Calpain-Mediated Proteolysis of Focal Adhesion Proteins. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2017, 1861, 2778–2788. [Google Scholar] [CrossRef] [PubMed]
- Mozzi, A.; Forcella, M.; Riva, A.; Difrancesco, C.; Molinari, F.; Martin, V.; Papini, N.; Bernasconi, B.; Nonnis, S.; Tedeschi, G.; et al. NEU3 Activity Enhances EGFR Activation without Affecting EGFR Expression and Acts on Its Sialylation Levels. Glycobiology 2015, 25, 855–868. [Google Scholar] [CrossRef] [PubMed]
- Crespo, P.M.; Demichelis, V.T.; Daniotti, J.L. Neobiosynthesis of Glycosphingolipids by Plasma Membrane-associated Glycosyltransferases. J. Biol. Chem. 2010, 285, 29179–29190. [Google Scholar] [CrossRef] [PubMed]
- Vilcaes, A.A.; Garbarino-Pico, E.; Torres Demichelis, V.; Daniotti, J.L. Ganglioside Synthesis by Plasma Membrane-Associated Sialyltransferase in Macrophages. Int. J. Mol. Sci. 2020, 21, 1063. [Google Scholar] [CrossRef]
- Bos, P.D.; Zhang, X.H.-F.; Nadal, C.; Shu, W.; Gomis, R.R.; Nguyen, D.X.; Minn, A.J.; van de Vijver, M.J.; Gerald, W.L.; Foekens, J.A.; et al. Genes That Mediate Breast Cancer Metastasis to the Brain. Nature 2009, 459, 1005–1009. [Google Scholar] [CrossRef]
- Drolez, A.; Vandenhaute, E.; Delannoy, C.P.; Dewald, J.H.; Gosselet, F.; Cecchelli, R.; Julien, S.; Dehouck, M.-P.; Delannoy, P.; Mysiorek, C. ST6GALNAC5 Expression Decreases the Interactions between Breast Cancer Cells and the Human Blood-Brain Barrier. Int. J. Mol. Sci. 2016, 17, 1309. [Google Scholar] [CrossRef]
- Zhang, C.; Lin, X.; Song, Y.; Zhang, X.; Li, H.; Wang, Q. Overexpression of Glucosylceramide Synthase and Its Significance in the Clinical Outcome of Non-Small Cell Lung Cancer. Chin. Med. J. 2014, 127, 3071–3076. [Google Scholar]
- Han, Y.; Li, Z.; Wu, Q.; Liu, H.; Sun, Z.; Wu, Y.; Luo, J. B4GALT5 High Expression Associated with Poor Prognosis of Hepatocellular Carcinoma. BMC Cancer 2022, 22, 392. [Google Scholar] [CrossRef]
- Yi, H.; Lin, Y.; Li, Y.; Guo, Y.; Yuan, L.; Mao, Y. Pan-Cancer Analysis of B4GALNT1 as a Potential Prognostic and Immunological Biomarker. J. Immunol. Res. 2022, 2022, 4355890. [Google Scholar] [CrossRef] [PubMed]
- Sha, Y.-L.; Liu, Y.; Yang, J.-X.; Wang, Y.-Y.; Gong, B.-C.; Jin, Y.; Qu, T.-Y.; Xia, F.-T.; Han, L.; Zhao, Q. B3GALT4 Remodels the Tumor Microenvironment through GD2-Mediated Lipid Raft Formation and the c-Met/AKT/MTOR/IRF-1 Axis in Neuroblastoma. J. Exp. Clin. Cancer Res. 2022, 41, 314. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.-J.; Ding, Y.; Levery, S.B.; Lobaton, M.; Handa, K.; Hakomori, S. Differential Expression Profiles of Glycosphingolipids in Human Breast Cancer Stem Cells vs. Cancer Non-Stem Cells. Proc. Natl. Acad. Sci. USA 2013, 110, 4968–4973. [Google Scholar] [CrossRef] [PubMed]
- Mehta, K.A.; Patel, K.A.; Pandya, S.J.; Patel, P.S. Aberrant Sialylation Plays a Significant Role in Oral Squamous Cell Carcinoma Progression. J. Oral Pathol. Med. 2020, 49, 253–259. [Google Scholar] [CrossRef]
- Deschuyter, M.; Leger, D.Y.; Verboom, A.; Chaunavel, A.; Maftah, A.; Petit, J.M. ST3GAL2 Knock-down Decreases Tumoral Character of Colorectal Cancer Cells in Vitro and in Vivo. Am. J. Cancer Res. 2022, 12, 280–302. [Google Scholar]
- Liu, J.; Li, M.; Wu, J.; Qi, Q.; Li, Y.; Wang, S.; Liang, S.; Zhang, Y.; Zhu, Z.; Huang, R.; et al. Identification of ST3GAL5 as a Prognostic Biomarker Correlating with CD8+ T Cell Exhaustion in Clear Cell Renal Cell Carcinoma. Front. Immunol. 2022, 13, 979605. [Google Scholar] [CrossRef]
- Li, W.; Zheng, X.; Ren, L.; Fu, W.; Liu, J.; Xv, J.; Liu, S.; Wang, J.; Du, G. Epigenetic Hypomethylation and Upregulation of GD3s in Triple Negative Breast Cancer. Ann. Transl. Med. 2019, 7, 723. [Google Scholar] [CrossRef]
- Sarkar, T.R.; Battula, V.L.; Werden, S.J.; Vijay, G.V.; Ramirez-Peña, E.Q.; Taube, J.H.; Chang, J.T.; Miura, N.; Porter, W.; Sphyris, N. GD3 Synthase Regulates Epithelial–Mesenchymal Transition and Metastasis in Breast Cancer. Oncogene 2015, 34, 2958–2967. [Google Scholar] [CrossRef]
- Battula, V.L.; Shi, Y.; Evans, K.W.; Wang, R.-Y.; Spaeth, E.L.; Jacamo, R.O.; Guerra, R.; Sahin, A.A.; Marini, F.C.; Hortobagyi, G. Ganglioside GD2 Identifies Breast Cancer Stem Cells and Promotes Tumorigenesis. J. Clin. Investig. 2012, 122, 2066–2078. [Google Scholar] [CrossRef]
- Hugonnet, M.; Singh, P.; Haas, Q.; von Gunten, S. The Distinct Roles of Sialyltransferases in Cancer Biology and Onco-Immunology. Front. Immunol. 2021, 12, 799861. [Google Scholar] [CrossRef]
- Tringali, C.; Lupo, B.; Silvestri, I.; Papini, N.; Anastasia, L.; Tettamanti, G.; Venerando, B. The Plasma Membrane Sialidase NEU3 Regulates the Malignancy of Renal Carcinoma Cells by Controlling Β1 Integrin Internalization and Recycling. J. Biol. Chem. 2012, 287, 42835–42845. [Google Scholar] [CrossRef]
- Asano, M. Various Biological Functions of Carbohydrate Chains Learned from Glycosyltransferase-Deficient Mice. Exp. Anim. 2020, 69, 261–268. [Google Scholar] [CrossRef]
- Lingwood, C.A. Aglycone Modulation of Glycolipid Receptor Function. Glycoconj. J. 1996, 13, 495–503. [Google Scholar] [CrossRef]
- Mullen, T.D.; Hannun, Y.A.; Obeid, L.M. Ceramide Synthases at the Centre of Sphingolipid Metabolism and Biology. Biochem. J. 2012, 441, 789–802. [Google Scholar] [CrossRef] [PubMed]
- Sassa, T.; Hirayama, T.; Kihara, A. Enzyme Activities of the Ceramide Synthases CERS2-6 Are Regulated by Phosphorylation in the C-Terminal Region. J. Biol. Chem. 2016, 291, 7477–7487. [Google Scholar] [CrossRef] [PubMed]
- Ogretmen, B. Sphingolipid Metabolism in Cancer Signalling and Therapy. Nat. Rev. Cancer 2018, 18, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Sarbu, M.; Petrica, L.; Clemmer, D.E.; Vukelić, Ž.; Zamfir, A.D. Gangliosides of Human Glioblastoma Multiforme: A Comprehensive Mapping and Structural Analysis by Ion Mobility Tandem Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2021, 32, 1249–1257. [Google Scholar] [CrossRef]
- Reza, S.; Ugorski, M.; Suchański, J. Glucosylceramide and Galactosylceramide, Small Glycosphingolipids with Significant Impact on Health and Disease. Glycobiology 2021, 31, 1416–1434. [Google Scholar] [CrossRef]
- Pinto, S.N.; Laviad, E.L.; Stiban, J.; Kelly, S.L.; Merrill, A.H., Jr.; Prieto, M.; Futerman, A.H.; Silva, L.C. The Sphingoid Bases of Sphingolipids, Including Ceramides, Can Vary in Length from 12 to >20 Carbons. J. Lipid Res. 2014, 55, 53–61. [Google Scholar] [CrossRef]
- Valentino, L.; Moss, T.; Olson, E.; Wang, H.-J.; Elashoff, R.; Ladisch, S. Shed Tumor Gangliosides and Progression of Human Neuroblastoma. Blood 1990, 75, 1564–1567. [Google Scholar] [CrossRef]
- Bergelson, L.D. Serum Gangliosides as Endogenous Immunomodulators. Immunol. Today 1995, 16, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Sun, M.; Yu, M.; Fu, Q.; Jiang, H.; Yu, G.; Li, G. Gangliosides Profiling in Serum of Breast Cancer Patient: GM3 as a Potential Diagnostic Biomarker. Glycoconj. J. 2019, 36, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Balis, F.M.; Busch, C.M.; Desai, A.V.; Hibbitts, E.; Naranjo, A.; Bagatell, R.; Irwin, M.; Fox, E. The Ganglioside GD2 as a Circulating Tumor Biomarker for Neuroblastoma. Pediatr. Blood Cancer 2020, 67, e28031. [Google Scholar] [CrossRef]
- Kong, Y.; Li, R.; Ladisch, S. Natural Forms of Shed Tumor Gangliosides. Biochim. Biophys. Acta 1998, 1394, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Yohe, H.C.; Rosenberg, A. Interaction of triiodide anion with gangliosides in aqueous iodine. Chem. Phys. Lipids 1972, 9, 279–294. [Google Scholar] [CrossRef] [PubMed]
- Crocker, P.R.; Paulson, J.C.; Varki, A. Siglecs and Their Roles in the Immune System. Nat. Rev. Immunol. 2007, 7, 255–266. [Google Scholar] [CrossRef]
- Daly, J.; Carlsten, M.; O’Dwyer, M. Sugar Free: Novel Immunotherapeutic Approaches Targeting Siglecs and Sialic Acids to Enhance Natural Killer Cell Cytotoxicity against Cancer. Front. Immunol. 2019, 10, 1047. [Google Scholar] [CrossRef]
- Lim, J.; Sari-Ak, D.; Bagga, T. Siglecs as Therapeutic Targets in Cancer. Biology 2021, 10, 1178. [Google Scholar] [CrossRef]
- Bakker, T.R.; Piperi, C.; Davies, E.A.; van der Merwe, P.A. Comparison of CD22 Binding to Native CD45 and Synthetic Oligosaccharide. Eur. J. Immunol. 2002, 32, 1924–1932. [Google Scholar] [CrossRef]
- Lee, Y.C.; Lee, R.T. Carbohydrate-Protein Interactions: Basis of Glycobiology. Acc. Chem. Res. 1995, 28, 321–327. [Google Scholar] [CrossRef]
- Crocker, P.R.; Feizi, T. Carbohydrate Recognition Systems: Functional Triads in Cell—Cell Interactions. Curr. Opin. Struct. Biol. 1996, 6, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Zang, H.; Siddiqui, M.; Gummuluru, S.; Wong, W.W.; Reinhard, B.M. Ganglioside-Functionalized Nanoparticles for Chimeric Antigen Receptor T-Cell Activation at the Immunological Synapse. ACS Nano 2022, 16, 18408–18420. [Google Scholar] [CrossRef] [PubMed]
- Affandi, A.J.; Grabowska, J.; Olesek, K.; Lopez Venegas, M.; Barbaria, A.; Rodríguez, E.; Mulder, P.P.G.; Pijffers, H.J.; Ambrosini, M.; Kalay, H.; et al. Selective Tumor Antigen Vaccine Delivery to Human CD169+ Antigen-Presenting Cells Using Ganglioside-Liposomes. Proc. Natl. Acad. Sci. USA 2020, 117, 27528–27539. [Google Scholar] [CrossRef] [PubMed]
- Collins, B.E.; Kiso, M.; Hasegawa, A.; Tropak, M.B.; Roder, J.C.; Crocker, P.R.; Schnaar, R.L. Binding Specificities of the Sialoadhesin Family of I-Type Lectins: Sialic Acid Linkage and Substructure Requirements for Binding of Myelin-Associated Glycoprotein, Schwann Cell Myelin Protein, and Sialoadhesin. J. Biol. Chem. 1997, 272, 16889–16895. [Google Scholar] [CrossRef] [PubMed]
- Powell, L.D.; Sgroi, D.; Sjoberg, E.R.; Stamenkovic, I.; Varki, A. Natural Ligands of the B Cell Adhesion Molecule CD22 Beta Carry N-Linked Oligosaccharides with Alpha-2,6-Linked Sialic Acids That Are Required for Recognition. J. Biol. Chem. 1993, 268, 7019–7027. [Google Scholar] [CrossRef]
- Hernández-Caselles, T.; Martínez-Esparza, M.; Pérez-Oliva, A.B.; Quintanilla-Cecconi, A.M.; García-Alonso, A.; Alvarez-López, D.M.; García-Peñarrubia, P. A Study of CD33 (SIGLEC-3) Antigen Expression and Function on Activated Human T and NK Cells: Two Isoforms of CD33 Are Generated by Alternative Splicing. J. Leukoc. Biol. 2006, 79, 46–58. [Google Scholar] [CrossRef]
- Macauley, M.S.; Crocker, P.R.; Paulson, J.C. Siglec-Mediated Regulation of Immune Cell Function in Disease. Nat. Rev. Immunol. 2014, 14, 653–666. [Google Scholar] [CrossRef]
- Schnaar, R.L. Gangliosides as Siglec Ligands. Glycoconj. J. 2023, 40, 159–167. [Google Scholar] [CrossRef]
- Rapoport, E.; Mikhalyov, I.; Zhang, J.; Crocker, P.; Bovin, N. Ganglioside Binding Pattern of CD33-Related Siglecs. Bioorganic Med. Chem. Lett. 2003, 13, 675–678. [Google Scholar] [CrossRef]
- Yamaji, T.; Teranishi, T.; Alphey, M.S.; Crocker, P.R.; Hashimoto, Y. A Small Region of the Natural Killer Cell Receptor, Siglec-7, Is Responsible for Its Preferred Binding to a2,8-Disialyl and Branched A2,6-Sialyl Residues: A comparison with Siglec-9. J. Biol. Chem. 2002, 277, 6324–6332. [Google Scholar] [CrossRef]
- Munday, J.; Kerr, S.; Ni, J.; Cornish, A.L.; Zhang, J.Q.; Nicoll, G.; Floyd, H.; Mattei, M.G.; Moore, P.; Liu, D.; et al. Identification, Characterization and Leucocyte Expression of Siglec-10, a Novel Human Sialic Acid-Binding Receptor. Biochem. J. 2001, 355 Pt 2, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Kohler, G.; Milstein, C. Continuous Cultures of Fused Cells Secreting Antibody of Predefined Specificity. Nature 1975, 256, 495–497. [Google Scholar] [CrossRef] [PubMed]
- Couzin-Frankel, J. Cancer Immunotherapy. Science 2013, 342, 1432–1433. [Google Scholar] [CrossRef] [PubMed]
- Schulz, G.; Cheresh, D.A.; Varki, N.M.; Yu, A.; Staffileno, L.K.; Reisfeld, R.A. Detection of Ganglioside GD2 in Tumor Tissues and Sera of Neuroblastoma Patients. Cancer Res. 1984, 44, 5914–5920. [Google Scholar]
- Longee, D.C.; Wikstrand, C.J.; M»nsson, J.-E.; He, X.; Fuller, G.N.; Bigner, S.H.; Fredman, P.; Svennerholm, L.; Bigner, D.D. Disialoganglioside GD2 in Human Neuroectodermal Tumor Cell Lines and Gliomas. Acta Neuropathol. 1991, 82, 45–54. [Google Scholar] [CrossRef]
- Cheresh, D.A.; Pierschbacher, M.D.; Herzig, M.A.; Mujoo, K. Disialogangliosides GD2 and GD3 Are Involved in the Attachment of Human Melanoma and Neuroblastoma Cells to Extracellular Matrix Proteins. J. Cell Biol. 1986, 102, 688–696. [Google Scholar] [CrossRef]
- Sorkin, L.S.; Otto, M.; Baldwin, W.M., 3rd; Vail, E.; Gillies, S.D.; Handgretinger, R.; Barfield, R.C.; Ming Yu, H.; Yu, A.L. Anti-GD(2) with an FC Point Mutation Reduces Complement Fixation and Decreases Antibody-Induced Allodynia. Pain 2010, 149, 135–142. [Google Scholar] [CrossRef]
- Desai, A.V.; Gilman, A.L.; Ozkaynak, M.F.; Naranjo, A.; London, W.B.; Tenney, S.C.; Diccianni, M.; Hank, J.A.; Parisi, M.T.; Shulkin, B.L.; et al. Outcomes Following GD2-Directed Postconsolidation Therapy for Neuroblastoma After Cessation of Random Assignment on ANBL0032: A Report From the Children’s Oncology Group. J. Clin. Oncol. 2022, 40, 4107–4118. [Google Scholar] [CrossRef]
- Thompson, J.P.; Schengrund, C.-L. Oligosaccharide-Derivatized Dendrimers: Defined Multivalent Inhibitors of the Adherence of the Cholera Toxin B Subunit and the Heat Labile Enterotoxin of E. coli to GM1. Glycoconj. J. 1997, 14, 837–845. [Google Scholar] [CrossRef]
- Kitov, P.I.; Sadowska, J.M.; Mulvey, G.; Armstrong, G.D.; Ling, H.; Pannu, N.S.; Read, R.J.; Bundle, D.R. Shiga-like Toxins Are Neutralized by Tailored Multivalent Carbohydrate Ligands. Nature 2000, 403, 669–672. [Google Scholar] [CrossRef]
- Kholodenko, I.V.; Kalinovsky, D.V.; Svirshchevskaya, E.V.; Doronin, I.I.; Konovalova, M.V.; Kibardin, A.V.; Shamanskaya, T.V.; Larin, S.S.; Deyev, S.M.; Kholodenko, R.V. Multimerization through Pegylation Improves Pharmacokinetic Properties of ScFv Fragments of GD2-Specific Antibodies. Molecules 2019, 24, 3835. [Google Scholar] [CrossRef]
- Kholodenko, R.V.; Kalinovsky, D.V.; Doronin, I.I.; Ponomarev, E.D.; Kholodenko, I.V. Antibody Fragments as Potential Biopharmaceuticals for Cancer Therapy: Success and Limitations. Curr. Med. Chem. 2019, 26, 396–426. [Google Scholar] [CrossRef] [PubMed]
- Siebert, N.; Jensen, C.; Troschke-Meurer, S.; Zumpe, M.; Jüttner, M.; Ehlert, K.; Kietz, S.; Müller, I.; Lode, H.N. Neuroblastoma Patients with High-Affinity FCGR2A, -3A and Stimulatory KIR 2DS2 Treated by Long-Term Infusion of Anti-GD2 Antibody Ch14.18/ CHO Show Higher ADCC Levels and Improved Event- Free Survival. Oncoimmunology 2016, 5, e1235108. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Fukumoto, S.; Kawaguchi, H.; Sato, S.; Ueda, R.; Furukawa, K. Ganglioside GD2 in Small Cell Lung Cancer Cell Lines: Enhancement of Cell Proliferation and Mediation of Apoptosis. Cancer Res. 2001, 61, 4244–4252. [Google Scholar] [PubMed]
- Kailayangiri, S.; Altvater, B.; Meltzer, J.; Pscherer, S.; Luecke, A.; Dierkes, C.; Titze, U.; Leuchte, K.; Landmeier, S.; Hotfilder, M.; et al. The Ganglioside Antigen GD2 Is Surface-Expressed in Ewing Sarcoma and Allows for MHC-Independent Immune Targeting. Br. J. Cancer 2012, 106, 1123–1133. [Google Scholar] [CrossRef]
- Reppel, L.; Tsahouridis, O.; Akulian, J.; Davis, I.J.; Lee, H.; Fucà, G.; Weiss, J.; Dotti, G.; Pecot, C.V.; Savoldo, B. Targeting Disialoganglioside GD2 with Chimeric Antigen Receptor-Redirected T Cells in Lung Cancer. J. Immunother. Cancer 2022, 10, e003897. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.; Maira, M.; Roychoudhury, R.; Galan, A.; Brahimi, F.; Gilbert, M.; Cunningham, A.-M.; Josephy, S.; Pirvulescu, I.; Moffett, S.; et al. Vaccination with Tumor-Ganglioside Glycomimetics Activates a Selective Immunity that Affords Cancer Therapy. Cell Chem. Biol. 2019, 26, 1013–1026. [Google Scholar] [CrossRef]
- Weir, H.K.; Thompson, T.D.; Stewart, S.L.; White, M.C. Peer Reviewed: Cancer Incidence Projections in the United States between 2015 and 2050. Prev. Chronic Dis. 2021, 18, 210006. [Google Scholar] [CrossRef]
- Pietrobono, S.; Stecca, B. Aberrant Sialylation in Cancer: Biomarker and Potential Target for Therapeutic Intervention? Cancers 2021, 13, 2014. [Google Scholar] [CrossRef]
- Duan, S.; Paulson, J.C. Siglecs as Immune Cell Checkpoints in Disease. Annu. Rev. Immunol. 2020, 38, 365–395. [Google Scholar] [CrossRef]
- Hatano, K.; Miyamoto, Y.; Nonomura, N.; Kaneda, Y. Expression of Gangliosides, GD1a, and Sialyl Paragloboside Is Regulated by NF-ΚB-Dependent Transcriptional Control of α2,3-Sialyltransferase I, II, and VI in Human Castration-Resistant Prostate Cancer Cells. Int. J. Cancer 2011, 129, 1838–1847. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Josson, S.; Fang, F.; Oberley, T.D.; St Clair, D.K.; Wan, X.S.; Sun, Y.; Bakthavatchalu, V.; Muthuswamy, A.; St Clair, W.H. RelB Enhances Prostate Cancer Growth: Implications for the Role of the Nuclear Factor-KappaB Alternative Pathway in Tumorigenicity. Cancer Res. 2009, 69, 3267–3271. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Shan, Z.; Yang, H.; Xu, J.; Li, W.; Guo, F. RelB plays an oncogenic role and conveys chemo-resistance to DLD-1 colon cancer cells. Cancer Cell Int. 2018, 18, 181. [Google Scholar] [CrossRef] [PubMed]
- Dewald, J.H.; Cavdarli, S.; Steenackers, A.; Delannoy, C.P.; Mortuaire, M.; Spriet, C.; Noël, M.; Groux-Degroote, S.; Delannoy, P. TNF differentially regulates ganglioside biosynthesis and expression in breast cancer cell lines. PLoS ONE 2018, 13, e0196369. [Google Scholar] [CrossRef]
- Eluard, B.; Nuan-Aliman, S.; Faumont, N.; Collares, D.; Bordereaux, D.; Montagne, A.; Martins, I.; Cagnard, N.; Caly, M.; Taoui, O.; et al. The alternative RelB NF-κB subunit is a novel critical player in diffuse large B-cell lymphoma. Blood 2022, 139, 384–398. [Google Scholar] [CrossRef]
- Goleva, E.; Lyubchenko, T.; Kraehenbuehl, L.; Lacouture, M.E.; Leung, D.Y.M.; Kern, J.A. Our current understanding of checkpoint inhibitor therapy in cancer immunotherapy. Ann. Allergy Asthma Immunol. 2021, 126, 630–638. [Google Scholar] [CrossRef]
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017, 168, 707–723. [Google Scholar] [CrossRef]
- Yu, H.; Gonzalez-Gil, A.; Wei, Y.; Fernandes, S.M.; Porell, R.N.; Vajn, K.; Paulson, J.C.; Nycholat, C.M.; Schnaar, R.L. Siglec-8 and Siglec-9 binding specificities and endogenous airway ligand distributions and properties. Glycobiology 2017, 27, 657–668. [Google Scholar] [CrossRef]
- Ibarlucea-Benitez, I.; Weitzenfeld, P.; Smith, P.; Ravetch, J.V. Siglecs-7/9 function as inhibitory immune checkpoints in vivo and can be targeted to enhance therapeutic antitumor immunity. Proc. Natl. Acad. Sci. USA 2021, 118, e2107424118. [Google Scholar] [CrossRef]
- Theruvath, J.; Menard, M.; Smith, A.H.; Linde, M.H.; Coles, G.L.; Dalton, G.N.; Wu, W.; Kiru, L.; Delaidelli, A.; Sotillo, E.; et al. Anti-GD2 synergizes with CD47 blockade to mediate tumor eradication. Nat. Med. 2022, 28, 333–344. [Google Scholar] [CrossRef]
- Folkman, J. Tumor angiogenesis: Therapeutic implications. N. Eng. J. Med. 1971, 285, 1182–1186. [Google Scholar] [CrossRef]
- Ziche, M.; Morbidelli, L.; Alessandri, G.; Gullino, P.M. Angiogenesis can be stimulated or repressed in vivo by a change in GM3:GD3 ganglioside ratio. Lab. Investig. 1992, 67, 711–715. [Google Scholar] [PubMed]
- Seyfried, T.N.; Mukherjee, P. Ganglioside GM3 Is Antiangiogenic in Malignant Brain Cancer. J. Oncol. 2010, 2010, 961243. [Google Scholar] [CrossRef]
- Fabris, D.; Karmelić, I.; Muharemović, H.; Sajko, T.; Jurilj, M.; Potočki, S.; Novak, R.; Vukelić, Ž. Ganglioside Composition Distinguishes Anaplastic Ganglioglioma Tumor Tissue from Peritumoral Brain Tissue: Complementary Mass Spectrometry and Thin-Layer Chromatography Evidence. Int. J. Mol. Sci. 2021, 22, 8844. [Google Scholar] [CrossRef] [PubMed]
- Tagawa, Y.; Laroy, W.; Nimrichter, L.; Fromholt, S.E.; Moser, A.B.; Moser, H.W.; Schnaar, R.L. Anti-Ganglioside Antibodies Bind with Enhanced Affinity to Gangliosides Containing Very Long Chain Fatty Acids. Neurochem. Res. 2002, 27, 847–855. [Google Scholar] [CrossRef]
- Ladisch, S.; Li, R.; Olson, E. Ceramide structure predicts tumor ganglioside immunosuppressive activity. Proc. Natl. Acad. Sci. USA 1994, 91, 1974–1978. [Google Scholar] [CrossRef]
- Yeh, S.C.; Wang, P.Y.; Lou, Y.W.; Khoo, K.H.; Hsiao, M.; Hsu, T.L.; Wong, C.-H. Glycolipid GD3 and GD3 synthase are key drivers for glioblastoma stem cells and tumorigenicity. Proc. Natl. Acad. Sci. USA 2016, 113, 5592–5597. [Google Scholar] [CrossRef]
- Croft, D.; Lodhia, P.; Lourenco, S.; MacKay, C. Effectively utilizing publicly available databases for cancer target evaluation. NAR Cancer 2023, 5, zcad035. [Google Scholar] [CrossRef]
- Salazar, B.M.; Balczewski, E.A.; Ung, C.Y.; Zhu, S. Neuroblastoma, a Paradigm for Big Data Science in Pediatric Oncology. Int. J. Mol.Sci. 2017, 18, 37. [Google Scholar] [CrossRef]
- Goldsmith, K.C.; Park, J.R.; Kayser, K.; Malvar, J.; Chi, Y.Y.; Groshen, S.G.; Villablanca, J.G.; Krytska, K.; Lai, L.M.; Acharya, P.T.; et al. Lorlatinib with or without chemotherapy in ALK-driven refractory/relapsed neuroblastoma: Phase 1 trial results. Nat. Med. 2023, 29, 1092–1102. [Google Scholar] [CrossRef]
- Porębska, N.; Ciura, K.; Chorążewska, A.; Zakrzewska, M.; Otlewski, J.; Opaliński, Ł. Multivalent protein-drug conjugates—An emerging strategy for the upgraded precision and efficiency of drug delivery to cancer cells. Biotechnol. Adv. 2023, 67, 108213. [Google Scholar] [CrossRef] [PubMed]
- Molica, M.; Perrone, S.; Mazzone, C.; Niscola, P.; Cesini, L.; Abruzzese, E.; de Fabritiis, P. CD33 Expression and Gentuzumab Ozogamicin in Acute Myeloid Leukemia: Two Sides of the Same Coin. Cancers 2021, 13, 3214. [Google Scholar] [CrossRef] [PubMed]
- Kalinovsky, D.V.; Kibardin, A.V.; Kholodenko, I.V.; Svirshchevskaya, E.V.; Doronin, I.I.; Konovalova, M.V.; Grechikhina, M.V.; Rozov, F.N.; Larin, S.S.; Deyev, S.M.; et al. Therapeutic efficacy of antibody-drug conjugates targeting GD2-positive tumors. J. Immunother. Cancer 2022, 10, e004646. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, A.; Żebrowska, U.; Ussowicz, M.; Sokół, A.; Stypińska, M.; Dembowska-Bagińska, B.; Pawińska-Wąsikowska, K.; Balwierz, W. Dinutuximab Beta Maintenance Therapy in Patients with High-Risk Neuroblastoma in First-Line and Refractory/Relapsed Settings—Real-World Data. J. Clin. Med. 2023, 12, 5252. [Google Scholar] [CrossRef]
- Shreve, J.T.; Khanani, S.A.; Haddad, T.C. Artificial Intelligence in Oncology: Current Capabilities, Future Opportunities, and Ethical Considerations. Am. Soc. Clin. Oncol. Educ. Book 2022, 42, 842–851. [Google Scholar] [CrossRef]

| Ganglioside | Saccharide Composition |
|---|---|
| GA2 | GalNAcβ1-4Galβ1-4Glcβ1- a |
| GA1 | Galβ1-3GalNAcβ1-4Galβ1-4Glcβ1- |
| GM1b | Neu5Acα2-3Galβ1-3GalNAcβ1-4Galβ1-4Glcβ1- b |
| GD1aα | Neu5Acα2-3Galβ1-3(Neu5Acα2-6)GalNAcβ1-4Galβ1-4Glcβ1- |
| GM3 | Neu5Acα2-3Galβ1-4Glcβ1- |
| GM2 | GalNAcβ1-4(Neu5Acα2-3)Galβ1-4Glcβ1- |
| GM1a | Galβ1-3GalNAcβ1-4(Neu5Acα2-3)Galβ1-4Glcβ1- |
| GD1a | Neu5Acα2-3Galβ1-3GalNAcβ1-4(Neu5Acα2-3)Galβ1-4Glcβ1- |
| GT1a | Neu5Acα2-8Neu5Acα2-3Galβ1-3GalNAcβ1-4(Neu5Acα2-3)Galβ1-4Glcβ1- |
| GD3 | Neu5Acα2-8Neu5Acα2-3Galβ1-4Glcβ1- |
| GD2 | GalNAcβ1-4 (Neu5Acα2-8Neu5Acα2-3)Galβ1-4Glcβ1- |
| GD1b | Galβ1-3GalNAcβ1-4 (Neu5Acα2-8Neu5Acα2-3)Galβ1-4Glcβ1- |
| GT1b | Neu5Acα2-3Galβ1-3GalNAcβ1-4 (Neu5Acα2-8Neu5Acα2-3)Galβ1-4Glcβ1- |
| GQ1b | Neu5Acα2-8Neu5Acα2-3Galβ1-3GalNAcβ1-4 (Neu5Acα2-8Neu5Acα2-3)Galβ1-4Glcβ1- |
| GT3 | Neu5Acα2-8Neu5Acα2-8Neu5Acα2-3Galβ1-4Glcβ1- |
| GT2 | GalNAcβ1-4(Neu5Acα2-8Neu5Acα2-8Neu5Acα2-3)Galβ1-4Glcβ1- |
| GT1c | Galβ1-3GalNAcβ1-4(Neu5Acα2-8Neu5Acα2-8Neu5Acα2-3)Galβ1-4Glcβ1- |
| GQ1c | Neu5Acα2-3Galβ1-3GalNAcβ1-4(Neu5Acα2-8Neu5Acα2-8Neu5Acα2-3)Galβ1-4Glcβ1- c |
| Pathway Affected | Ganglioside | Effect | Cell Type | Reference |
|---|---|---|---|---|
| ↑ a Akt b activity | GlcCer | ↑ Proliferation | Breast cancer | [41] |
| ↓ Proangiogenic effects of VEGF/VEGFR -2 and GD1a | GM3 | ↓ Angiogenesis | HUVECs c | [8,9] |
| ↓ EGFR phosphorylation | GM3 | ↓ Mitogenesis | Swiss 3T3 Human epidermoid carcinoma | [18,32,34] |
| FGF | GM3 | ↓ Proliferation | BHK and Swiss 3T3 | [32,42] |
| FGF2 | GM3 | ↑ Proliferation | Bovine aortic endothelial | [43] |
| ↓ Dimerization of PDGFR | GM3 | ↑ Proliferation | Human glioma | [44] |
| uPA ↑ P70S6 kinase signaling | Over-expressed GM3 | ↑ Proliferation | Carcinoma SCC12 | [45] |
| ↑ EGFR kinase | de-N-Acetyl-GM3 | ↑ Proliferation | Melanoma | [46] |
| Binds integrin receptor ↑ FAK, Erk and Src phosphorylation | GM2 | ↑ Migration | Renal carcinoma | [33] |
| TGF-b1 | GM2 | ↑ Growth and invasiveness | Pancreatic ductal adenocarcinoma | [47] |
| GM1 binds TrkA | GM1 | ↑ NGF receptor | Neuroblastoma | [48,49] |
| FGF2 | GM1 | ↓ Proliferation | CHO | [50] |
| PDGF | GM1 | ↓ Proliferation | Swiss 3T3 | [32] |
| EGFR moves to caveolae | GM1 | ↓ Proliferation | Human breast epi- thelial | [51] |
| TrkA | GM1 | ↑ Neuronal differentiation | Neuro2A | [52] |
| ↑ Akt, Erk1/2 phosphorylation | GD3 + HGF collagen 1 | ↑ Proliferation | Melanoma N1 | [53] |
| Paxillin | GD3 | ↑ Migration | Melanoma N1 | [7] |
| Mediates propagation of CD95-induced apoptosis | GD3 | ↑ Apoptosis | Lymphoblasts | [54] |
| Siglec-7 receptor on NK cells | GD3 | ↓ Immuno-suppressive | Natural killer | [55] |
| PDGFRα complexes with Yes kinase | GD3 | ↑ Proliferation and invasion | Glioma | [56] |
| Src | GD2 | ↑ Neurite retraction | Neuroblastoma | [57] |
| P13K/Akt mTOR | GD2 | ↑ Proliferation | Neuroblastoma | [58] |
| VEGF | GD1a | ↑ Proliferation | HUVECs | [8] |
| HGF | GD1a | ↓ Motility | FBJ osteosarcoma | [59] |
| Caspase-8,7 and PARP | GD1b | ↓ Proliferation and ↑ apoptosis | Human breast cancer MCF-7 | [60] |
| uPA | GT1b | ↑ Apoptosis | Lung cancer A549 | [61] |
| ↓ interleukin 8 promoter | GQ1b | ↓ Proliferation | Human melanoma | [62] |
| Siglec | Cell Type Expressed on | Ganglioside Bound | Reference |
|---|---|---|---|
| 1 (CD169) | Macrophage | GM3, GD1a, GD1b, and GT1b fairly equally | [118] |
| 2 (CD22) | Primarily B cells | Strong preference for Neu5Nac- and Neu5Gcα2-6Gal | [119] |
| 3 (CD33) | Mitogen-activated T and natural killer (NK) cells | GM3, GD3, GQ1b, GT1b (α2-3 and α2-6 sialylated gangliosides) | [120,121] |
| 4 Myelin-associated glycoprotein | Myelinating cells | GD1a, GT1b, stabilizes axon-myelin interactions | [122] |
| 5 (CD170) | T cells | GQ1b, weakly to GT1b | [123] |
| 7 (CD328) | NK cells | GD3, GD2, GD1b, GT1b (preferentially binds α2-8 sialylated gangliosides) | [94,111,124] |
| 8 | Eosinophils and mast cells, less on basophils | Low affinity to GM2, GM3, GD3, GT1b, GQ1b | [123] |
| 9 (CD329) | Monocytes, neutrophils, lesser amounts of NK, B, and T cells | GD1a, GT1b | [124] |
| 10 (CD330) | Eosinophils, monocytes, subpopulation of NK cells | Only GT1b | [123,125] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schengrund, C.-L. The Ying and Yang of Ganglioside Function in Cancer. Cancers 2023, 15, 5362. https://doi.org/10.3390/cancers15225362
Schengrund C-L. The Ying and Yang of Ganglioside Function in Cancer. Cancers. 2023; 15(22):5362. https://doi.org/10.3390/cancers15225362
Chicago/Turabian StyleSchengrund, Cara-Lynne. 2023. "The Ying and Yang of Ganglioside Function in Cancer" Cancers 15, no. 22: 5362. https://doi.org/10.3390/cancers15225362
APA StyleSchengrund, C. -L. (2023). The Ying and Yang of Ganglioside Function in Cancer. Cancers, 15(22), 5362. https://doi.org/10.3390/cancers15225362