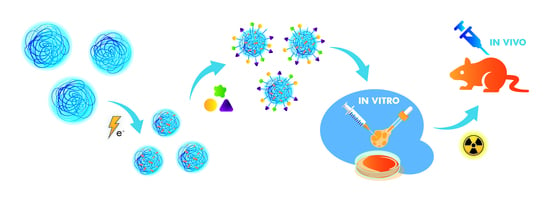
Towards Cancer Nanoradiopharmaceuticals—Radioisotope Nanocarrier System for Prostate Cancer Theranostics Based on Radiation-Synthesized Polymer Nanogels
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electron Beam Dosimetry
2.3. Synthesis and Characterization of PAA Nanogels
2.4. Synthesis and Characterization of Nanocarriers
- -
- 450NG nanogels at 170 BN and BD peptides per nanoparticle (450NGBN and 450NGBD, respectively);
- -
- 30PAA molecules at 4 BN and BD peptides per chain (30PAABN and 30PAABD, respectively).
2.4.1. Multistep Reaction with EDC/NHS
2.4.2. One-Pot Reaction with EDC/NHS
2.4.3. One-Pot Reaction with DMTMM
2.5. Characterization of Nanocarriers
2.6. Radiolabeling
2.7. Biological Studies
2.7.1. Cell Culture
2.7.2. Real-Time Quantitative Polymerase Chain Reaction (RTqPCR)
2.7.3. Western Blotting
2.7.4. Cellular Uptake
2.8. In Vivo Studies
2.9. Statistical Analysis
3. Results and Discussion
3.1. Nanocarrier Functionalization
3.2. Radiolabeling
3.3. Targeted Delivery of Radiolabeled Nanocarriers
3.3.1. Gastrin-Releasing Peptide Receptor Expression
3.3.2. Nanocarrier Uptake in GRPR-Positive Cell Line
3.3.3. In Vivo
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lankoff, A.; Czerwińska, M.; Kruszewski, M. Nanoparticle-based radioconjugates for targeted imaging and therapy of prostate cancer. Molecules 2023, 28, 4122. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lu, B.; He, M.; Wang, Y.; Wang, Z.; Du, L. Prostate cancer incidence and mortality: Global status and temporal trends in 89 countries from 2000 to 2019. Front. Public Health 2022, 10, 811044. [Google Scholar] [CrossRef] [PubMed]
- Mehtälä, J.; Zong, J.; Vassilev, Z.; Brobert, G.; Gabarró, M.S.; Stattin, P.; Khanfir, H. Overall survival and second primary malignancies in men with metastatic prostate cancer. PLoS ONE 2020, 15, e0227552. [Google Scholar] [CrossRef] [PubMed]
- Rurarz, B.P.; Bukowczyk, M.; Gibka, N.; Piastowska-Ciesielska, A.W.; Karczmarczyk, U.; Ulański, P. Nanostrategies for therapeutic and diagnostic targeting of gastrin-releasing peptide receptor. Int. J. Mol. Sci. 2023, 24, 3455. [Google Scholar] [CrossRef] [PubMed]
- Schollhammer, R.; Quintyn Ranty, M.L.; de Clermont Gallerande, H.; Cavelier, F.; Valverde, I.E.; Vimont, D.; Hindié, E.; Morgat, C. Theranostics of primary prostate cancer: Beyond PSMA and GRP-R. Cancers 2023, 15, 2345. [Google Scholar] [CrossRef] [PubMed]
- Mansi, R.; Fleischmann, A.; Mäcke, H.R.; Reubi, J.C. Targeting GRPR in urological cancers—From basic research to clinical application. Nat. Rev. Urol. 2013, 10, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Halmos, G.; Schally, A.V.; Wang, X.; Martinez, M. Presence of receptors for bombesin/gastrin-releasing peptide and mRNA for three receptor subtypes in human prostate cancers. Prostate 2000, 42, 295–303. [Google Scholar] [CrossRef]
- Baratto, L.; Jadvar, H.; Iagaru, A. Prostate cancer theranostics targeting gastrin-releasing peptide receptors. Mol. Imaging Biol. 2018, 20, 501–509. [Google Scholar] [CrossRef]
- De Visser, M.; Bernard, H.F.; Erion, J.L.; Schmidt, M.A.; Srinivasan, A.; Waser, B.; Reubi, J.C.; Krenning, E.P.; De Jong, M. Novel 111In-labelled bombesin analogues for molecular imaging of prostate tumours. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 1228–1238. [Google Scholar] [CrossRef]
- Mansi, R.; Nock, B.A.; Dalm, S.U.; Busstra, M.B.; van Weerden, W.M.; Maina, T. Radiolabeled bombesin analogs. Cancers 2021, 13, 5766. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Z.; Merkens, H.; Colpo, N.; Zeisler, J.; Zhang, C.; Roxin, A.; Tan, R.; Bendre, S.; Benard, F.; et al. Synthesis and evaluation of novel 68Ga-labeled bombesin analogs for imaging gastrin-releasing peptide receptor expression with positron emission tomography. J. Nucl. Med. 2022, 63 (Suppl. S2), 2453. [Google Scholar]
- Carlucci, G.; Kuipers, A.; Ananias, H.J.K.; De Paula Faria, D.; Dierckx, R.A.J.O.; Helfrich, W.; Rink, R.; Moll, G.N.; De Jong, I.J.; Elsinga, P.H. GRPR-selective PET imaging of prostate cancer using [18F]-lanthionine-bombesin analogs. Peptides 2015, 67, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, R.; Ruffani, A.; Graham, B.; Spiccia, L.; Steinbach, J.; Pietzsch, J.; Stephan, H. Synthesis and radiopharmacological evaluation of 64Cu-labeled bombesin analogs featuring a bis(2-pyridylmethyl)-1,4,7-triazacyclononane chelator. Eur. J. Med. Chem. 2013, 70, 434–446. [Google Scholar] [CrossRef] [PubMed]
- Däpp, S.; Müller, C.; Garayoa, E.G.; Bläuenstein, P.; Maes, V.; Brans, L.; Tourwé, D.A.; Schibli, R. PEGylation, increasing specific activity and multiple dosing as strategies to improve the risk-benefit profile of targeted radionuclide therapy with 177Lu- DOTA-bombesin analogues. EJNMMI Res. 2012, 2, 24. [Google Scholar] [CrossRef]
- Huynh, T.T.; van Dam, E.M.; Sreekumar, S.; Mpoy, C.; Blyth, B.J.; Muntz, F.; Harris, M.J.; Rogers, B.E. Copper-67-labeled bombesin peptide for targeted radionuclide therapy of prostate pancer. Pharmaceuticals 2022, 15, 728. [Google Scholar] [CrossRef] [PubMed]
- Wild, D.; Frischknecht, M.; Zhang, H.; Morgenstern, A.; Bruchertseifer, F.; Boisclair, J.; Provencher-Bolliger, A.; Reubi, J.C.; Maecke, H.R. Alpha- versus beta-particle radiopeptide therapy in a human prostate cancer model (213Bi-DOTA-PESIN and 213Bi-AMBA versus 177Lu-DOTA-PESIN). Cancer Res. 2011, 71, 1009–1018. [Google Scholar] [CrossRef]
- Morgenstern, A.; Apostolidis, C.; Kratochwil, C.; Sathekge, M.; Krolicki, L.; Bruchertseifer, F. An overview of targeted alpha therapy with 225actinium and 213bismuth. Curr. Radiopharm. 2018, 11, 200–208. [Google Scholar] [CrossRef]
- Gibbens-Bandala, B.; Morales-Avila, E.; Ferro-Flores, G.; Santos-Cuevas, C.; Meléndez-Alafort, L.; Trujillo-Nolasco, M.; Ocampo-García, B. 177Lu-bombesin-PLGA (paclitaxel): A targeted controlled-release nanomedicine for bimodal therapy of breast cancer. Mater. Sci. Eng. C 2019, 105, 110043. [Google Scholar] [CrossRef]
- Gibbens-Bandala, B.; Morales-Avila, E.; Ferro-Flores, G.; Santos-Cuevas, C.; Luna-Gutiérrez, M.; Ramírez-Nava, G.; Ocampo-García, B. Synthesis and evaluation of 177Lu-DOTA-DN(PTX)-BN for selective and concomitant radio and drug-therapeutic effect on breast cancer cells. Polymers 2019, 11, 1572. [Google Scholar] [CrossRef]
- Mendoza-Nava, H.; Ferro-Flores, G.; Ramírez, F.D.M.; Ocampo-García, B.; Santos-Cuevas, C.; Aranda-Lara, L.; Azorín-Vega, E.; Morales-Avila, E.; Isaac-Olivé, K. 177Lu-dendrimer conjugated to folate and bombesin with gold nanoparticles in the dendritic cavity: A potential theranostic radiopharmaceutical. J. Nanomater. 2016, 2016, 1039258. [Google Scholar] [CrossRef]
- Mendoza-Nava, H.; Ferro-Flores, G.; Ramírez, F.d.M.; Ocampo-García, B.; Santos-Cuevas, C.; Azorín-Vega, E.; Jiménez-Mancilla, N.; Luna-Gutiérrez, M.; Isaac-Olivé, K. Fluorescent, plasmonic, and radiotherapeutic properties of the 177 Lu–dendrimer-AuNP–folate–bombesin nanoprobe located inside cancer cells. Mol. Imaging 2017, 16, 153601211770476. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ye, M.; Ma, D.; Shen, J.; Fang, F. Engineering of 177Lu-labeled gold encapsulated into dendrimeric nanomaterials for the treatment of lung cancer. J. Biomater. Sci. Polym. Ed. 2022, 33, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.; Paulo, A.; Pallier, A.; Même, S.; Tóth, É.; Gano, L.; Marques, F.; Geraldes, C.F.G.C.; Castro, M.M.C.A.; Cardoso, A.M.; et al. Dual imaging gold nanoplatforms for targeted radiotheranostics. Materials 2020, 13, 513. [Google Scholar] [CrossRef] [PubMed]
- Accardo, A.; Mansi, R.; Salzano, G.; Morisco, A.; Aurilio, M.; Parisi, A.; Maione, F.; Cicala, C.; Ziaco, B.; Tesauro, D.; et al. Bombesin peptide antagonist for target-selective delivery of liposomal doxorubicin on cancer cells. J. Drug Target. 2013, 21, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Piroonpan, T.; Rimdusit, P.; Taechutrakul, S.; Pasanphan, W. pH-responsive water-soluble chitosan amphiphilic core–shell nanoparticles: Radiation-assisted green synthesis and drug-controlled release studies. Pharmaceutics 2023, 15, 847. [Google Scholar] [CrossRef] [PubMed]
- Fazolin, G.N.; Varca, G.H.C.; Kadlubowski, S.; Sowinski, S.; Lugão, A.B. The effects of radiation and experimental conditions over papain nanoparticle formation: Towards a new generation synthesis. Radiat. Phys. Chem. 2020, 169, 107984. [Google Scholar] [CrossRef]
- De Freitas, L.F.; Varca, G.H.C.; Batista, J.G.D.S.; Lugão, A.B. An overview of the synthesis of gold nanoparticles using radiation technologies. Nanomaterials 2018, 8, 939. [Google Scholar] [CrossRef]
- Soto Espinoza, S.L.; Sánchez, M.L.; Risso, V.; Smolko, E.E.; Grasselli, M. Radiation synthesis of seroalbumin nanoparticles. Radiat. Phys. Chem. 2012, 81, 1417–1421. [Google Scholar] [CrossRef]
- Dispenza, C.; Sabatino, M.A.; Ajovalasit, A.; Ditta, L.A.; Ragusa, M.; Purrello, M.; Costa, V.; Conigliaro, A.; Alessandro, R. Nanogel-antimiR-31 conjugates affect colon cancer cells behaviour. RSC Adv. 2017, 7, 52039–52047. [Google Scholar] [CrossRef]
- Ghaffarlou, M.; Sütekin, S.D.; Güven, O. Preparation of nanogels by radiation-induced cross-linking of interpolymer complexes of poly (acrylic acid) with poly (vinyl pyrrolidone) in aqueous medium. Radiat. Phys. Chem. 2018, 142, 130–136. [Google Scholar] [CrossRef]
- Ulański, P.; Janik, I.; Rosiak, J.M. Radiation formation of polymeric nanogels. Radiat. Phys. Chem. 1998, 52, 289–294. [Google Scholar] [CrossRef]
- Ulański, P.; Kadlubowski, S.; Rosiak, J.M. Synthesis of poly(acrylic acid) nanogels by preparative pulse radiolysis. Radiat. Phys. Chem. 2002, 63, 533–537. [Google Scholar] [CrossRef]
- Ghaffarlou, M.; Sütekin, S.D.; Karacaoğlu, E.; Karahisar Turan, S.; İnci, Ö.G.; Güven, O.; Barsbay, M. Folic acid-modified biocompatible pullulan/poly(acrylic acid) nanogels for targeted delivery to MCF-7 cancer cells. Eur. J. Pharm. Biopharm. 2023, 184, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Abo-Zaid, O.A.R.; Moawed, F.S.M.; Barakat, W.E.M.; Ghobashy, M.M.; Ahmed, E.S.A. Antitumor activity of 5-fluorouracil polymeric nanogel synthesized by gamma radiation on a rat model of colon carcinoma: A proposed mechanism. Discov. Oncol. 2023, 14, 138. [Google Scholar] [CrossRef] [PubMed]
- El-Sattar, N.E.A.A.; El-Hddad, S.E.S.A.; Ghobashy, M.M.; Zaher, A.A.; El-Adl, K. Nanogel-mediated drug delivery system for anticancer agent: pH stimuli responsive poly(ethylene glycol/acrylic acid) nanogel prepared by gamma irradiation. Bioorg. Chem. 2022, 127, 105972. [Google Scholar] [CrossRef]
- Kadlubowski, S.; Grobelny, J.; Olejniczak, W.; Cichomski, M.; Ulanski, P. Pulses of fast electrons as a tool to synthesize poly(acrylic acid) nanogels. Intramolecular cross-linking of linear polymer chains in additive-free aqueous solution. Macromolecules 2003, 36, 2484–2492. [Google Scholar] [CrossRef]
- Matusiak, M.; Kadlubowski, S.; Ulanski, P. Radiation-induced synthesis of poly(acrylic acid) nanogels. Radiat. Phys. Chem. 2018, 142, 125–129. [Google Scholar] [CrossRef]
- Matusiak, M.; Rurarz, B.P.; Kadłubowski, S.; Wolszczak, M.; Karczmarczyk, U.; Maurin, M.; Kolesińska, B.; Ulański, P. Synthesis and properties of targeted radioisotope carriers based on poly(acrylic acid) nanogels. Pharmaceutics 2021, 13, 1240. [Google Scholar] [CrossRef]
- Rurarz, B.P.; Gibka, N.; Bukowczyk, M.; Kadłubowski, S.; Ulański, P. Radiation synthesis of poly(acrylic acid) nanogels for drug delivery applications—Post-synthesis product colloidal stability. Nukleonika 2021, 66, 179–186. [Google Scholar] [CrossRef]
- Elliott, J.E.; MacDonald, M.; Nie, J.; Bowman, C.N. Structure and swelling of poly(acrylic acid) hydrogels: Effect of pH, ionic strength, and dilution on the crosslinked polymer structure. Polymer 2004, 45, 1503–1510. [Google Scholar] [CrossRef]
- Tang, S.H.; Wang, J.; Yang, C.X.; Dong, L.X.; Kong, D.; Yan, X.P. Ultrasonic assisted preparation of lanthanide-oleate complexes for the synthesis of multifunctional monodisperse upconversion nanoparticles for multimodal imaging. Nanoscale 2014, 6, 8037–8044. [Google Scholar] [CrossRef]
- Matusiak, M.; Kadłubowski, S.; Ulański, P. Recombination of poly(acrylic acid) radicals in acidic aqueous solutions: A pulse radiolysis study. Appl. Sci. 2021, 11, 10142. [Google Scholar] [CrossRef]
- Nikravan, G.; Haddadi-Asl, V.; Salami-Kalajahi, M. Stimuli-responsive DOX release behavior of cross-linked poly(acrylic acid) nanoparticles. E-Polymers 2019, 19, 203–214. [Google Scholar] [CrossRef]
- De, T.K.; Hoffman, A.S. An ophthalmic formulation of a beta-adrenoceptor antagonist, levobetaxolol, using poly(acrylic acid) nanoparticles as carrier: Loading and release studies. J. Bioact. Compat. Polym. 2001, 16, 20–31. [Google Scholar] [CrossRef]
- Wu, H.; Jin, H.; Wang, C.; Zhang, Z.; Ruan, H.; Sun, L.; Yang, C.; Li, Y.; Qin, W.; Wang, C. Synergistic cisplatin/doxorubicin combination chemotherapy for multidrug-resistant cancer via polymeric nanogels targeting delivery. ACS Appl. Mater. Interfaces 2017, 9, 9426–9436. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Guan, X.; Wu, Y.; Zhuang, S.; Wu, Y.; Du, L.; Zhao, J.; Rong, J.; Zhao, J.; Tu, M. An alginate/poly(N-isopropylacrylamide)-based composite hydrogel dressing with stepwise delivery of drug and growth factor for wound repair. Mater. Sci. Eng. C 2020, 115, 111123. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Hwang, G.; Woo, J.; Park, J.; Kim, J. Characterization of responsive hydrogel nanoparticles upon polyelectrolyte complexation. Polymers 2017, 9, 66. [Google Scholar] [CrossRef]
- Cuggino, J.C.; Molina, M.; Wedepohl, S.; Igarzabal, C.I.A.; Calderón, M.; Gugliotta, L.M. Responsive nanogels for application as smart carriers in endocytic pH-triggered drug delivery systems. Eur. Polym. J. 2016, 78, 14–24. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Q.; Huang, S.; Xiao, A.; Li, F.; Gan, L.; Yang, X. Smart pH/redox dual-responsive nanogels for on-demand intracellular anticancer drug release. ACS Appl. Mater. Interfaces 2016, 8, 7729–7738. [Google Scholar] [CrossRef]
- Park, J.S.; Yang, H.N.; Woo, D.G.; Jeon, S.Y.; Park, K.H. Poly(N-isopropylacrylamide-co-acrylic acid) nanogels for tracing and delivering genes to human mesenchymal stem cells. Biomaterials 2013, 34, 8819–8834. [Google Scholar] [CrossRef]
- Nurkeeva, Z.S.; Khutoryanskiy, V.V.; Mun, G.A.; Bitekenova, A.B.; Kadlubowski, S.; Shilina, Y.A.; Ulanski, P.; Rosiak, J.M. Interpolymer complexes of poly(acrylic acid) nanogels with some non-ionic polymers in aqueous solutions. Colloids Surf. A Physicochem. Eng. Asp. 2004, 236, 141–146. [Google Scholar] [CrossRef]
- Rattanawongwiboon, T.; Ghaffarlou, M.; Sütekin, S.D.; Pasanphan, W.; Güven, O. Preparation of multifunctional poly(acrylic acid)-poly(ethylene oxide) nanogels from their interpolymer complexes by radiation-induced intramolecular crosslinking. Colloid Polym. Sci. 2018, 296, 1599–1608. [Google Scholar] [CrossRef]
- Ma, T.; Li, X.; Zhao, D.; Qiu, G.; Shi, X.; Lu, X. A novel method to in situ synthesis of magnetic poly(N-isopropylacrylamide-co-acrylic acid) nanogels. Colloid Polym. Sci. 2016, 294, 1251–1257. [Google Scholar] [CrossRef]
- Li, F.; Xing, Q.; Han, Y.; Li, Y.; Wang, W.; Perera, T.S.H.; Dai, H. Ultrasonically assisted preparation of poly(acrylic acid)/calcium phosphate hybrid nanogels as pH-responsive drug carriers. Mater. Sci. Eng. C 2017, 80, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Nan, J.; Lu, Y.; Wang, C.; Chu, F.; Gu, Z. Hybrid Fe3O4-poly(acrylic acid) nanogels for theranostic cancer treatment. J. Biomed. Nanotechnol. 2015, 11, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Dispenza, C.; Adamo, G.; Sabatino, M.A.; Grimaldi, N.; Bulone, D.; Bondì, M.L.; Rigogliuso, S.; Ghersi, G. Oligonucleotides-decorated-poly(N-vinyl pyrrolidone) nanogels for gene delivery. J. Appl. Polym. Sci. 2014, 131, 39774. [Google Scholar] [CrossRef]
- Hermanson, G.T. Chapter 4—Zero-length crosslinkers. In Bioconjugate Techniques; Academic Press: Cambridge, MA, USA, 2013; pp. 259–273. [Google Scholar] [CrossRef]
- Kunishima, M.; Kawachi, C.; Morita, J.; Terao, K.; Iwasaki, F.; Tani, S. 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride: An efficient condensing agent leading to the formation of amides and esters. Tetrahedron 1999, 55, 13159–13170. [Google Scholar] [CrossRef]
- Kolesinska, B.; Rozniakowski, K.K.; Fraczyk, J.; Relich, I.; Papini, A.M.; Kaminski, Z.J. The effect of counterion and tertiary amine on the efficiency of N-triazinylammonium sulfonates in solution and solid-phase peptide synthesis. Eur. J. Org. Chem. 2015, 2015, 401–408. [Google Scholar] [CrossRef]
- Kamiński, Z.J.; Kolesińska, B.; Kolesińska, J.; Sabatino, G.; Chelli, M.; Rovero, P.; Błaszczyk, M.; Główka, M.L.; Papini, A.M. N-triazinylammonium tetrafluoroborates. A new generation of efficient coupling reagents useful for peptide synthesis. J. Am. Chem. Soc. 2005, 127, 16912–16920. [Google Scholar] [CrossRef]
- Katagiri, W.; Lee, J.H.; Tétrault, M.A.; Kang, H.; Jeong, S.; Evans, C.L.; Yokomizo, S.; Santos, S.; Jones, C.; Hu, S.; et al. Real-time imaging of vaccine biodistribution using zwitterionic NIR nanoparticles. Adv. Healthc. Mater. 2019, 8, 1900035. [Google Scholar] [CrossRef]
- Gao, Q.Q.; Zhang, C.M.; Zhang, E.X.; Chen, H.Y.; Zhen, Y.H.; Zhang, S.B.; Zhang, S.F. Zwitterionic pH-responsive hyaluronic acid polymer micelles for delivery of doxorubicin. Colloids Surf. B 2019, 178, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Benito, A.B.; Aiertza, M.K.; Marradi, M.; Gil-Iceta, L.; Shekhter Zahavi, T.; Szczupak, B.; Jiménez-González, M.; Reese, T.; Scanziani, E.; Passoni, L.; et al. Functional single-chain polymer nanoparticles: Targeting and imaging pancreatic tumors in vivo. Biomacromolecules 2016, 17, 3213–3221. [Google Scholar] [CrossRef] [PubMed]
- Chai, H.J.; Kiew, L.V.; Chin, Y.; Norazit, A.; Noor, S.M.; Lo, Y.L.; Looi, C.Y.; Lau, Y.S.; Lim, T.M.; Wong, W.F.; et al. Renal targeting potential of a polymeric drug carrier, poly-L-glutamic acid, in normal and diabetic rats. Int. J. Nanomed. 2017, 12, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ouyang, J.; Chen, Q.; Deng, C.; Meng, F.; Zhang, J.; Cheng, R.; Lan, Q.; Zhong, Z. EGFR and CD44 dual-targeted multifunctional hyaluronic acid nanogels boost protein delivery to ovarian and breast cancers in vitro and in vivo. ACS Appl. Mater. Interfaces 2017, 9, 24140–24147. [Google Scholar] [CrossRef] [PubMed]
- Matusiak, M.; Kadlubowski, S.; Rosiak, J.M. Nanogels synthesized by radiation-induced intramolecular crosslinking of water-soluble polymers. Radiat. Phys. Chem. 2018, 169, 108099. [Google Scholar] [CrossRef]
- Kratochvil, P. Classical Light Scattering from Polymer Solutions; Elsevier: Amsterdam, The Netherlands, 1987; ISBN 9780444418326. [Google Scholar]
- Ulanski, P.; Bothe, E.; Hildenbrand, K.; Rosiak, J.M.; von Sonntag, C. Hydroxyl radical- induced reactions of poly(acrylic acid); a pulse radiolysis, EPR and product study. Part I. Deoxygenated aqueous solutions. J. Chem. Soc. Perkin. Trans. 1996, 2, 13–22. [Google Scholar] [CrossRef]
- Nagórniewicz, B.; Mardhian, D.F.; Booijink, R.; Storm, G.; Prakash, J.; Bansal, R. Engineered relaxin as theranostic nanomedicine to diagnose and ameliorate liver cirrhosis. Nanomed. Nanotechnol. Biol. Med. 2019, 17, 106–118. [Google Scholar] [CrossRef]
- Thompson, K.; Michielsen, S. Novel synthesis of N-substituted polyacrylamides: Derivatization of poly(acrylic acid) with amines using a triazine-based condensing reagent. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 126–136. [Google Scholar] [CrossRef]
- Lewinski, N.; Colvin, V.; Drezek, R. Cytotoxicity of nanoparticles. Small 2008, 4, 26–49. [Google Scholar] [CrossRef]
- Kowalska, K.; Habrowska-Górczyńska, D.E.; Domińska, K.; Piastowska-Ciesielska, A.W. The dose-dependent effect of zearalenone on mitochondrial metabolism, plasma membrane permeabilization and cell cycle in human prostate cancer cell lines. Chemosphere 2017, 180, 455–466. [Google Scholar] [CrossRef]
- Stefanello, T.F.; Couturaud, B.; Szarpak-Jankowska, A.; Fournier, D.; Louage, B.; Garcia, F.P.; Nakamura, C.V.; De Geest, B.G.; Woisel, P.; Van Der Sanden, B.; et al. Coumarin-containing thermoresponsive hyaluronic acid-based nanogels as delivery systems for anticancer chemotherapy. Nanoscale 2017, 9, 12150–12162. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Watbled, B.; Baussanne, I.; Hediger, S.; Demeunynck, M.; De Paëpe, G. Optimizing chemistry at the surface of prodrug-loaded cellulose nanofibrils with MAS-DNP. Commun. Chem. 2023, 6, 58. [Google Scholar] [CrossRef] [PubMed]
- Reile, H.; Armatis, P.E.; Schally, A.V. Characterization of high-affinity receptors for bombesin/gastrin releasing peptide on the human prostate cancer cell lines PC-3 and DU- 145: Internalization of receptor bound 125I-(Tyr4) bombesin by tumor cells. Prostate 1994, 25, 29–38. [Google Scholar] [CrossRef]
- Nakajima, T.; Ninomiya, Y.; Nenoi, M. Radiation-induced reactions in the liver—Modulation of radiation effects by lifestyle-related factors. Int. J. Mol. Sci. 2018, 19, 3855. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jung, Y. Radiation-induced liver disease: Current understanding and future perspectives. Exp. Mol. Med. 2017, 49, e359. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-N.; Poon, W.; Tavares, A.J.; McGilvray, I.D.; Chan, W.C.W. Nanoparticle–liver interactions: Cellular uptake and hepatobiliary elimination. J. Control. Release 2016, 240, 332–348. [Google Scholar] [CrossRef]
- Cheng, Y.H.; He, C.; Riviere, J.E.; Monteiro-Riviere, N.A.; Lin, Z. Meta-analysis of nanoparticle delivery to tumors using a physiologically based pharmacokinetic modeling and simulation approach. ACS Nano 2020, 14, 3075–3095. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Li, Y.; Wan, J.; Wang, F.; Guo, J.; Wang, C. Effect of increasing liver blood flow on nanodrug clearance by the liver for enhanced antitumor therapy. Biomater. Sci. 2019, 7, 1507–1515. [Google Scholar] [CrossRef]
- Cornelio, D.B.; De Farias, C.B.; Prusch, D.S.; Heinen, T.E.; Dos Santos, R.P.; Abujamra, A.L.; Schwartsmann, G.; Roesler, R. Influence of GRPR and BDNF/TrkB signaling on the viability of breast and gynecologic cancer cells. Mol. Clin. Oncol. 2013, 1, 148–152. [Google Scholar] [CrossRef]
- Du Sert, N.P.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; Emerson, M.; et al. Reporting animal research: Explanation and elaboration for the arrive guidelines 2.0. PLoS Biol. 2020, 18, e3000411. [Google Scholar] [CrossRef]










| 250NG | 450NG | |
|---|---|---|
| Concentration [mmol of monomer units/L] | 10.0 | 17.5 |
| Total absorbed dose [kGy] | 6.18 | 7.97 |
| Reaction Scheme | pH at Activation | Buffer Used | pH at Conjugation | Buffer Used | Temperature |
|---|---|---|---|---|---|
| M1 | not controlled | -(pure water) | 7.4 | PBS | 4 °C |
| M2 | 8.2 | 0.1 M TES buffer | |||
| M3 | pH 5.5 | 0.1 M MES buffer | 7.4 | PBS | |
| M4 | 8.2 | 0.1 M TES buffer | |||
| M5 | pH 6.3 | 0.1 M MES buffer | 7.4 | PBS | |
| M6 | 8.2 | 0.1 M TES buffer |
| Reaction Scheme | pH at Activation | Buffer Used | Temperature |
|---|---|---|---|
| EN1 | pH 5.5 | 0.1 M MES buffer | 4 °C |
| EN2 | pH 6.3 | ||
| EN3 | pH 7.4 | PBS |
| Reaction Scheme | pH at Activation | Buffer Used | Temperature |
|---|---|---|---|
| D1 | pH 5.5 | 0.1 M MES buffer | 4 °C |
| D2 | pH 6.3 | ||
| D3 | pH 7.4 | PBS |
| Gene | Sequence (5′-3′) | Product Size (bp) |
|---|---|---|
| GRPR | For TGATCCAGAGTGCTTACAATC Rev CGAACAGGCCCACAAACAC | 111 |
| RPLP0 | For ACGGATTACACCTTCCCACTTGCTAAAAGGTC Rev AGCCACAAAGGCAGATGGATCAGCCAAG | 69 |
| RPS17 | For AAGCGCGTGTGCGAGGAGATCG Rev TCGCTTCATCAGAT GCGTGACATAACCTG | 87 |
| H3F3A | For AGGACTTTAAAAGATCTGCGCTTCCAGAG Rev ACCAGATAGGCCTCACTTGCCTCCTGC | 74 |
| Protein | Primary Antibody | Secondary Antibody |
|---|---|---|
| GAPDH | Mouse monoclonal anti-GAPDH antibody (overnight at 4 °C, 1:10,000 dilution; sc-59540, Santa Cruz Biotechnology Inc., Dallas, TX, USA) | Alkaline phosphatase-conjugated goat anti-mouse polyclonal antibody (4 h at 4 °C, 1:15,000 dilution; A3688, Sigma Aldrich, St. Louis, MO, USA) |
| GRPR | Rabbit polyclonal anti-GRPR antibody (overnight at 4 °C, 1:1000 dilution; PA5-33833, Thermo Fisher Scientific Inc., Waltham, MA, USA) | Alkaline phosphatase-conjugated goat anti-rabbit polyclonal antibody (4 h at 4 °C, 1:15,000 dilution; A3687, Sigma Aldrich, St. Louis, MO, USA) |
| 250NG | 450NG | |
|---|---|---|
| Weight-average molecular weight [kDa] | 239 ± 3 | 1449 ± 21 |
| Hydrodynamic diameter [nm] | 67 ± 5 | 124 ± 16 |
| RCP [%] Labeling with 177Lu | ||||||
|---|---|---|---|---|---|---|
| 1 h | 1 Day | 4 Days | 7 Days | 14 Days | ||
| 30PAABD | AAB | 100.0 ± 0.00 (96.1 ± 1.72) | 99.1 ± 0.39 | 98.9 ± 1.27 | 97.3 ± 1.55 | 96.0 ± 0.70 |
| HS | 99.0 ± 1.87 | 95.4 ± 1.47 | 87.1 ± 4.66 | 86.9 ± 6.45 | n.d. | |
| 250NGBD | AAB | 99.5 ± 0.10 | 98.8 ± 1.00 | 97.4 ± 0.36 | 96.0 ± 2.93 | 96.0 ± 0.06 |
| HS | 98.9 ± 0.43 | 98.6 ± 0.00 | n.d. | 96.9 ± 0.20 | 87.8 ± 1.81 | |
| 450NGBD | AAB | 100.0 ± 0.00 | 100.0 ± 0.00 | 98.5 ± 1.12 | 97.7 ± 0.15 | 99.3 ± 0.15 |
| HS | n.d. | 98.9 ± 0.78 | n.d. | 94.7 ± 1.22 | 93.0 ± 2.03 | |
| RCP [%] Labeling with 90Y | ||||||
|---|---|---|---|---|---|---|
| 1 h | 1 Day | 4 Days | 7 Days | 14 Days | ||
| 30PAABD | AAB | 99.8 ± 0.24 (94.5 ± 1.36) | 97.6 ± 2.37 | 98.4 ± 0.88 | 95.4 ± 0.63 | 94.6 ± 0.20 |
| HS | 95.9 ± 1.17 | 94.0 ± 0.25 | 91.3 ± 0.68 | 93.1 ± 1.21 | n.d. | |
| 250NGBD | AAB | 98.2 ± 1.32 | 98.8 ± 0.60 | 98.4 ± 0.36 | 98.2 ± 1.71 | 97.9 ± 0.96 |
| HS | 93.2 ± 0.14 | 97.4 ± 0.00 | n.d. | 95.8 ± 2.67 | 96.0 ± 0.78 | |
| 450NGBD | AAB | 100 ± 0.00 | 99.4 ± 0.00 | 99.2 ± 0.06 | 98.3 ± 0.17 | 99.0 ± 0.26 |
| HS | 99.8 ± 0.00 | 99.7 ± 0.38 | 97.8 ± 1.20 | 92.9 ± 0.25 | 96.3 ± 1.37 | |
| 450NGBD | 250NGBD | 30PAABD | |
|---|---|---|---|
| p-value | 0.8028 | 0.0222 | 0.0167 |
| rs | −0.1429 | −0.9276 | −1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rurarz, B.P.; Urbanek, K.A.; Karczmarczyk, U.; Raczkowska, J.; Habrowska-Górczyńska, D.E.; Kozieł, M.J.; Kowalska, K.; Kadłubowski, S.; Sawicka, A.; Maurin, M.; et al. Towards Cancer Nanoradiopharmaceuticals—Radioisotope Nanocarrier System for Prostate Cancer Theranostics Based on Radiation-Synthesized Polymer Nanogels. Cancers 2023, 15, 5646. https://doi.org/10.3390/cancers15235646
Rurarz BP, Urbanek KA, Karczmarczyk U, Raczkowska J, Habrowska-Górczyńska DE, Kozieł MJ, Kowalska K, Kadłubowski S, Sawicka A, Maurin M, et al. Towards Cancer Nanoradiopharmaceuticals—Radioisotope Nanocarrier System for Prostate Cancer Theranostics Based on Radiation-Synthesized Polymer Nanogels. Cancers. 2023; 15(23):5646. https://doi.org/10.3390/cancers15235646
Chicago/Turabian StyleRurarz, Beata Paulina, Kinga Anna Urbanek, Urszula Karczmarczyk, Joanna Raczkowska, Dominika Ewa Habrowska-Górczyńska, Marta Justyna Kozieł, Karolina Kowalska, Sławomir Kadłubowski, Agnieszka Sawicka, Michał Maurin, and et al. 2023. "Towards Cancer Nanoradiopharmaceuticals—Radioisotope Nanocarrier System for Prostate Cancer Theranostics Based on Radiation-Synthesized Polymer Nanogels" Cancers 15, no. 23: 5646. https://doi.org/10.3390/cancers15235646
APA StyleRurarz, B. P., Urbanek, K. A., Karczmarczyk, U., Raczkowska, J., Habrowska-Górczyńska, D. E., Kozieł, M. J., Kowalska, K., Kadłubowski, S., Sawicka, A., Maurin, M., Piastowska-Ciesielska, A. W., & Ulański, P. (2023). Towards Cancer Nanoradiopharmaceuticals—Radioisotope Nanocarrier System for Prostate Cancer Theranostics Based on Radiation-Synthesized Polymer Nanogels. Cancers, 15(23), 5646. https://doi.org/10.3390/cancers15235646