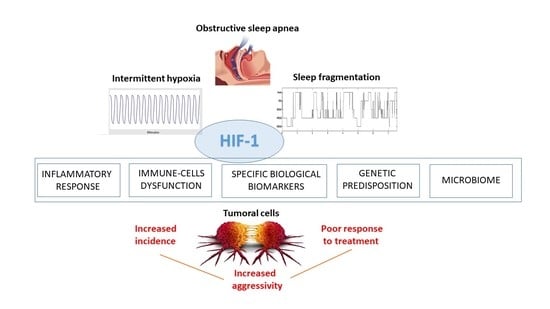
Potential Pathophysiological Pathways in the Complex Relationships between OSA and Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Quantitative or Qualitative Cell Dysfunction
3. Role of Specific Biological Biomarkers
| Biomarker | Main Functions | Biomarker in OSA and Cancer |
|---|---|---|
| HIF-1α [87] |
|
|
| VEGF [89] |
|
|
| VCAM-1 [94] |
|
|
| PD-1/PD-L1 [33,84] |
|
|
| TGF-β [95] |
|
|
| PSPC1 [11] |
|
|
| TNF-α [101] |
|
|
| COX-2/PGE2 [71] |
|
|
| Cannabinoid receptors [103] |
|
|
| Endostatin [104] |
|
|
| Endothelin-1 [28] |
|
|
4. Genetic Factors
5. Exosomes
6. Microbiota
7. Conclusions and Future Challenges
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Romero-Peralta, S.; García-Rio, F.; Resano Barrio, P.; Viejo-Ayuso, E.; Izquierdo, J.L.; Sabroso, R.; Castelao, J.; Fernández Francés, J.; Mediano, O. Defining the Heterogeneity of Sleep Apnea Syndrome: A Cluster Analysis with Implications for Patient Management. Arch. Bronconeumol. 2022, 58, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Pien, G.W.; Ratcliffe, S.J.; Björnsdottir, E.; Armardottir, E.S.; Pack, A.I.; Benediktsdottir, B.; Gislason, T. The different clinical faces of obstructive sleep apnoea: A cluster analysis. Eur. Res. J. 2014, 44, 1600–1607. [Google Scholar] [CrossRef] [PubMed]
- Mediano, O.; González Mangado, N.; Montserrat, J.M.; Alonso-Álvarez, M.L.; Almendros, I.; Alonso-Fernández, A.; Barbé, F.; Borsini, E.; Caballero-Eraso, C.; Cano-Pumarega, I.; et al. International Consensus Document on Obstructive Sleep Apnea. Arch. Bronconeumol. 2022, 58, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Marin, J.M.; Carrizo, S.J. Guidelines, Recommendations and Consensus on Obstructive Sleep Apnea. Arch. Bronconeumol. 2022, 58, 3–4. [Google Scholar] [CrossRef]
- Javaheri, S.; Barbe, F.; Campos-Rodriguez, F.; Dempsey, J.A.; Khayat, R.; Javaheri, S. Sleep Apnea: Types, Mechanisms, and Clinical Cardiovascular Consequences. J. Am. Coll. Cardiol. 2017, 69, 841–858. [Google Scholar] [CrossRef]
- Grassi, G.; Quarti-Trevano, F.; Mancia, G. Obstructive Sleep Apnea, CPAP and Arterial Hypertension: A Cardiologist’s View Point. Arch. Bronconeumol. 2022, 58, 461–462. [Google Scholar] [CrossRef]
- Velásquez-Rodríguez, J.; Ortiz-Maraima, T.; Rodríguez-Viñoles, M.P.; Bucce, R.; Jorquera, A.; Rodríguez, B. Serum Leptin and Ultrasound Markers of Early Atherosclerosis in Patients with Sleep Apnea Hypopnea Syndrome. Arch. Bronconeumol. 2021, 57, 230–231. [Google Scholar] [CrossRef]
- Sanchez-de-la-Torre, M.; Campos-Rodriguez, F.; Barbe, F. Obstructive sleep apnea and cardiovascular disease. Lancet Respir. Med. 2013, 1, 61–72. [Google Scholar] [CrossRef]
- Grau, N.; Martí-Almor, J.; Félez, M.A. Relationship between SAHS and cardiac arrhythmias. Arch. Bronconeumol. 2021, 57, 513–514. [Google Scholar] [CrossRef]
- Lloberes, P.; Silveira, M.G.; Sampol, J.; Esquinas, C.; Espinel, E.; Ferrer, R.; Gonzalo, M.; Sampol, G. Is There an Association Between Nocturia and Nighttime Hypertension in Patients with Moderate to Severe Sleep Apnea? Arch. Bronconeumol. 2022, 58, 369–371. [Google Scholar] [CrossRef]
- Pengo, M.F.; Steier, J.; Parati, G.; ANDANTE Collaborators; Researchers Collaborating in the ANDANTE PROJECT. The ANDANTE Project: A Worldwide Individual Data Meta-Analysis of the Effect of Sleep Apnea Treatment on Blood Pressure. Arch. Bronconeumol. 2021, 57, 673–676. [Google Scholar] [CrossRef] [PubMed]
- Parish, J.M.; Somers, V.K. Obstructive sleep apnea and cardiovascular disease. Mayo Clin. Proc. 2004, 79, 1036–1046. [Google Scholar] [CrossRef] [PubMed]
- Sapiña-Beltrán, E.; Gracia-Lavedan, E.; Torres, G.; Gaeta, A.M.; Paredes, J.; Mayoral, A.; Fernández, E.; Bermúdez-López, M.; Valdivielso, J.M.; Farràs-Salles, C.; et al. Prevalence of Obstructive Sleep Apnoea and Its Association with Atherosclerotic Plaques in a Cohort of Subjects With Mild-Moderate Cardiovascular Risk. Arch. Bronconeumol. 2022, 58, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Bello, I.; Monzón Manzano, E.; García Río, F.; Justo Sanz, R.; Cubillos-Zapata, C.; Casitas, R.; Sánchez, B.; Jaureguizar, A.; Acuña, P.; Alonso-Fernández, A.; et al. Procoagulant State of Sleep Apnea Depends on Systemic Inflammation and Endothelial Damage. Arch. Bronconeumol. 2022, 58, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Marin, J.M.; Carrizo, S.J.; Vicente, E.; Agusti, A.G. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: An observational study. Lancet 2005, 365, 1046–1053. [Google Scholar] [CrossRef]
- Navarro-Soriano, C.; Martínez-García, M.A.; Torres, G.; Barbé, F.; Sánchez-de-la-Torre, M.; Caballero-Eraso, C.; Lloberes, P.; Cambriles, T.D.; Somoza, M.; Masa, J.F.; et al. Long-term Effect of CPAP Treatment on Cardiovascular Events in Patients with Resistant Hypertension and Sleep Apnea. Data From the HIPARCO-2 Study. Arch. Bronconeumol. Engl. Ed. 2021, 57, 165–171. [Google Scholar] [CrossRef]
- Gaines, J.; Vgontzas, A.N.; Fernandez, J.; Bixler, O. Obstructive sleep apnea and the metabolic syndrome: The road to clinically-meaningful phenotyping, improved prognosis, and personalized treatment. Sleep Med. Rev. 2018, 42, 211–219. [Google Scholar] [CrossRef]
- Alonso-Fernández, A.; Cerdá Moncadas, M.; Álvarez Ruiz De Larrinaga, A.; Sánchez Barón, A.; Codina Marcet, M.; Rodríguez Rodríguez, P.; Gil Gómez, A.V.; Giménez Carrero, M.P.; Pía Martínez, C.; Cubero Marín, J.P.; et al. Impact of Obstructive Sleep Apnea on Gestational Diabetes Mellitus. Arch. Bronconeumol. 2022, 58, 219–227. [Google Scholar] [CrossRef]
- Zhou, J.; Camacho, M.; Tang, X.; Kushida, A. A review of neurocognitive function and obstructive sleep apnea with or without daytime sleepiness. Sleep Med. 2016, 23, 99–108. [Google Scholar] [CrossRef]
- Osorio, R.S.; Martínez-García, M.Á.; Rapoport, D.M. Sleep apnoea in the elderly: A great challenge for the future. Eur. Respir. J. 2021, 24, 2101649. [Google Scholar] [CrossRef]
- Gonzalez Bosc, L.V.; Resta, T.; Walker, B.; Kanagy, N.L. Mechanisms of intermittent hypoxia induced hypertension. J. Cell Mol. Med. 2010, 14, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Gómez Olivas, J.D.; Campos-Rodriguez, F.; Nagore, E.; Hernández, L.; Cabriada, V.; Abad, J.; Mediano, O.; Pastor, E.; Chiner, E.; De la Torre, M.S.; et al. Sleep Duration and Cutaneous Melanoma Aggressiveness. A Prospective Observational Study in 443 Patients. Arch. Bronconeumol. 2021, 57, 776–778. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Babapoor-Farrokhran, S.; Rodrigues, M.; Deshpande, M.; Puchner, B.; Kashiwabuchi, F. Hypoxia-inducible factor 1 upregulation of both VEGF and ANGPTL4 is required to promote the angiogenic phenotype in uveal melanoma. Oncotarget 2016, 7, 7816–7828. [Google Scholar] [CrossRef] [PubMed]
- Eckert, D.J.; Sands, S.A. Hypoxia and Sleep-disordered Breathing: Friend or Foe? Am. J. Respir. Crit. Care Med. 2022, 205, 869–872. [Google Scholar] [CrossRef]
- Marhuenda, E.; Campillo, N.; Gabasa, M.; Martínez-García, M.A.; Campos-Rodríguez, F.; Gozal, D.; Navajas, D.; Alcaraz, J.; Farré, R.; Almendros, I. Effects of Sustained and Intermittent Hypoxia on Human Lung Cancer Cells. Am. J. Respir. Cell Mol. Biol. 2019, 61, 540–544. [Google Scholar] [CrossRef]
- Almendros, I.; Gileles-Hillel, A.; Khalyfa, A.; Wang, Y.; Zhang, S.X.; Carreras, A.; Farre, R.; Gozal, D. Adipose tissue macrophage polarization by intermittent hypoxia in a mouse model of OSA: Effect of tumor microenvironment. Cancer Lett. 2015, 361, 233–239. [Google Scholar] [CrossRef]
- Ji, Y.; Liang, Y.; Chu, P.H.; Ge, M.; Yeung, S.C.; Man Ip, M.S.; Wo Mak, J.C. The effects of intermittent hypoxia on hepatic expression of fatty acid translocase CD36 in lean and diet-induced obese mice. Biomed. J. 2022; in press. [Google Scholar] [CrossRef]
- Minoves, M.; Kotzki, S.; Hazane-Puch, F.; Lemarie, E.; Bouyon, S.; Vollaire, J.; Gonthier, B.; Pepin, J.L.; Josserand, V.; Briancon-Marjollet, A.; et al. Chronic intermittent hypoxia, a hallmark of obstructive sleep apnea, promotes 4T1 breast cancer development through endothelin-1 receptors. Sci. Rep. 2022, 12, 12916. [Google Scholar] [CrossRef]
- Prabhakar, N.R.; Peng, Y.J.; Nanduri, J. Hypoxia-inducible factors and obstructive sleep apnea. J. Clin. Investig. 2020, 130, 5042–5051. [Google Scholar] [CrossRef]
- Ryan, S.; Taylor, C.T.; McNicholas, W.T. Predictors of elevated nuclear factor-kappaB-dependent genes in obstructive sleep apnea syndrome. Am. J. Respir. Crit. Care Med. 2006, 174, 824–830. [Google Scholar] [CrossRef]
- Ryan, S. Mechanisms of cardiovascular disease in obstructive sleep apnoea. J. Thorac. Dis. 2018, 10, S4201–S4211. [Google Scholar] [CrossRef] [PubMed]
- Cubillos-Zapata, C.; Almendros, I.; Diaz-Garcia, E.; Toledano, V.; Casitas, R.; Galera, R.; Lopez-Collazo, E.; Farre, R.; Gozal, D.; Garcia-Rio, F. Differential effect of intermittent hypoxia and sleep fragmentation on PD-1/PD-L1 upregulation. Sleep 2020, 43, zsz285. [Google Scholar] [CrossRef] [PubMed]
- Cubillos-Zapata, C.; Avendano-Ortiz, J.; Hernandez-Jimenez, E.; Toledano, V.; Casas-Martin, J.; Varela-Serrano, A.; Torres, M.; Almendros, I.; Casitas, R.; Fernandez-Navarro, I.; et al. Hypoxia-induced PD-L1/PD-1 crosstalk impairs T-cell function in sleep apnoea. Eur. Respir. J. 2017, 50, 1700833. [Google Scholar] [CrossRef] [PubMed]
- Cubillos-Zapata, C.; Balbas-Garcia, C.; Avendano-Ortiz, J.; Toledano, V.; Torres, M.; Almendros, I.; Casitas, R.; Zamarron, E.; Garcia-Sanchez, A.; Feliu, J.; et al. Age-dependent hypoxia-induced PD-L1 upregulation in patients with obstructive sleep apnoea. Respirology 2019, 24, 684–692. [Google Scholar] [CrossRef]
- Diaz-Garcia, E.; Garcia-Tovar, S.; Alfaro, E.; Jaureguizar, A.; Casitas, R.; Sanchez-Sanchez, B.; Zamarron, E.; Fernandez-Lahera, J.; Lopez-Collazo, E.; Cubillos-Zapata, C.; et al. Inflammasome Activation: A Keystone of Proinflammatory Response in Obstructive Sleep Apnea. Am. J. Respir. Crit. Care Med. 2022, 205, 1337–1348. [Google Scholar] [CrossRef]
- Diaz-Garcia, E.; Garcia-Tovar, S.; Casitas, R.; Jaureguizar, A.; Zamarron, E.; Sanchez-Sanchez, B.; Sastre-Perona, A.; Lopez-Collazo, E.; Garcia-Rio, F.; Cubillos-Zapata, C. Intermittent Hypoxia Mediates Paraspeckle Protein-1 Upregulation in Sleep Apnea. Cancers 2021, 13, 3888. [Google Scholar] [CrossRef]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef]
- Yuen, V.W.; Wong, C.C. Hypoxia-inducible factors and innate immunity in liver cancer. J. Clin. Investig. 2020, 130, 5052–5062. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Carmeliet, P. Hypoxia and inflammation. N. Engl. J. Med. 2011, 364, 656–665. [Google Scholar] [CrossRef]
- Cummins, E.P.; Berra, E.; Comerford, K.M.; Ginouves, A.; Fitzgerald, K.T.; Seeballuck, F.; Godson, C.; Nielsen, J.E.; Moynagh, P.; Pouyssegur, J.; et al. Prolyl hydroxylase-1 negatively regulates IkappaB kinase-beta, giving insight into hypoxia-induced NFkappaB activity. Proc. Natl. Acad. Sci. USA 2006, 103, 18154–18159. [Google Scholar] [CrossRef]
- D’Ignazio, L.; Bandarra, D.; Rocha, S. NF-kappaB and HIF crosstalk in immune responses. FEBS J. 2016, 283, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Palazon, A.; Goldrath, A.W.; Nizet, V.; Johnson, R.S. HIF transcription factors, inflammation, and immunity. Immunity 2014, 41, 518–528. [Google Scholar] [CrossRef]
- Fitzpatrick, S.F.; King, A.D.; O’Donnell, C.; Roche, H.M.; Ryan, S. Mechanisms of intermittent hypoxia-mediated macrophage activation—Potential therapeutic targets for obstructive sleep apnoea. J. Sleep Res. 2021, 30, e13202. [Google Scholar] [CrossRef] [PubMed]
- Akinnusi, M.; Jaoude, P.; Kufel, T.; El-Solh, A.A. Toll-like receptor activity in patients with obstructive sleep apnea. Sleep Breath. 2013, 17, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Imamura, T.; Poulsen, O.; Haddad, G.G. Intermittent hypoxia induces murine macrophage foam cell formation by IKK-beta-dependent NF-kappaB pathway activation. J. Appl. Physiol. 2016, 121, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.; Arnaud, C.; Fitzpatrick, S.F.; Gaucher, J.; Tamisier, R.; Pepin, J.L. Adipose tissue as a key player in obstructive sleep apnoea. Eur. Respir. Rev. 2019, 28, 190006. [Google Scholar] [CrossRef]
- Gaines, J.; Vgontzas, A.N.; Fernandez-Mendoza, J.; Calhoun, S.L.; He, F.; Liao, D.; Sawyer, M.D.; Bixler, E.O. Inflammation mediates the association between visceral adiposity and obstructive sleep apnea in adolescents. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E851–E858. [Google Scholar] [CrossRef]
- Ryan, S.; Taylor, C.T.; McNicholas, W.T. Systemic inflammation: A key factor in the pathogenesis of cardiovascular complications in obstructive sleep apnoea syndrome? Postgrad. Med. J. 2009, 85, 693–698. [Google Scholar] [CrossRef]
- Lee, M.Y.; Wang, Y.; Mak, J.C.; Ip, M.S. Intermittent hypoxia induces NF-kappaB-dependent endothelial activation via adipocyte-derived mediators. Am. J. Physiol. Cell Physiol. 2016, 310, C446–C455. [Google Scholar] [CrossRef]
- Ryan, S.; Taylor, C.T.; McNicholas, W.T. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation 2005, 112, 2660–2667. [Google Scholar] [CrossRef]
- Song, J.Q.; Jiang, L.Y.; Fu, C.P.; Wu, X.; Liu, Z.L.; Xie, L.; Wu, X.D.; Hao, S.Y.; Li, S.Q. Heterozygous SOD2 deletion deteriorated chronic intermittent hypoxia-induced lung inflammation and vascular remodeling through mtROS-NLRP3 signaling pathway. Acta Pharmacol. Sin. 2020, 41, 1197–1207. [Google Scholar] [CrossRef] [PubMed]
- Sapin, E.; Peyron, C.; Roche, F.; Gay, N.; Carcenac, C.; Savasta, M.; Levy, P.; Dematteis, M. Chronic Intermittent Hypoxia Induces Chronic Low-Grade Neuroinflammation in the Dorsal Hippocampus of Mice. Sleep 2015, 38, 1537–1546. [Google Scholar] [CrossRef] [PubMed]
- Orecchioni, M.; Ghosheh, Y.; Pramod, A.B.; Ley, K. Macrophage Polarization: Different Gene Signatures in M1(LPS+) vs. Classically and M2(LPS-) vs. Alternatively Activated Macrophages. Front. Immunol. 2019, 10, 1084. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J.; Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Gileles-Hillel, A.; Almendros, I.; Khalyfa, A.; Nigdelioglu, R.; Qiao, Z.; Hamanaka, R.B.; Mutlu, G.M.; Akbarpour, M.; Gozal, D. Prolonged Exposures to Intermittent Hypoxia Promote Visceral White Adipose Tissue Inflammation in a Murine Model of Severe Sleep Apnea: Effect of Normoxic Recovery. Sleep 2017, 40, zsw074. [Google Scholar] [CrossRef]
- Carreras, A.; Zhang, S.X.; Almendros, I.; Wang, Y.; Peris, E.; Qiao, Z.; Gozal, D. Resveratrol attenuates intermittent hypoxia-induced macrophage migration to visceral white adipose tissue and insulin resistance in male mice. Endocrinology 2015, 156, 437–443. [Google Scholar] [CrossRef]
- Gozal, D.; Gileles-Hillel, A.; Cortese, R.; Li, Y.; Almendros, I.; Qiao, Z.; Khalyfa, A.A.; Andrade, J.; Khalyfa, A. Visceral White Adipose Tissue after Chronic Intermittent and Sustained Hypoxia in Mice. Am. J. Respir. Cell Mol. Biol. 2017, 56, 477–487. [Google Scholar] [CrossRef]
- Delprat, V.; Tellier, C.; Demazy, C.; Raes, M.; Feron, O.; Michiels, C. Cycling hypoxia promotes a pro-inflammatory phenotype in macrophages via JNK/p65 signaling pathway. Sci. Rep. 2020, 10, 882. [Google Scholar] [CrossRef]
- Kolb, R.; Sutterwala, F.S.; Zhang, W. Obesity and cancer: Inflammation bridges the two. Curr. Opin. Pharmacol. 2016, 29, 77–89. [Google Scholar] [CrossRef]
- Avgerinos, K.I.; Spyrou, N.; Mantzoros, C.S.; Dalamaga, M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism 2019, 92, 121–135. [Google Scholar] [CrossRef]
- Trzepizur, W.; Cortese, R.; Gozal, D. Murine models of sleep apnea: Functional implications of altered macrophage polarity and epigenetic modifications in adipose and vascular tissues. Metabolism 2018, 84, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, K.; Huppertz, T.; Radsak, M.; Gouveris, H. Cellular Immune Dysfunction in Obstructive Sleep Apnea. Front Surg. 2022, 9, 890377. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, E.; Wu, W.; Mark, C.; Yang, A.; DiGiacomo, E.; Carlton-Smith, C.; Salloum, S.; Brisac, C.; Lin, W.; Corey, K.E.; et al. Intermittent hypoxia is a proinflammatory stimulus resulting in IL-6 expression and M1 macrophage polarization. Hepatol. Commun. 2017, 1, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lee, M.Y.K.; Mak, J.C.W.; Ip, M.S.M. Low-Frequency Intermittent Hypoxia Suppresses Subcutaneous Adipogenesis and Induces Macrophage Polarization in Lean Mice. Diabetes Metab. J. 2019, 43, 659–674. [Google Scholar] [CrossRef] [PubMed]
- Hammarstedt, A.; Gogg, S.; Hedjazifar, S.; Nerstedt, A.; Smith, U. Impaired Adipogenesis and Dysfunctional Adipose Tissue in Human Hypertrophic Obesity. Physiol. Rev. 2018, 98, 1911–1941. [Google Scholar] [CrossRef]
- Zhang, S.X.; Khalyfa, A.; Wang, Y.; Carreras, A.; Hakim, F.; Neel, B.A.; Brady, M.J.; Qiao, Z.; Hirotsu, C.; Gozal, D. Sleep fragmentation promotes NADPH oxidase 2-mediated adipose tissue inflammation leading to insulin resistance in mice. Int. J. Obes. Lond. 2014, 38, 619–624. [Google Scholar] [CrossRef]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Verduzco, D.; Lloyd, M.; Xu, L.; Ibrahim-Hashim, A.; Balagurunathan, Y.; Gatenby, R.A.; Gillies, R.J. Intermittent hypoxia selects for genotypes and phenotypes that increase survival, invasion, and therapy resistance. PLoS ONE 2015, 10, e0120958. [Google Scholar] [CrossRef]
- Picado, C.; Roca-Ferrer, J. Role of the Cyclooxygenase Pathway in the Association of Obstructive Sleep Apnea and Cancer. J. Clin. Med. 2020, 9, 3237. [Google Scholar] [CrossRef]
- Almendros, I.; Wang, Y.; Becker, L.; Lennon, F.E.; Zheng, J.; Coats, B.R.; Schoenfelt, K.S.; Carreras, A.; Hakim, F.; Zhang, S.X.; et al. Intermittent hypoxia-induced changes in tumor-associated macrophages and tumor malignancy in a mouse model of sleep apnea. Am. J. Respir. Crit. Care Med. 2014, 189, 593–601. [Google Scholar] [CrossRef]
- Campillo, N.; Torres, M.; Vilaseca, A.; Nonaka, P.N.; Gozal, D.; Roca-Ferrer, J.; Picado, C.; Montserrat, J.M.; Farre, R.; Navajas, D.; et al. Role of Cyclooxygenase-2 on Intermittent Hypoxia-Induced Lung Tumor Malignancy in a Mouse Model of Sleep Apnea. Sci. Rep. 2017, 7, 44693. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Yin, J.; Bai, Y.; Peng, H.; Zhou, X.; Bai, J. Differential expression of immune markers in the patients with obstructive sleep apnea/hypopnea syndrome. Eur. Arch. Otorhinolaryngol. 2019, 276, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Galati, D.; Zanotta, S.; Canora, A.; Polistina, G.E.; Nicoletta, C.; Ghinassi, G.; Ciasullo, E.; Bocchino, M. Severe depletion of peripheral blood dendritic cell subsets in obstructive sleep apnea patients: A new link with cancer? Cytokine 2020, 125, 154831. [Google Scholar] [CrossRef]
- Dyugovskaya, L.; Lavie, P.; Lavie, L. Phenotypic and functional characterization of blood gammadelta T cells in sleep apnea. Am. J. Respir. Crit. Care Med. 2003, 168, 242–249. [Google Scholar] [CrossRef]
- Dyugovskaya, L.; Lavie, P.; Hirsh, M.; Lavie, L. Activated CD8+ T-lymphocytes in obstructive sleep apnoea. Eur. Respir. J. 2005, 25, 820–828. [Google Scholar] [CrossRef]
- Staats, R.; Rodrigues, R.; Barros, A.; Bacelar-Nicolau, L.; Aguiar, M.; Fernandes, D.; Moreira, S.; Simoes, A.; Silva-Santos, B.; Rodrigues, J.V.; et al. Decrease of perforin positive CD3(+)gammadelta-T cells in patients with obstructive sleep disordered breathing. Sleep Breath 2018, 22, 211–221. [Google Scholar] [CrossRef]
- Hernandez-Jimenez, E.; Cubillos-Zapata, C.; Toledano, V.; Perez de Diego, R.; Fernandez-Navarro, I.; Casitas, R.; Carpio, C.; Casas-Martin, J.; Valentin, J.; Varela-Serrano, A.; et al. Monocytes inhibit NK activity via TGF-beta in patients with obstructive sleep apnoea. Eur. Respir. J. 2017, 49, 1602456. [Google Scholar] [CrossRef]
- Gaoatswe, G.; Kent, B.D.; Corrigan, M.A.; Nolan, G.; Hogan, A.E.; McNicholas, W.T.; O’Shea, D. Invariant Natural Killer T Cell Deficiency and Functional Impairment in Sleep Apnea: Links to Cancer Comorbidity. Sleep 2015, 38, 1629–1634. [Google Scholar] [CrossRef]
- Akbarpour, M.; Khalyfa, A.; Qiao, Z.; Gileles-Hillel, A.; Almendros, I.; Farre, R.; Gozal, D. Altered CD8+ T-Cell Lymphocyte Function and TC1 Cell Stemness Contribute to Enhanced Malignant Tumor Properties in Murine Models of Sleep Apnea. Sleep 2017, 40, zsw040. [Google Scholar] [CrossRef]
- Cubillos-Zapata, C.; Martinez-Garcia, M.A.; Diaz-Garcia, E.; Garcia-Tovar, S.; Campos-Rodriguez, F.; Sanchez-de-la-Torre, M.; Nagore, E.; Martorell-Calatayud, A.; Blasco, L.H.; Pastor, E.; et al. Obstructive sleep apnea is related to melanoma aggressiveness through paraspeckle protein-1 upregulation. Eur. Respir. J. 2022, 61, 2200707. [Google Scholar] [CrossRef]
- Liu, Y.L.; Luo, S.H.; Ou, Q.; Yuan, P.; Lu, M.Z.; Chen, J.N.; Luo, Z.R.; Lao, M.C.; Cui, J.H.; Gao, X.L. The expressions of CTLA-4 and PD-1 on CD(4)(+) T cells and the level of plasma VEGF in patients with obstructive sleep apnea hypopnea syndrome. Zhonghua Jie He He Hu Xi Za Zhi 2019, 42, 268–274. [Google Scholar] [PubMed]
- Polasky, C.; Steffen, A.; Loyal, K.; Lange, C.; Bruchhage, K.L.; Pries, R. Redistribution of Monocyte Subsets in Obstructive Sleep Apnea Syndrome Patients Leads to an Imbalanced PD-1/PD-L1 Cross-Talk with CD4/CD8 T Cells. J. Immunol. 2021, 206, 51–58. [Google Scholar] [CrossRef]
- Wunder, J.S.; Lee, M.J.; Nam, J.; Lau, B.Y.; Dickson, B.C.; Pinnaduwage, D.; Bull, S.B.; Ferguson, P.C.; Seto, A.; Gokgoz, N.; et al. Osteosarcoma and soft-tissue sarcomas with an immune infiltrate express PD-L1: Relation to clinical outcome and Th1 pathway activation. Oncoimmunology 2020, 9, 1737385. [Google Scholar] [CrossRef] [PubMed]
- Cubillos-Zapata, C.; Martinez-Garcia, M.A.; Campos-Rodriguez, F.; Sanchez de la Torre, M.; Nagore, E.; Martorell-Calatayud, A.; Hernandez Blasco, L.; Chiner Vives, E.; Abad-Capa, J.; Montserrat, J.M.; et al. Soluble PD-L1 is a potential biomarker of cutaneous melanoma aggressiveness and metastasis in obstructive sleep apnoea patients. Eur. Respir. J. 2019, 53, 1801298. [Google Scholar] [CrossRef] [PubMed]
- Cagney, D.N.; Sul, J.; Huang, R.Y.; Ligon, K.L.; Wen, P.Y.; Alexander, B.M. The FDA NIH Biomarkers, EndpointS, and other Tools (BEST) resource in neuro-oncology. Neuro-Oncology 2017, 20, 1162–1172. [Google Scholar] [CrossRef] [PubMed]
- Dzhalilova, D.; Makarova, O. Differences in Tolerance to Hypoxia: Physiological, Biochemical, and Molecular-Biological Characteristics. Biomedicines 2020, 8, 428. [Google Scholar] [CrossRef]
- Almendros, I.; Martínez-García, M.Á.; Campos-Rodríguez, F.; Riveiro-Falkenbach, E.; Rodríguez-Peralto, J.L.; Nagore, E.; Martorell-Calatayud, A.; Blasco, L.; Roca, J.; Vives, E.; et al. Intermittent Hypoxia Is Associated with High Hypoxia Inducible Factor-1α but Not High Vascular Endothelial Growth Factor Cell Expression in Tumors of Cutaneous Melanoma Patients. Front. Neurol. 2018, 9, 272. [Google Scholar] [CrossRef]
- Martínez-García, M.Á.; Riveiro-Falkenbach, E.; Rodriguez-Peralto, J.L.; Nagore, E.; Martorell-Calatayud, A.; Campos-Rodriguez, F.; Farré, R.; Blasco, L.H.; Roca, J.B.; Vives, E.C.; et al. A prospective multicenter cohort study of cutaneous melanoma: Clinical staging and potential associations with HIF-1α and VEGF expressions. Melanoma Res. 2017, 27, 558–564. [Google Scholar] [CrossRef]
- Gozal, D.; Lipton, A.J.; Jones, K.L. Circulating Vascular Endothelial Growth Factor Levels in Patients with Obstructive Sleep Apnea. Sleep 2002, 25, 59–65. [Google Scholar] [CrossRef]
- Cubillos-Zapata, C.; Hernandez-Jimenez, E.; Avendano-Ortiz, J.; Toledano, V.; Varela-Serrano, A.; Fernandez-Navarro, I.; Casitas, R.; Carpio, C.; Aguirre, L.A.; Garcia-Rio, F.; et al. Obstructive Sleep Apnea Monocytes Exhibit High Levels of Vascular Endothelial Growth Factor Secretion, Augmenting Tumor Progression. Mediat. Inflamm. 2018, 2018, 7373921. [Google Scholar] [CrossRef]
- Santamaria-Martos, F.; Benítez, I.; Girón, C.; Barbé, F.; Martínez-García, M.-A.; Hernández, L. Biomarkers of carcinogenesis and tumour growth in patients with cutaneous melanoma and obstructive sleep apnoea. Eur. Respir. J. 2018, 51, 1701885. [Google Scholar] [CrossRef] [PubMed]
- Konstantina, A.; Lazaris, A.C.; Ioannidis, E.; Liossi, A.; Aroni, K. Immunohistochemical expression of VEGF, HIF1-a, and PlGF in malignant melanomas and dysplastic nevi. Melanoma Res. 2011, 21, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Brychtova, S.; Bezdekova, M.; Brychta, T.; Tichy, M. The role of vascular endothelial growth factors and their receptors in malignant melanomas. Neoplasma 2008, 55, 273–279. [Google Scholar] [PubMed]
- Schlesinger, M.; Bendas, G. Vascular cell adhesion molecule-1 (VCAM-1)—An increasing insight into its role in tumorigenicity and metastasis. Int. J. Cancer 2015, 136, 2504–2514. [Google Scholar] [CrossRef] [PubMed]
- Audrito, V.; Serra, S.; Stingi, A.; Orso, F.; Gaudino, F.; Bologna, C. PD-L1 up-regulation in melanoma increases disease aggressiveness and is mediated through miR-17-5p. Oncotarget 2017, 8, 15894–15911. [Google Scholar] [CrossRef]
- Massi, D.; Brusa, D.; Merelli, B.; Ciano, M.; Audrito, V.; Serra, S. PD-L1 marks a subset of melanomas with a shorter overall survival and distinct genetic and morphological characteristics. Ann. Oncol. 2014, 25, 2433–2442. [Google Scholar] [CrossRef]
- Colak, S.; Ten Dijke, P. Targeting TGF-β Signaling in Cancer. Trends Cancer 2017, 3, 56–71. [Google Scholar] [CrossRef]
- Moustakas, A.; Pardali, K.; Gaal, A.; Heldin, C.H. Mechanisms of TGF-beta signaling in regulation of cell growth and differentiation. Immunol. Lett. 2002, 82, 85–91. [Google Scholar] [CrossRef]
- Cubillos-Zapata, C.; Martínez-García, M.Á.; Díaz-García, E.; Jaureguizar, A.; Campos-Rodríguez, F.; Sánchez-de-la-Torre, M. Obesity attenuates the effect of sleep apnea on active TGF-ß1 levels and tumor aggressiveness in patients with melanoma. Sci. Rep. 2020, 10, 15528. [Google Scholar] [CrossRef]
- Deng, B.; Zhu, J.-M.; Wang, Y.; Liu, T.-T.; Ding, Y.-B.; Xiao, W.-M. Intratumor Hypoxia Promotes Immune Tolerance by Inducing Regulatory T Cells via TGF-β1 in Gastric Cancer. PLoS ONE 2013, 8, e63777. [Google Scholar] [CrossRef]
- Cao, Y.; Song, Y.; Ning, P.; Zhang, L.; Wu, S.; Quan, J. Association between tumor necrosis factor alpha and obstructive sleep apnea in adults: A meta-analysis update. BMC Pulm. Med. 2020, 20, 215. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Martínez, E.; Martín-Ruiz, A.; Martín, P.; Calvo, V.; Provencio, M.; García, J.M. CB2 cannabinoid receptor activation promotes colon cancer progression via AKT/GSK3β signaling pathway. Oncotarget 2016, 7, 68781–68791. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-T.; Zhao, F.-F.; Jia, Z.-M.; Qi, L.-Q.; Zhang, X.-Z.; Zhang, L. Cannabinoid receptors promote chronic intermittent hypoxia-induced breast cancer metastasis via IGF-1R/AKT/GSK-3β. Mol. Ther. Oncolytics 2021, 23, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-B.; Yang, Y.-Y.; Zeng, Y.; Zeng, H.-Q.; Fu, B.-B.; Ko, C.-Y. Anti-tumor effect of endostatin in a sleep-apnea mouse model with tumor. Clin. Transl. Oncol. 2019, 21, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Gharib, S.A.; Seiger, A.N.; Hayes, A.L.; Mehra, R.; Patel, S.R. Treatment of Obstructive Sleep Apnea Alters Cancer-associated Transcriptional Signatures in Circulating Leukocytes. Sleep 2014, 37, 709–714. [Google Scholar] [CrossRef]
- Douglas, N.J.; Luke, M.; Mathur, R. Is the Sleep-Apnea Hypopnea Syndrome Inherited. Thorax 1993, 48, 719–721. [Google Scholar] [CrossRef] [PubMed]
- Gislason, T.; Johannsson, J.H.; Haraldsson, A.; Olafsdottir, B.R.; Jonsdottir, H.; Kong, A. Familial predisposition and cosegregation analysis of adult obstructive sleep apnea and the sudden infant death syndrome. Am. J. Respir. Crit. Care Med. 2002, 166, 833–838. [Google Scholar] [CrossRef]
- Mathur, R.; Douglas, N.J. Family studies in patients with the sleep apnea-hypopnea syndrome. Ann. Intern Med. 1995, 122, 174–178. [Google Scholar] [CrossRef]
- Guilleminault, C.; Partinen, M.; Hollman, K.; Powell, N.; Stoohs, R. Familial aggregates in obstructive sleep apnea syndrome. Chest 1995, 107, 1545–1551. [Google Scholar] [CrossRef]
- Redline, S.; Tishler, P.V.; Tosteson, T.D.; Williamson, J.; Kump, K.; Browner, I. The familial aggregation of obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 1995, 151 Pt 1, 682–687. [Google Scholar] [CrossRef]
- Schwab, R.J.; Pasirstein, M.; Kaplan, L.; Pierson, R.; Mackley, A.; Hachadoorian, R. Family aggregation of upper airway soft tissue structures in normal subjects and patients with sleep apnea. Am. J. Respir. Crit. Care Med. 2006, 173, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Chi, L.Q.; Comyn, F.L.; Keenan, B.T.; Cater, J.; Maislin, G.; Pack, A.I. Heritability of Craniofacial Structures in Normal Subjects and Patients with Sleep Apnea. Sleep 2014, 37, 1689–1698. [Google Scholar] [CrossRef] [PubMed]
- King, L.; Harris, E.F.; Tolley, E.A. Heritability of Cephalometric and Occlusal Variables as Assessed from Siblings with Overt Malocclusions. Am. J. Orthod. Dentofac. 1993, 104, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Johannsdottir, B.; Thorarinsson, F.; Thordarson, A.; Magnusson, T.E. Heritability of craniofacial characteristics between parents and offspring estimated from lateral cephalograms. Am. J. Orthod. Dentofac. 2005, 127, 200–207. [Google Scholar] [CrossRef]
- Malis, C.; Rasmussen, E.L.; Poulsen, P.; Petersen, I.; Christensen, K.; Beck-Nielsen, H. Total and regional fat distribution is strongly influenced by genetic factors in young and elderly twins. Obes. Res. 2005, 13, 2139–2145. [Google Scholar] [CrossRef]
- Min, J.; Chiu, D.T.; Wang, Y. Variation in the heritability of body mass index based on diverse twin studies: A systematic review. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2013, 14, 871–882. [Google Scholar] [CrossRef]
- Cade, B.E.; Lee, J.; Sofer, T.; Wang, H.; Zhang, M.; Chen, H. Whole-genome association analyses of sleep-disordered breathing phenotypes in the NHLBI TOPMed program. Genome Med. 2021, 13, 136. [Google Scholar] [CrossRef]
- Campos, A.I.; Ingold, N.; Huang, Y.; Mitchell, B.L.; Kho, P.-F.; Han, X. Discovery of genomic loci associated with sleep apnoea risk through multi-trait GWAS analysis with snoring. medRxiv 2022. [Google Scholar] [CrossRef]
- Farias Tempaku, P.; Leite Santoro, M.; Bittencourt, L.; D’Almeida, V.; Iole Belangero, S.; Tufik, S. Genome-wide association study reveals two novel risk alleles for incident obstructive sleep apnea in the EPISONO cohort. Sleep Med. 2020, 66, 24–32. [Google Scholar] [CrossRef]
- Xu, H.; Liu, F.; Li, Z.; Li, X.; Liu, Y.; Li, N. Genome-Wide Association Study of Obstructive Sleep Apnea and Objective Sleep-related Traits Identifies Novel Risk Loci in Han Chinese Individuals. Am. J. Respir. Crit. Care Med. 2022, 206, 1534–1545. [Google Scholar] [CrossRef]
- Semenza, G.L. Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer 2003, 3, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Li, F.; Jiang, P.; Gao, J. Effect of chronic intermittent hypoxia-induced HIF-1alpha/ATAD2 expression on lung cancer stemness. Cell Mol. Biol. Lett. 2022, 27, 44. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Cao, H.; Zhang, Q.; Wang, B. The effect of intermittent hypoxia and fecal microbiota of OSAS on genes associated with colorectal cancer. Sleep Breath. 2021, 25, 1075–1087. [Google Scholar] [CrossRef] [PubMed]
- Nanduri, J.; Wang, N.; Wang, B.L.; Prabhakar, N.R. Lysine demethylase KDM6B regulates HIF-1alpha-mediated systemic and cellular responses to intermittent hypoxia. Physiol. Genom. 2021, 53, 385–394. [Google Scholar] [CrossRef]
- Li, L.; Lu, J.; Xue, W.; Wang, L.; Zhai, Y.; Fan, Z. Target of obstructive sleep apnea syndrome merge lung cancer: Based on big data platform. Oncotarget 2017, 8, 21567–21578. [Google Scholar] [CrossRef]
- Paatero, I.; Jokilammi, A.; Heikkinen, P.T.; Iljin, K.; Kallioniemi, O.P.; Jones, F.E. Interaction with ErbB4 promotes hypoxia-inducible factor-1alpha signaling. J. Biol. Chem. 2012, 287, 9659–9671. [Google Scholar] [CrossRef]
- Rosas, D.; Raez, L.E.; Russo, A.; Rolfo, C. Neuregulin 1 Gene (NRG1). A Potentially New Targetable Alteration for the Treatment of Lung Cancer. Cancers 2021, 13, 5038. [Google Scholar] [CrossRef]
- Liu, J.; Carnero-Montoro, E.; van Dongen, J.; Lent, S.; Nedeljkovic, I.; Ligthart, S. An integrative cross-omics analysis of DNA methylation sites of glucose and insulin homeostasis. Nat. Commun. 2019, 10, 2581. [Google Scholar] [CrossRef]
- Chen, Y.C.; Hsu, P.Y.; Hsiao, C.C.; Lin, M.C. Epigenetics: A Potential Mechanism Involved in the Pathogenesis of Various Adverse Consequences of Obstructive Sleep Apnea. Int. J. Mol. Sci. 2019, 20, 2937. [Google Scholar] [CrossRef]
- Yan, M.; Yang, X.; Wang, H.; Shao, Q. The critical role of histone lysine demethylase KDM2B in cancer. Am. J. Transl. Res. 2018, 10, 2222–2233. [Google Scholar]
- Di Rosa, M.C.; Zimbone, S.; Saab, M.W.; Tomasello, M.F. The Pleiotropic Potential of BDNF beyond Neurons: Implication for a Healthy Mind in a Healthy Body. Life 2021, 11, 1256. [Google Scholar] [CrossRef] [PubMed]
- Khalaji, A.; Behnoush, A.H.; Shobeiri, P.; Saeedian, B.; Teixeira, A.L.; Rezaei, N. Association between brain-derived neurotrophic factor levels and obstructive sleep apnea: A systematic review and meta-analysis. Sleep Breath. 2022. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Liu, B.; Ji, R.; Jiang, X.; Yan, X.; Xin, Y. Targeting the BDNF/TrkB pathway for the treatment of tumors. Oncol. Lett. 2019, 17, 2031–2039. [Google Scholar] [CrossRef] [PubMed]
- Freitas, L.S.; Silveira, A.C.; Martins, F.C.; Costa-Hong, V.; Lebkuchen, A.; Cardozo, K.H.M. Severe obstructive sleep apnea is associated with circulating microRNAs related to heart failure, myocardial ischemia, and cancer proliferation. Sleep Breath. 2020, 24, 1463–1472. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Huang, K.; Wen, F.; Cui, G.; Guo, H.; He, Z. Intermittent hypoxia-induced downregulation of microRNA-320b promotes lung cancer tumorigenesis by increasing CDT1 via USP37. Mol. Ther. Nucleic. Acids. 2021, 24, 528–541. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Zeng, C.; Li, W.; Song, W.; Xu, P. Manuscript Title: A 4-miRNAs Serum Panel for Obstructive Sleep Apnea Syndrome Screening. Nat. Sci. Sleep. 2022, 14, 2055–2064. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.B.; Lin, X.L.; Wu, X.Y.; Zeng, Y.M.; Chen, X.Y.; Luo, X. Differential expression of microRNAs in xenografted Lewis lung carcinomas subjected to intermittent hypoxia: A next-generation sequence analysis. Transl. Cancer Res. 2020, 9, 4354–4365. [Google Scholar] [CrossRef]
- Khalyfa, A.; Trzepizur, W.; Gileles-Hillel, A.; Qiao, Z.; Sanz-Rubio, D.; Marin, J.M. Heterogeneity of Melanoma Cell Responses to Sleep Apnea-Derived Plasma Exosomes and to Intermittent Hypoxia. Cancers 2021, 13, 4781. [Google Scholar] [CrossRef]
- Albini, A.; Sporn, M.B. The tumour microenvironment as a target for chemoprevention. Nat. Rev. Cancer 2007, 7, 139–147. [Google Scholar] [CrossRef]
- Aguilar-Cazares, D.; Chavez-Dominguez, R.; Marroquin-Muciño, M.; Perez-Medina, M.; Benito-Lopez, J.J.; Camarena, A.; Rumbo-Nava, U.; Lopez-Gonzalez, J.S. The systemic-level repercussions of cancer-associated inflammation mediators produced in the tumor microenvironment. Front. Endocrinol. Lausanne 2022, 13, 929572. [Google Scholar] [CrossRef]
- Tao, J.; Zhu, L.; Yakoub, M.; Reißfelder, C.; Loges, S.; Schölch, S. Cell-Cell Interactions Drive Metastasis of Circulating Tumor Microemboli. Cancer Res. 2022, 82, 2661–2671. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.T.; Jin, W.L.; Li, X. Intercellular communication in the tumour microecosystem: Mediators and therapeutic approaches for hepatocellular carcinoma. Biochim. Biophys. Acta Mol. Basis. Dis. 2022, 1868, 166528. [Google Scholar] [CrossRef] [PubMed]
- Hanse, E.A.; Kong, M. A happy cell stays home: When metabolic stress creates epigenetic advantages in the tumor microenvironment. Front. Oncol. 2022, 12, 962928. [Google Scholar] [CrossRef] [PubMed]
- Barnestein, R.; Galland, L.; Kalfeist, L.; Ghiringhelli, F.; Ladoire, S.; Limagne, E. Immunosuppressive tumor microenvironment modulation by chemotherapies and targeted therapies to enhance immunotherapy effectiveness. Oncoimmunology 2022, 11, 2120676. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Meng, L.; Li, B.; Li, Y.; Shen, T.; Zhao, B. The Exosome Journey: From Biogenesis to Regulation and Function in Cancers. J. Oncol. 2022, 2022, 9356807. [Google Scholar] [CrossRef]
- Majood, M.; Rawat, S.; Mohanty, S. Delineating the role of extracellular vesicles in cancer metastasis: A comprehensive review. Front. Immunol. 2022, 13, 966661. [Google Scholar] [CrossRef]
- Lopatina, T.; Sarcinella, A.; Brizzi, M.F. Tumour Derived Extracellular Vesicles: Challenging Target to Blunt Tumour Immune Evasion. Cancers 2022, 14, 4020. [Google Scholar] [CrossRef]
- Chen, X.; Feng, J.; Chen, W.; Shao, S.; Chen, L.; Wan, H. Small extracellular vesicles: From promoting pre-metastatic niche formation to therapeutic strategies in breast cancer. Cell Commun. Signal. 2022, 20, 141. [Google Scholar] [CrossRef]
- Mansoori, B.; Baradaran, B.; Nazari, A.; Gaballu, F.A.; Cho, W.C.; Mansoori, B. MicroRNAs in the cancer cell-to-cell communication: An insight into biological vehicles. Biomed. Pharmacother. 2022, 153, 113449. [Google Scholar] [CrossRef]
- Pitt, J.M.; Kroemer, G.; Zitvogel, L. Extracellular vesicles: Masters of intercellular communication and potential clinical interventions. J. Clin. Investig. 2016, 126, 1139–1143. [Google Scholar] [CrossRef]
- Salido-Guadarrama, I.; Romero-Cordoba, S.; Peralta-Zaragoza, O.; Hidalgo-Miranda, A.; Rodríguez-Dorantes, M. MicroRNAs transported by exosomes in body fluids as mediators of intercellular communication in cancer. Onco Targets Ther. 2014, 7, 1327–1338. [Google Scholar] [CrossRef] [PubMed]
- Al-Nedawi, K. The yin-yang of microvesicles (exosomes) in cancer biology. Front. Oncol. 2014, 4, 172. [Google Scholar] [CrossRef] [PubMed]
- Braicu, C.; Tomuleasa, C.; Monroig, P.; Cucuianu, A.; Berindan-Neagoe, I.; Calin, G.A. Exosomes as divine messengers: Are they the Hermes of modern molecular oncology? Cell Death Differ. 2015, 22, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Cortese, R.; Almendros, I.; Wang, Y.; Gozal, D. Tumor circulating DNA profiling in xenografted mice exposed to intermittent hypoxia. Oncotarget 2015, 6, 556–569. [Google Scholar] [CrossRef] [PubMed]
- Hermann, D.M.; Xin, W.; Bähr, M.; Giebel, B.; Doeppner, T.R. Emerging roles of extracellular vesicle-associated non-coding RNAs in hypoxia: Insights from cancer, myocardial infarction and ischemic stroke. Theranostics 2022, 12, 5776–5802. [Google Scholar] [CrossRef] [PubMed]
- Khalyfa, A.; Gozal, D. Exosomal miRNAs as potential biomarkers of cardiovascular risk in children. J. Transl. Med. 2014, 12, 162. [Google Scholar] [CrossRef]
- Khalyfa, A.; Kheirandish-Gozal, L.; Gozal, D. Exosome and Macrophage Crosstalk in Sleep-Disordered Breathing-Induced Metabolic Dysfunction. Int. J. Mol. Sci. 2018, 19, 3383. [Google Scholar] [CrossRef]
- Khalyfa, A.; Kheirandish-Gozal, L.; Gozal, D. Circulating exosomes in obstructive sleep apnea as phenotypic biomarkers and mechanistic messengers of end-organ morbidity. Respir. Physiol. Neurobiol. 2018, 256, 143–156. [Google Scholar] [CrossRef]
- Khalyfa, A.; Kheirandish-Gozal, L.; Khalyfa, A.A.; Philby, M.F.; Alonso-Alvarez, M.L.; Mohammadi, M.; Bhattacharjee, R.; Teran-Santos, J.; Huang, L.; Andrade, J.; et al. Circulating Plasma Extracellular Microvesicle MicroRNA Cargo and Endothelial Dysfunction in Children with Obstructive Sleep Apnea. Am. J. Respir. Crit. Care Med. 2016, 194, 1116–1126. [Google Scholar] [CrossRef]
- Khalyfa, A.; Castro-Grattoni, A.L.; Gozal, D. Cardiovascular morbidities of obstructive sleep apnea and the role of circulating extracellular vesicles. Ther. Adv. Respir. Dis. 2019, 13, 1753466619895229. [Google Scholar] [CrossRef]
- Sanz-Rubio, D.; Khalyfa, A.; Qiao, Z.; Ullate, J.; Marin, J.M.; Kheirandish-Gozal, L.; Gozal, D. Cell-Selective Altered Cargo Properties of Extracellular Vesicles Following In Vitro Exposures to Intermittent Hypoxia. Int. J. Mol. Sci. 2021, 22, 5604. [Google Scholar] [CrossRef] [PubMed]
- Khalyfa, A.; Ericsson, A.; Qiao, Z.; Almendros, I.; Farré, R.; Gozal, D. Circulating exosomes and gut microbiome induced insulin resistance in mice exposed to intermittent hypoxia: Effects of physical activity. EBioMedicine 2021, 64, 103208. [Google Scholar] [CrossRef] [PubMed]
- Khalyfa, A.; Gaddameedhi, S.; Crooks, E.; Zhang, C.; Li, Y.; Qiao, Z.; Trzepizur, W.; Kay, S.A.; Andrade, J.; Satterfield, B.C.; et al. Circulating Exosomal miRNAs Signal Circadian Misalignment to Peripheral Metabolic Tissues. Int. J. Mol. Sci. 2020, 21, 6396. [Google Scholar] [CrossRef] [PubMed]
- Khalyfa, A.; Gozal, D.; Masa, J.F.; Marin, J.M.; Qiao, Z.; Corral, J.; González, M.; Marti, S.; Kheirandish-Gozal, L.; Egea, C.; et al. Sleep-disordered breathing, circulating exosomes, and insulin sensitivity in adipocytes. Int. J. Obes. Lond. 2018, 42, 1127–1139. [Google Scholar] [CrossRef] [PubMed]
- Khalyfa, A.; Zhang, C.; Khalyfa, A.A.; Foster, G.E.; Beaudin, A.E.; Andrade, J.; Hanly, P.J.; Poulin, M.J.; Gozal, D. Effect on Intermittent Hypoxia on Plasma Exosomal Micro RNA Signature and Endothelial Function in Healthy Adults. Sleep 2016, 39, 2077–2090. [Google Scholar] [CrossRef] [PubMed]
- Almendros, I.; Khalyfa, A.; Trzepizur, W.; Gileles-Hillel, A.; Huang, L.; Akbarpour, M.; Andrade, J.; Farré, R.; Gozal, D. Tumor Cell Malignant Properties Are Enhanced by Circulating Exosomes in Sleep Apnea. Chest 2016, 150, 1030–1041. [Google Scholar] [CrossRef] [PubMed]
- Khalyfa, A.; Almendros, I.; Gileles-Hillel, A.; Akbarpour, M.; Trzepizur, W.; Mokhlesi, B.; Huang, L.; Andrade, J.; Farré, R.; Gozal, D. Circulating exosomes potentiate tumor malignant properties in a mouse model of chronic sleep fragmentation. Oncotarget 2016, 7, 54676–54690. [Google Scholar] [CrossRef]
- Khalyfa, A.; Masa, J.F.; Qiao, Z.; González, M.; Marti, S.; Khalyfa, A.A.; Kheirandish-Gozal, L.; Gozal, D. Plasma exosomes in obesity hypoventilation syndrome patients drive lung cancer cell malignant properties: Effect of long-term adherent CPAP treatment. Biochim. Biophys. Acta Mol. Basis. Dis. 2022, 1868, 166479. [Google Scholar] [CrossRef]
- Słomka, A.; Kornek, M.; Cho, W.C. Small Extracellular Vesicles and Their Involvement in Cancer Resistance: An Up-to-Date Review. Cells 2022, 11, 2913. [Google Scholar] [CrossRef]
- Yi, X.; Chen, J.; Huang, D.; Feng, S.; Yang, T.; Li, Z.; Wang, X.; Zhao, M.; Wu, J.; Zhong, T. Current perspectives on clinical use of exosomes as novel biomarkers for cancer diagnosis. Front. Oncol. 2022, 12, 966981. [Google Scholar] [CrossRef]
- Dai, X.; Ye, Y.; He, F. Emerging innovations on exosome-based onco-therapeutics. Front. Immunol. 2022, 13, 865245. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Hebbandi Nanjundappa, R.; Sokke Umeshappa, C.; Geuking, M.B. The impact of the gut microbiota on T cell ontogeny in the thymus. Cell Mol. Life Sci. 2022, 79, 221. [Google Scholar] [CrossRef]
- Yu, L.W.; Agirman, G.; Hsiao, E.Y. The Gut Microbiome as a Regulator of the Neuroimmune Landscape. Annu. Rev. Immunol. 2022, 40, 143–167. [Google Scholar] [CrossRef]
- Santacroce, L.; Man, A.; Charitos, I.A.; Haxhirexha, K.; Topi, S. Current knowledge about the connection between health status and gut microbiota from birth to elderly. A narrative review. Front. Biosci. Landmark Ed. 2021, 26, 135–148. [Google Scholar]
- Nathan, N.N.; Philpott, D.J.; Girardin, S.E. The intestinal microbiota: From health to disease, and back. Microbes Infect. 2021, 23, 104849. [Google Scholar] [CrossRef]
- Dicks, L.M.T. Gut Bacteria and Neurotransmitters. Microorganisms 2022, 10, 1838. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Z.; Lu, T.; Chen, W.; Yan, W.; Yuan, K.; Shi, L.; Liu, X.; Zhou, X.; Shi, J.; et al. The microbiota-gut-brain axis in sleep disorders. Sleep Med. Rev. 2022, 65, 101691. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, S. Diet, Gut Microbiome, and Cognitive Decline. Curr. Nutr. Rep. 2022, 11, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Sadrekarimi, H.; Gardanova, Z.R.; Bakhshesh, M.; Ebrahimzadeh, F.; Yaseri, A.F.; Thangavelu, L.; Hasanpoor, Z.; Zadeh, F.A.; Kahrizi, M.S. Emerging role of human microbiome in cancer development and response to therapy: Special focus on intestinal microflora. J. Transl. Med. 2022, 20, 301. [Google Scholar] [CrossRef]
- Cao, Z.; Sugimura, N.; Burgermeister, E.; Ebert, M.P.; Zuo, T.; Lan, P. The gut virome: A new microbiome component in health and disease. EBioMedicine 2022, 81, 104113. [Google Scholar] [CrossRef] [PubMed]
- Goto, T. Microbiota and lung cancer. Semin. Cancer Biol. 2022, 86, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Denk, D.; Greten, F.R. Inflammation: The incubator of the tumor microenvironment. Trends Cancer 2022, 8, 901–914. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Hu, Y.; Wang, X.; Shen, L.; Liao, X.; Zhu, Y.; Yu, J.; Zhao, F.; Zhou, Y.; Shen, H.; et al. Microbiome in cancer: An exploration of carcinogenesis, immune responses an immunotherapy. Front. Immunol. 2022, 13, 877939. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Lin, W.; Li, N.; Wang, Q.; Zhu, S.; Zeng, A.; Song, L. Therapeutic approaches to colorectal cancer via strategies based on modulation of gut microbiota. Front. Microbiol. 2022, 13, 945533. [Google Scholar] [CrossRef]
- Aghamajidi, A.; Maleki Vareki, S. The Effect of the Gut Microbiota on Systemic and Anti-Tumor Immunity and Response to Systemic Therapy against Cancer. Cancers 2022, 14, 3563. [Google Scholar] [CrossRef]
- Najmi, M.; Tran, T.; Witt, R.G.; Nelson, K.C. Modulation of the Gut Microbiome to Enhance Immunotherapy Response in Metastatic Melanoma Patients: A Clinical Review. Dermatol. Ther. 2022, 12, 2489–2497. [Google Scholar] [CrossRef]
- Ziegler, S.; Bereswill, S.; Heimesaat, M.M. Modulation of the intestinal microbiota impacts the efficacy of immunotherapy in cancer patients—A recent literature survey. Eur. J. Microbiol. Immunol. 2022. Epub ahead of print. [Google Scholar] [CrossRef]
- Wu, H.; Ganguly, S.; Tollefsbol, T.O. Modulating Microbiota as a New Strategy for Breast Cancer Prevention and Treatment. Microorganisms 2022, 10, 1727. [Google Scholar] [CrossRef]
- Xu, X.; Ying, J. Gut Microbiota and Immunotherapy. Front. Microbiol. 2022, 13, 945887. [Google Scholar] [CrossRef]
- Li, X.; Zhang, S.; Guo, G.; Han, J.; Yu, J. Gut microbiome in modulating immune checkpoint inhibitors. EBioMedicine 2022, 82, 104163. [Google Scholar] [CrossRef] [PubMed]
- Khalyfa, A.; Qiao, Z.; Raju, M.; Shyu, C.R.; Coghill, L.; Ericsson, A.; Gozal, D. Monocarboxylate Transporter-2 Expression Restricts Tumor Growth in a Murine Model of Lung Cancer: A Multi-Omic Analysis. Int. J. Mol. Sci. 2021, 22, 10616. [Google Scholar] [CrossRef]
- Badran, M.; Khalyfa, A.; Ericsson, A.; Gozal, D. Fecal microbiota transplantation from mice exposed to chronic intermittent hypoxia elicits sleep disturbances in naïve mice. Exp. Neurol. 2020, 334, 113439. [Google Scholar] [CrossRef] [PubMed]
- Mashaqi, S.; Gozal, D. Obstructive Sleep Apnea and Systemic Hypertension: Gut Dysbiosis as the Mediator? J. Clin. Sleep Med. 2019, 15, 1517–1527. [Google Scholar] [CrossRef] [PubMed]
- Mashaqi, S.; Gozal, D. “Circadian misalignment and the gut microbiome. A bidirectional relationship triggering inflammation and metabolic disorders”—A literature review. Sleep Med. 2020, 72, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Farré, N.; Gozal, D. Sleep and the Microbiome: A Two-Way Relationship. Arch. Bronconeumol. 2019, 55, 7–8, (In English, Spanish). [Google Scholar] [CrossRef]
- Farré, N.; Farré, R.; Gozal, D. Sleep Apnea Morbidity: A Consequence of Microbial Immune Cross-Talk? Chest 2018, 154, 754–759. [Google Scholar] [CrossRef]
- Khalyfa, A.; Poroyko, V.A.; Qiao, Z.; Gileles-Hillel, A.; Khalyfa, A.A.; Akbarpour, M.; Almendros, I.; Farré, R.; Gozal, D. Exosomes and Metabolic Function in Mice Exposed to Alternating Dark-Light Cycles Mimicking Night Shift Work Schedules. Front. Physiol. 2017, 8, 882. [Google Scholar] [CrossRef]
- Moreno-Indias, I.; Torres, M.; Montserrat, J.M.; Sanchez-Alcoholado, L.; Cardona, F.; Tinahones, F.J.; Gozal, D.; Poroyko, V.A.; Navajas, D.; Queipo-Ortuño, M.I.; et al. Intermittent hypoxia alters gut microbiota diversity in a mouse model of sleep apnoea. Eur. Respir. J. 2015, 45, 1055–1065. [Google Scholar] [CrossRef]
- Moreno-Indias, I.; Torres, M.; Sanchez-Alcoholado, L.; Cardona, F.; Almendros, I.; Gozal, D.; Montserrat, J.M.; Queipo-Ortuño, M.I.; Farré, R. Normoxic Recovery Mimicking Treatment of Sleep Apnea Does Not Reverse Intermittent Hypoxia-Induced Bacterial Dysbiosis and Low-Grade Endotoxemia in Mice. Sleep 2016, 39, 1891–1897. [Google Scholar] [CrossRef]
- Poroyko, V.A.; Carreras, A.; Khalyfa, A.; Khalyfa, A.A.; Leone, V.; Peris, E.; Almendros, I.; Gileles-Hillel, A.; Qiao, Z.; Hubert, N.; et al. Chronic Sleep Disruption Alters Gut Microbiota, Induces Systemic and Adipose Tissue Inflammation and Insulin Resistance in Mice. Sci. Rep. 2016, 6, 35405. [Google Scholar] [CrossRef]
- Mashaqi, S.; Laubitz, D.; Morales, E.J.D.; De Armond, R.; Alameddin, H.; Ghishan, F.K.; Kiela, P.R.; Parthasarathy, S. Interactive Effect of Combined Intermittent and Sustained Hypoxia and High-Fat Diet on the Colonic Mucosal Microbiome and Host Gene Expression in Mice. Nat. Sci. Sleep 2022, 14, 1623–1639. [Google Scholar] [CrossRef] [PubMed]
- Van Meijel, R.L.J.; Venema, K.; Canfora, E.E.; Blaak, E.E.; Goossens, G.H. Mild intermittent hypoxia exposure alters gut microbiota composition in men with overweight and obesity. Benef. Microbes 2022, 13, 355–363. [Google Scholar] [CrossRef]
- Tang, S.S.; Liang, C.H.; Liu, Y.L.; Wei, W.; Deng, X.R.; Shi, X.Y.; Wang, L.M.; Zhang, L.J.; Yuan, H.J. Intermittent hypoxia is involved in gut microbial dysbiosis in type 2 diabetes mellitus and obstructive sleep apnea-hypopnea syndrome. World J. Gastroenterol. 2022, 28, 2320–2333. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Luo, H.; Niu, Y.; Yang, X.; Li, Z.; Wang, K.; Bi, H.; Pang, X. Chronic intermittent hypoxia induces gut microbial dysbiosis and infers metabolic dysfunction in mice. Sleep Med. 2022, 91, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zou, J.; Xu, H.; Huang, W.; Zhang, X.; Wei, Z.; Li, X.; Liu, Y.; Zou, J.; Liu, F.; et al. Effects of Chronic Intermittent Hypoxia and Chronic Sleep Fragmentation on Gut Microbiome, Serum Metabolome, Liver and Adipose Tissue Morphology. Front. Endocrinol. 2022, 13, 820939. [Google Scholar] [CrossRef]
- Allaband, C.; Lingaraju, A.; Martino, C.; Russell, B.; Tripathi, A.; Poulsen, O.; Dantas Machado, A.C.; Zhou, D.; Xue, J.; Elijah, E.; et al. Intermittent Hypoxia and Hypercapnia Alter Diurnal Rhythms of Luminal Gut Microbiome and Metabolome. mSystems 2021, 6, e0011621. [Google Scholar] [CrossRef]
- Xue, J.; Allaband, C.; Zhou, D.; Poulsen, O.; Martino, C.; Jiang, L.; Tripathi, A.; Elijah, E.; Dorrestein, P.C.; Knight, R.; et al. Influence of Intermittent Hypoxia/Hypercapnia on Atherosclerosis, Gut Microbiome, and Metabolome. Front. Physiol. 2021, 12, 663950. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, S.; Xu, H.; Yi, H.; Guan, J.; Yin, S. Metabolomics and microbiome profiling as biomarkers in obstructive sleep apnoea: A comprehensive review. Eur. Respir. Rev. 2021, 30, 200220. [Google Scholar] [CrossRef]
- Cai, Y.; Juszczak, H.M.; Cope, E.K.; Goldberg, A.N. The microbiome in obstructive sleep apnea. Sleep 2021, 44, zsab061. [Google Scholar] [CrossRef]
- Tripathi, A.; Melnik, A.V.; Xue, J.; Poulsen, O.; Meehan, M.J.; Humphrey, G.; Jiang, L.; Ackermann, G.; McDonald, D.; Zhou, D.; et al. Intermittent Hypoxia and Hypercapnia, a Hallmark of Obstructive Sleep Apnea, Alters the Gut Microbiome and Metabolome. mSystems 2018, 3, e00020-18. [Google Scholar] [CrossRef] [PubMed]
- Szentirmai, É.; Millican, N.S.; Massie, A.R.; Kapás, L. Butyrate, a metabolite ofintestinal bacteria, enhances sleep. Sci. Rep. 2019, 9, 7035. [Google Scholar] [CrossRef]
- Durgan, D.J.; Ganesh, B.P.; Cope, J.L.; Ajami, N.J.; Phillips, S.C.; Petrosino, J.F.; Hollister, E.B.; Bryan, R.M., Jr. Role of the Gut Microbiome in Obstructive Sleep Apnea-Induced Hypertension. Hypertension 2016, 67, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, B.P.; Nelson, J.W.; Eskew, J.R.; Ganesan, A.; Ajami, N.J.; Petrosino, J.F.; Bryan, R.M., Jr.; Durgan, D.J. Prebiotics, Probiotics, and Acetate Supplementation Prevent Hypertension in a Model of Obstructive Sleep Apnea. Hypertension 2018, 72, 1141–1150. [Google Scholar] [CrossRef]
- Badran, M.; Mashaqi, S.; Gozal, D. The gut microbiome as a target for adjuvant therapy in obstructive sleep apnea. Expert Opin. Ther. Targets 2020, 24, 1263–1282. [Google Scholar] [CrossRef]
- Badran, M.; Khalyfa, A.; Ericsson, A.; Puech, C.; McAdams, Z.; Bender, S.B.; Gozal, D. Gut microbiota mediate vascular dysfunction in a murine model of sleep apnea: Effect of probiotics. Eur. Respir. J. 2022, 61, 2200002. [Google Scholar] [CrossRef]
- Xian, H.; Li, J.; Zhang, Y.; Li, D.; Zhu, Y.; Li, S.; Tan, Z.; Lin, Z.; Li, X.; Pan, Y. Antimetastatic Effects of Ganoderma lucidum Polysaccharide Peptide on B16-F10-luc-G5 Melanoma Mice with Sleep Fragmentation. Front. Pharmacol. 2021, 12, 650216. [Google Scholar] [CrossRef]
- Yao, Z.W.; Zhao, B.C.; Yang, X.; Lei, S.H.; Jiang, Y.M.; Liu, K.X. Relationships of sleep disturbance, intestinal microbiota, and postoperative pain in breast cancer patients: A prospective observational study. Sleep Breath. 2021, 25, 1655–1664. [Google Scholar] [CrossRef]
- González-Mercado, V.J.; Sarkar, A.; Penedo, F.J.; Pérez-Santiago, J.; McMillan, S.; Marrero, S.J.; Marrero-Falcón, M.A.; Munro, C.L. Gut microbiota perturbation is associated with acute sleep disturbance among rectal cancer patients. J. Sleep Res. 2020, 29, e12915. [Google Scholar] [CrossRef]

| Cell Dysfunction |
|---|
| Macrophage polarization |
| Natural killer T-cell deficiency |
| CD8 + T cells lymphocyte dysfunction |
| CD3 + gd-T-cell dysfunction |
| Stem-cell-like properties |
| Peripheral dendritic cell depletion |
| Biomarkers |
| VEGF and other pro-angiogenic molecules |
| TGF-beta 1 |
| TNF-alpha |
| Tryptophan metabolism |
| Cyclooxigenase-2 pathway |
| Cannabinoid receptors |
| Soluble PD-L1 |
| Endostatin |
| Endothelin-1 (and receptors) |
| Oxidative stress molecules |
| Paraspeckle protein-1 upregulation |
| Genetic |
| Glucose metabolism genes |
| HIF-1 gene expression (and variants) |
| Common key genes in OSA and cancer |
| Micro-RNA-320b and others |
| NF-kB factor genes |
| Other links |
| Exosomes |
| Microbiota |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-de-la-Torre, M.; Cubillos, C.; Veatch, O.J.; Garcia-Rio, F.; Gozal, D.; Martinez-Garcia, M.A. Potential Pathophysiological Pathways in the Complex Relationships between OSA and Cancer. Cancers 2023, 15, 1061. https://doi.org/10.3390/cancers15041061
Sánchez-de-la-Torre M, Cubillos C, Veatch OJ, Garcia-Rio F, Gozal D, Martinez-Garcia MA. Potential Pathophysiological Pathways in the Complex Relationships between OSA and Cancer. Cancers. 2023; 15(4):1061. https://doi.org/10.3390/cancers15041061
Chicago/Turabian StyleSánchez-de-la-Torre, Manuel, Carolina Cubillos, Olivia J. Veatch, Francisco Garcia-Rio, David Gozal, and Miguel Angel Martinez-Garcia. 2023. "Potential Pathophysiological Pathways in the Complex Relationships between OSA and Cancer" Cancers 15, no. 4: 1061. https://doi.org/10.3390/cancers15041061
APA StyleSánchez-de-la-Torre, M., Cubillos, C., Veatch, O. J., Garcia-Rio, F., Gozal, D., & Martinez-Garcia, M. A. (2023). Potential Pathophysiological Pathways in the Complex Relationships between OSA and Cancer. Cancers, 15(4), 1061. https://doi.org/10.3390/cancers15041061