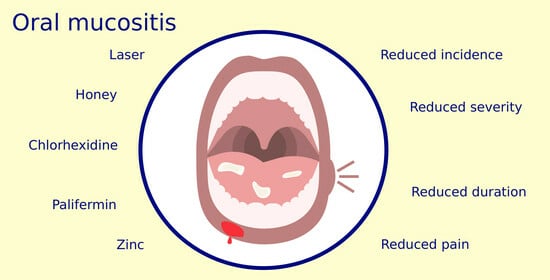
Oral Mucositis Management in Children under Cancer Treatment: A Systematic Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol Registation
2.2. Literature Search Strategy
2.3. Exclusion and Inclusion Criteria
2.4. Data Extraction and Quality Assessment
3. Results
3.1. Study Selection
3.2. Characteristics of the Included Studies
3.3. Therapeutic Efficacy of Each Intervention
3.4. Quality Assessement
4. Discussion
4.1. Low-Level Laser Therapy
4.2. Palifermin
4.3. Zinc
4.4. Chlorhexidine
4.5. Honey
4.6. Calcium Phosphate
4.7. Oral Cryotherapy
4.8. Olive Oil
4.9. Vitamin E
4.10. Glutamine
4.11. Other Therapies
4.12. Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Kalsbeek, R.J.; Hudson, M.M.; Mulder, R.L.; Ehrhardt, M.; Green, D.M.; Mulrooney, D.A.; Hakkert, J.; den Hartogh, J.; Nijenhuis, A.; van Santen, H.M.; et al. A Joint International Consensus Statement for Measuring Quality of Survival for Patients with Childhood Cancer. Nat. Med. 2023, 29, 1340–1348. [Google Scholar] [CrossRef]
- Silveira, F.M.; Wysocki, A.D.; Mendez, R.D.R.; Pena, S.B.; dos Santos, E.M.; Malaguti-Toffano, S.; dos Santos, V.B.; dos Santos, M.A. Impact of Chemotherapy Treatment on the Quality of Life of Patients with Cancer. ACTA Paul. Enferm. 2021, 34, eAPE00583. [Google Scholar] [CrossRef]
- Dyson, K.A.; Stover, B.D.; Grippin, A.; Mendez-Gomez, H.R.; Lagmay, J.; Mitchell, D.A.; Sayour, E.J. Emerging Trends in Immunotherapy for Pediatric Sarcomas. J. Hematol. Oncol. 2019, 12, 78. [Google Scholar] [CrossRef]
- Wang, J.J.; Lei, K.F.; Han, F. Tumor Microenvironment: Recent Advances in Various Cancer Treatments. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 3855–3864. [Google Scholar] [CrossRef]
- Ritwik, P. Dental Care for Patients with Childhood Cancers. Ochsner J. 2018, 18, 351–357. [Google Scholar] [CrossRef]
- Peterson, D.E.; Bensadoun, R.J.; Roila, F. Management of Oral and Gastrointestinal Mucositis: ESMO Clinical Practice Guidelines. Ann. Oncol. 2011, 22, vi78–vi84. [Google Scholar] [CrossRef]
- Chaveli-López, B. Oral Toxicity Produced by Chemotherapy: A Systematic Review. J. Clin. Exp. Dent. 2014, 6, e81–e90. [Google Scholar] [CrossRef]
- Miranda-Rius, J.; Brunet-Llobet, L.; Lahor-Soler, E.; Farré, M. Salivary Secretory Disorders, Inducing Drugs, and Clinical Management. Int. J. Med. Sci. 2015, 12, 811–824. [Google Scholar] [CrossRef]
- Riley, P.; Glenny, A.M.; Hua, F.; Worthington, H.V. Pharmacological Interventions for Preventing Dry Mouth and Salivary Gland Dysfunction Following Radiotherapy. Cochrane Database Syst. Rev. 2017, 7, CD012744. [Google Scholar] [CrossRef]
- Raj, R.; Thankappan, K.; Janakiram, C.; Iyer, S.; Mathew, A. Etiopathogenesis of Trismus in Patients with Head and Neck Cancer: An Exploratory Literature Review. Craniomaxillofac. Trauma. Reconstr. 2020, 13, 219–225. [Google Scholar] [CrossRef]
- Docimo, R.; Anastasio, M.D.; Bensi, C. Chemotherapy-Induced Oral Mucositis in Children and Adolescents: A Systematic Review. Eur. Arch. Paediatr. Dent. 2022, 23, 501–511. [Google Scholar] [CrossRef]
- Oronsky, B.; Goyal, S.; Kim, M.M.; Cabrales, P.; Lybeck, M.; Caroen, S.; Oronsky, N.; Burbano, E.; Carter, C.; Oronsky, A. A Review of Clinical Radioprotection and Chemoprotection for Oral Mucositis. Transl. Oncol. 2018, 11, 771–778. [Google Scholar] [CrossRef]
- Thomsen, M.; Vitetta, L. Adjunctive Treatments for the Prevention of Chemotherapy- and Radiotherapy-Induced Mucositis. Integr. Cancer Ther. 2018, 17, 1027–1047. [Google Scholar] [CrossRef]
- Elad, S.; Cheng, K.K.F.; Lalla, R.V.; Yarom, N.; Hong, C.; Logan, R.M.; Bowen, J.; Gibson, R.; Saunders, D.P.; Zadik, Y.; et al. MASCC/ISOO Clinical Practice Guidelines for the Management of Mucositis Secondary to Cancer Therapy. Cancer 2020, 126, 4423–4431. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Silva, W.; Gomes-Silva, W.; Zadik, Y.; Yarom, N.; Al-Azri, A.R.; Hong, C.H.L.; Ariyawardana, A.; Saunders, D.P.; Correa, M.E.; Arany, P.R.; et al. MASCC/ISOO Clinical Practice Guidelines for the Management of Mucositis: Sub-Analysis of Current Interventions for the Management of Oral Mucositis in Pediatric Cancer Patients. Support. Care Cancer 2021, 29, 3539–3562. [Google Scholar] [CrossRef]
- Idrees, M.; Kujan, O. A Curcumin-Based Oral Gel Has Potential Protective Efficacy against Oral Mucositis: In Vitro Study. J. Pers. Med. 2024, 14, 1. [Google Scholar] [CrossRef]
- Raza, A.; Karimyan, N.; Watters, A.; Emperumal, C.P.; Al-Eryani, K.; Enciso, R. Efficacy of Oral and Topical Antioxidants in the Prevention and Management of Oral Mucositis in Head and Neck Cancer Patients: A Systematic Review and Meta-Analyses. Support. Care Cancer 2022, 30, 8689–8703. [Google Scholar] [CrossRef]
- Ramezani, V.; Ghadirian, S.; Shabani, M.; Boroumand, M.A.; Daneshvar, R.; Saghafi, F. Efficacy of Curcumin for Amelioration of Radiotherapy-Induced Oral Mucositis: A Preliminary Randomized Controlled Clinical Trial. BMC Cancer 2023, 23, 354. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Wells, G.; Shea, J.B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analysis; The Ottawa Hospital Foundation: Ottawa ON, Canada, 2000. [Google Scholar]
- Abdulrhman, M.; Samir El Barbary, N.; Ahmed Amin, D.; Saeid Ebrahim, R. Honey and a Mixture of Honey, Beeswax, and Olive Oilpropolis Extract in Treatment of Chemotherapy-Induced Oral Mucositis: A Randomized Controlled Pilot Study. Pediatr. Hematol. Oncol. 2012, 29, 285–292. [Google Scholar] [CrossRef]
- Alkhouli, M.; Laflouf, M.; Alhaddad, M. Efficacy of Aloe-Vera Use for Prevention of Chemotherapy-Induced Oral Mucositis in Children with Acute Lymphoblastic Leukemia: A Randomized Controlled Clinical Trial. Compr. Child Adolesc. Nurs. 2021, 44, 49–62. [Google Scholar] [CrossRef]
- Alkhouli, M.; Laflouf, M.; Alhaddad, M. Evaluation of the Effectiveness of Olive Oil to Prevent Chemotherapy Induced Oral Mucositis: A Randomized Controlled Clinical Trial. Pediatr. Dent. J. 2019, 29, 123–131. [Google Scholar] [CrossRef]
- Amadori, F.; Bardellini, E.; Conti, G.; Pedrini, N.; Schumacher, R.F.; Majorana, A. Low-Level Laser Therapy for Treatment of Chemotherapy-Induced Oral Mucositis in Childhood: A Randomized Double-Blind Controlled Study. Lasers Med. Sci. 2016, 31, 1231–1236. [Google Scholar] [CrossRef]
- Badr, L.K.; El Asmar, R.; Hakim, S.; Saad, R.; Merhi, R.; Zahreddine, A.; Muwakkit, S. The Efficacy of Honey or Olive Oil on the Severity of Oral Mucositis and Pain Compared to Placebo (Standard Care) in Children with Leukemia Receiving Intensive Chemotherapy: A Randomized Controlled Trial (RCT). J. Pediatr. Nurs. 2023, 70, e48–e53. [Google Scholar] [CrossRef]
- Bardellini, E.; Amadori, F.; Schumacher, R.F.; D’Ippolito, C.; Porta, F.; Majorana, A. Efficacy of a Solution Composed by Verbascoside, Polyvinylpyrrolidone (PVP) and Sodium Hyaluronate in the Treatment of Chemotherapy-Induced Oral Mucositis in Children with Acute Lymphoblastic Leukemia. J. Pediatr. Hematol. Oncol. 2016, 38, 559–562. [Google Scholar] [CrossRef]
- Bostanabad, M.A.; Hiradfar, A.; Mohammadpoorasl, A.; Javadzadeh, Y.; Khalvati, B.; Alvandnezhad, T. The Effect of Mucoadhesive Gel Containing Satureja hortensis Extract 1% on Severity of Chemotherapy-Induced Mucositis Pain in Children: A Randomized Clinical Trial. Int. J. Pediatr. 2018, 6, 7605–7614. [Google Scholar] [CrossRef]
- Chang, Y.H.; Yu, M.S.; Wu, K.H.; Hsu, M.C.; Chiou, Y.H.; Wu, H.P.; Peng, C.T.; Chao, Y.H. Effectiveness of Parenteral Glutamine on Methotrexate-Induced Oral Mucositis in Children with Acute Lymphoblastic Leukemia. Nutr. Cancer. 2017, 69, 746–751. [Google Scholar] [CrossRef]
- Costa, E.M.M.d.B.; Fernandes, M.Z.; Quinder, L.B.; de Souza, L.B.; Pinto, L.P. Evaluation of an Oral Preventive Protocol in Children with Acute Lymphoblastic Leukemia. Pesqui. Odontol. Bras. 2003, 17, 147–150. [Google Scholar] [CrossRef]
- Cruz, L.B.; Ribeiro, A.S.; Rech, A.; Rosa, L.G.N.; Castro, C.G.; Brunetto, A.L. Influence of Low-Energy Laser in the Prevention of Oral Mucositis in Children with Cancer Receiving Chemotherapy. Pediatr. Blood Cancer 2007, 48, 435–440. [Google Scholar] [CrossRef]
- De Castro, J.F.L.; Abreu, E.G.F.; Correia, A.V.L.; Da Mota Vasconcelos Brasil, C.; Da Cruz Perez, D.E.; De Paula Ramos Pedrosa, F. Low-Level Laser in Prevention and Treatment of Oral Mucositis in Pediatric Patients with Acute Lymphoblastic Leukemia. Photomed. Laser Surg. 2013, 31, 613–618. [Google Scholar] [CrossRef] [PubMed]
- De Koning, B.A.E.; Philipsen-Geerling, B.; Hoijer, M.; Hählen, K.; Büller, H.A.; Pieters, R. Protection against Chemotherapy Induced Mucositis by TGF-Β2 in Childhood Cancer Patients: Results from a Randomized Cross-over Study. Pediatr. Blood Cancer 2007, 48, 532–539. [Google Scholar] [CrossRef]
- Funato, M.; Ozeki, M.; Suzuki, A.; Ishihara, M.; Kobayashi, R.; Nozawa, A.; Yasue, S.; Endo-Ohnishi, S.; Fukao, T.; Itoh, Y. Prophylactic Effect of Polaprezinc, a Zinc-l-Carnosine, against Chemotherapy-Induced Oral Mucositis in Pediatric Patients Undergoing Autologous Stem Cell Transplantation. Anticancer Res. 2018, 38, 4691–4697. [Google Scholar] [CrossRef]
- Gandemer, V.; Le Deley, M.-C.; Dollfus, C.; Auvrignon, A.; Bonnaure-Mallet, M.; Duval, M.; De Lumley, L.; Hartmann, O.; Mechinaud, F.; Sirvent, N.; et al. Multicenter Randomized Trial of Chewing Gum for Preventing Oral Mucositis in Children Receiving Chemotherapy. J. Pediatr. Hematol. Oncol. 2007, 29, 86–94. [Google Scholar] [CrossRef]
- Gobbo, M.; Verzegnassi, F.; Ronfani, L.; Zanon, D.; Melchionda, F.; Bagattoni, S.; Majorana, A.; Bardellini, E.; Mura, R.; Piras, A.; et al. Multicenter Randomized, Double-Blind Controlled Trial to Evaluate the Efficacy of Laser Therapy for the Treatment of Severe Oral Mucositis Induced by Chemotherapy in Children: LaMPO RCT. Pediatr. Blood Cancer 2018, 65, e27098. [Google Scholar] [CrossRef]
- Gutiérrez-Vargas, R.; Villasis-Keever, M.Á.; Portilla-Robertson, J.; Ascencio-Montiel, I.D.J.; Zapata-Tarrés, M. Effect of Zinc on Oropharyngeal Mucositis in Children with Acute Leukemia Undergoing Chemotherapy. Med. Oral. Patol. Oral. Cir. Bucal. 2020, 25, e791–e798. [Google Scholar] [CrossRef]
- Immonen, E.; Aine, L.; Nikkilä, A.; Parikka, M.; Grönroos, M.; Vepsäläinen, K.; Palmu, S.; Helminen, M.; Peltomäki, T.; Lohi, O. Randomized Controlled and Double-Blinded Study of Caphosol versus Saline Oral Rinses in Pediatric Patients with Cancer. Pediatr. Blood Cancer 2020, 67, e28520. [Google Scholar] [CrossRef]
- Kamsvåg, T.; Svanberg, A.; Legert, K.G.; Arvidson, J.; von Essen, L.; Mellgren, K.; Toporski, J.; Winiarski, J.; Ljungman, G. Prevention of Oral Mucositis with Cryotherapy in Children Undergoing Hematopoietic Stem Cell Transplantations—A Feasibility Study and Randomized Controlled Trial. Support. Care Cancer 2020, 28, 4869–4879. [Google Scholar] [CrossRef]
- Khurana, H.; Pandey, R.K.; Saksena, A.K.; Kumar, A. An Evaluation of Vitamin E and Pycnogenol in Children Suffering from Oral Mucositis during Cancer Chemotherapy. Oral. Dis. 2013, 19, 456–464. [Google Scholar] [CrossRef]
- Kobya, H.B.; Güdücü Tüfekci, F. Honey Prevents Oral Mocositis in Children Undergoing Chemotherapy: A Quasi-Experimental Study with a Control Group. Complement. Ther. Med. 2016, 29, 132–140. [Google Scholar] [CrossRef]
- Lauritano, D.; Petruzzi, M.; Di Stasio, D.; Lucchese, A. Clinical Effectiveness of Palifermin in Prevention and Treatment of Oral Mucositis in Children with Acute Lymphoblastic Leukaemia: A Case-Control Study. Int. J. Oral. Sci. 2014, 6, 27–30. [Google Scholar] [CrossRef]
- Lucchese, A.; Matarese, G.; Ghislanzoni, L.H.; Gastaldi, G.; Manuelli, M.; Gherlone, E. Efficacy and Effects of Palifermin for the Treatment of Oral Mucositis in Patients Affected by Acute Lymphoblastic Leukemia. Leuk. Lymphoma 2016, 57, 820–827. [Google Scholar] [CrossRef]
- Medeiros-Filho, J.B.; Maia Filho, E.M.; Ferreira, M.C. Laser and Photochemotherapy for the Treatment of Oral Mucositis in Young Patients: Randomized Clinical Trial. Photodiagnosis Photodyn. Ther. 2017, 18, 39–45. [Google Scholar] [CrossRef]
- Morris, J.; Rudebeck, M.; Neudorf, S.; Moore, T.; Duerst, R.; Shah, A.J.; Graham, M.; Aquino, V.; Morris, C.; Olsson, B. Safety, Pharmacokinetics, and Efficacy of Palifermin in Children and Adolescents with Acute Leukemias Undergoing Myeloablative Therapy and Allogeneic Hematopoietic Stem Cell Transplantation: A Pediatric Blood and Marrow Transplant Consortium Trial. Biol. Blood Marrow Transplant. 2016, 22, 1247–1256. [Google Scholar] [CrossRef]
- Mubaraki, S.; Pani, S.C.; Alseraihy, A.; Abed, H.; Alkhayal, Z. The Efficacy of Two Different Oral Hygiene Regimens on the Incidence and Severity of Oral Mucositis in Pediatric Patients Receiving Hematopoietic Stem Cell Transplantation: A Prospective Interventional Study. Spec. Care Dent. 2020, 40, 566–573. [Google Scholar] [CrossRef]
- Nunes, L.F.M.; de Arruda, J.A.A.; Souza, A.F.; Silva, R.C.C.; Lanza, C.R.M.; Kakehasi, F.M.; Mesquita, R.A.; Abreu, L.G.; Travassos, D.V.; Silva, T.A. Prophylactic Photobiomodulation Therapy Using 660 Nm Diode Laser for Oral Mucositis in Paediatric Patients under Chemotherapy: 5-Year Experience from a Brazilian Referral Service. Lasers Med. Sci. 2020, 35, 1857–1866. [Google Scholar] [CrossRef]
- Pereira Pinto, L.; de Souza, L.B.; Gordón-Núñez, M.A.; Soares, R.C.; de Brito Costa, E.M.M.; de Aquino, A.R.L.; Fernandes, M.Z. Prevention of Oral Lesions in Children with Acute Lymphoblastic Leukemia. Int. J. Pediatr. Otorhinolaryngol. 2006, 70, 1847–1851. [Google Scholar] [CrossRef]
- Prakash, S.; Meena, J.P.; Gupta, A.K.; Bakhshi, S.; Velpandian, T.; Pandey, R.M.; Seth, R. Ketamine Mouthwash versus Placebo in the Treatment of Severe Oral Mucositis Pain in Children with Cancer: A Randomized Double-Blind Placebo-Controlled Trial. Pediatr. Blood Cancer 2020, 67, e28573. [Google Scholar] [CrossRef]
- Raphael, M.F.; den Boer, A.M.; Kollen, W.J.W.; Mekelenkamp, H.; Abbink, F.C.H.; Kaspers, G.J.L.; Zomer-Kooijker, K.; Molmans, B.H.W.; Tissing, W.J.E. Caphosol, a Therapeutic Option in Case of Cancer Therapy-Induced Oral Mucositis in Children?: Results from a Prospective Multicenter Double Blind Randomized Controlled Trial. Support. Care Cancer 2014, 22, 3–6. [Google Scholar] [CrossRef]
- Rathe, M.; De Pietri, S.; Wehner, P.S.; Frandsen, T.L.; Grell, K.; Schmiegelow, K.; Sangild, P.T.; Husby, S.; Müller, K. Bovine Colostrum Against Chemotherapy-Induced Gastrointestinal Toxicity in Children with Acute Lymphoblastic Leukemia: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Parenter. Enter. Nutr. 2020, 44, 337–347. [Google Scholar] [CrossRef]
- Reyad, F.A.; Elsayed, N.M.; El Chazli, Y. Photobiomodulation for Chemotherapy-Induced Oral Mucositis in Leukemic Children: A Randomized Controlled Clinical Trial. Oral. Dis. 2023, 29, 2239–2247. [Google Scholar] [CrossRef]
- Sato, A.; Saisho-Hattori, T.; Koizumi, Y.; Minegishi, M.; Iinuma, K.; Imaizumi, M. Prophylaxis of Mucosal Toxicity by Oral Propantheline and Cryotherapy in Children with Malignancies Undergoing Myeloablative Chemo-Radiotherapy. Tohoku J. Exp. Med. 2006, 210, 315–320. [Google Scholar] [CrossRef]
- Shah, D.; Gupta, A.; Meena, J.P.; Kumar Gupta, A.; Velpandian, T.; Pandey, R.M.; Makkar, H.; Seth, R. Efficacy and Safety of Zinc in the Prevention of Oral Mucositis in Children with Cancer Receiving Intensified Chemotherapy: A Randomized Double-Blind Placebo-Controlled Trial. Pediatr. Blood Cancer 2023, 70, e30309. [Google Scholar] [CrossRef]
- Shahrabi, M.; Solduzian, M.; Babaie, M.H.; Mousavi, S.A.; Goodarzi, N.; Ravari, N.S.; Sadeghi, K. The Effects of a Combination Oral Spray (Mucosamin®) for the Prevention of Oral Mucositis in Pediatric Patients Undergoing Hematopoietic Stem Cell Transplantation: A Double Blind Randomized Clinical Trial. Support. Care Cancer 2022, 30, 7963–7972. [Google Scholar] [CrossRef]
- Soares, A.d.S.; Wanzeler, A.M.V.; Cavalcante, G.H.S.; Barros, E.M.d.S.; Carneiro, R.d.C.M.; Tuji, F.M. Therapeutic Effects of Andiroba (Carapa guianensis Aubl) Oil, Compared to Low Power Laser, on Oral Mucositis in Children Underwent Chemotherapy: A Clinical Study. J. Ethnopharmacol. 2021, 264, 113365. [Google Scholar] [CrossRef]
- Soto, M.; Lalla, R.V.; Gouveia, R.V.; Zecchin, V.G.; Seber, A.; Lopes, N.N.F. Pilot Study on the Efficacy of Combined Intraoral and Extraoral Low-Level Laser Therapy for Prevention of Oral Mucositis in Pediatric Patients Undergoing Hematopoietic Stem Cell Transplantation. Photomed. Laser Surg. 2015, 33, 540–546. [Google Scholar] [CrossRef]
- Sung, L.; Tomlinson, G.A.; Greenberg, M.L.; Koren, G.; Judd, P.; Ota, S.; Feldman, B.M. Serial Controlled N-of-1 Trials of Topical Vitamin E as Prophylaxis for Chemotherapy-Induced Oral Mucositis in Paediatric Patients. Eur. J. Cancer 2007, 43, 1269–1275. [Google Scholar] [CrossRef]
- Vitale, M.C.; Modaffari, C.; Decembrino, N.; Zhou, F.X.; Zecca, M.; Defabianis, P. Preliminary Study in a New Protocol for the Treatment of Oral Mucositis in Pediatric Patients Undergoing Hematopoietic Stem Cell Transplantation (HSCT) and Chemotherapy (CT). Lasers Med. Sci. 2017, 32, 1423–1428. [Google Scholar] [CrossRef]
- Widjaja, N.A.; Pratama, A.; Prihaningtyas, R.A.; Irawan, R.; Ugrasena, I. Efficacy Oral Glutamine to Prevent Oral Mucositis and Reduce Hospital Costs during Chemotherapy in Children with Acute Lymphoblastic Leukemia. Asian Pac. J. Cancer Prev. 2020, 21, 2117–2121. [Google Scholar] [CrossRef]
- Hafner, D.; Hrast, P.; Tomaževič, T.; Jazbec, J.; Kavčič, M. Photobiomodulation for Chemotherapy-Induced Oral Mucositis in Pediatric Patients. Biomolecules 2023, 13, 418. [Google Scholar] [CrossRef]
- Lai, C.C.; Chen, S.Y.; Tu, Y.K.; Ding, Y.W.; Lin, J.J. Effectiveness of Low Level Laser Therapy versus Cryotherapy in Cancer Patients with Oral Mucositis: Systematic Review and Network Meta-Analysis. Crit. Rev. Oncol. Hematol. 2021, 160, 103276. [Google Scholar] [CrossRef]
- Guimaraes, D.M.; Ota, T.M.N.; Da Silva, D.A.C.; Almeida, F.D.L.D.S.; Schalch, T.D.; Deana, A.M.; Junior, J.M.A.; Fernandes, K.P.S. Low-Level Laser or LED Photobiomodulation on Oral Mucositis in Pediatric Patients under High Doses of Methotrexate: Prospective, Randomized, Controlled Trial. Support. Care Cancer 2021, 29, 6441–6447. [Google Scholar] [CrossRef]
- Patel, P.; Robinson, P.D.; Baggott, C.; Gibson, P.; Ljungman, G.; Massey, N.; Ottaviani, G.; Phillips, R.; Revon-Rivière, G.; Treister, N.; et al. Clinical Practice Guideline for the Prevention of Oral and Oropharyngeal Mucositis in Pediatric Cancer and Hematopoietic Stem Cell Transplant Patients: 2021 Update. Eur. J. Cancer 2021, 154, 92–101. [Google Scholar] [CrossRef]
- Coutsouvelis, J.; Corallo, C.; Spencer, A.; Avery, S.; Dooley, M.; Kirkpatrick, C.M. A Meta-Analysis of Palifermin Efficacy for the Management of Oral Mucositis in Patients with Solid Tumours and Haematological Malignancy. Crit. Rev. Oncol. Hematol. 2022, 172, 103606. [Google Scholar] [CrossRef]
- Mazhari, F.; Shirazi, A.S.; Shabzendehdar, M. Management of Oral Mucositis in Pediatric Patients Receiving Cancer Therapy: A Systematic Review and Meta-Analysis. Pediatr. Blood Cancer 2019, 66, e27403. [Google Scholar] [CrossRef]
- Efthymakis, K.; Neri, M. The Role of Zinc L-Carnosine in the Prevention and Treatment of Gastrointestinal Mucosal Disease in Humans: A Review. Clin. Res. Hepatol. Gastroenterol. 2022, 46, 101954. [Google Scholar] [CrossRef]
- Yarom, N.; Hovan, A.; Bossi, P.; Ariyawardana, A.; Jensen, S.B.; Gobbo, M.; Saca-Hazboun, H.; Kandwal, A.; Majorana, A.; Ottaviani, G.; et al. Systematic Review of Natural and Miscellaneous Agents for the Management of Oral Mucositis in Cancer Patients and Clinical Practice Guidelines—Part 1: Vitamins, Minerals, and Nutritional Supplements. Support. Care Cancer 2019, 27, 3997–4010. [Google Scholar] [CrossRef]
- Cheng, K.K.F.; Chang, A.M. Palliation of Oral Mucositis Symptoms in Pediatric Patients Treated with Cancer Chemotherapy. Cancer Nurs. 2003, 26, 476–484. [Google Scholar] [CrossRef]
- Cheng, K.K.F.; Chang, A.M.; Yuen, M.P. Prevention of Oral Mucositis in Paediatric Patients Treated with Chemotherapy: A Randomised Crossover Trial Comparing Two Protocols of Oral Care. Eur. J. Cancer 2004, 40, 1208–1216. [Google Scholar] [CrossRef]
- Hurrell, L.; Burgoyne, L.; Logan, R.; Revesz, T.; Gue, S. The Management of Pediatric Oncology Inpatients with Oral Mucositis. J. Pediatr. Hematol. Oncol. 2019, 41, E510–E516. [Google Scholar] [CrossRef]
- Jicman, D.; Sârbu, M.I.; Fotea, S.; Nechifor, A.; Bălan, G.; Anghele, M.; Vasile, C.I.; Niculeț, E.; Sârbu, N.; Rebegea, L.F.; et al. Oral Mucositis Induced by Chemoradiotherapy in Head and Neck Cancer-A Short Review about the Therapeutic Management and the Benefits of Bee Honey. Medicina 2022, 58, 751. [Google Scholar] [CrossRef]



| Author and Publication Year | Study Type | Sample Size | Age Range and Mean (Years) | Antineoplastic Intervention | Location | Ref. |
|---|---|---|---|---|---|---|
| Abdulrhman M et al., 2012 | RCT | 90 (57 M, 33 F) | 2–18 (6.9 ± 3.8) | CT | Egypt | [21] |
| Alkhouli M et al., 2021 | RCT | 22 (14 M, 8 F) | 3–6 (4.6) | CT | Syria | [22] |
| Alkhouli M et al., 2019 | RCT | 22 (12 M, 10 F) | 4–6 (5.4) | CT | Syria | [23] |
| Amadori F et al., 2016 | RCT | 123 (56 M, 67 F) | 3–18 (9.8 ± 3.3) | CT or HSCT | Italy | [24] |
| Badr LK et al., 2023 | RCT | 42 (23 M, 19 F) | 5–17 (10.9 ± 4.1) | CT | Lebanon | [25] |
| Bardellini E et al., 2016 | RCT | 56 (22 M, 34 F) | 5–18 (7.0 ± 1.8) | CT | Italy | [26] |
| Bostanabad MA et al., 2018 | RCT | 60 (40 M, 20 F) | 3–14 (7.6 ± 3.4) | CT | Iran | [27] |
| Chang YH et al., 2017 | Retrospective | 96 (49 M, 37 F) | 0–18 (8.8 ± 5.2) | CT | Taiwan | [28] |
| Costa EM et al., 2003 | Clinical trial | 14 (n.a.) | 2–10 (7.0) | CT | Brazil | [29] |
| Cruz LB et al., 2007 | RCT | 60 (39 M, 21 F) | 3–18 (8.7 ± 4.3) | CT or HSCT | Brazil | [30] |
| de Castro JFL et al., 2013 | Clinical trial | 40 (27 M, 13 F) | 1–18 (6.8) | CT | Brazil | [31] |
| de Koning BA et al., 2007 | RCT | 25 (17 M, 8 F) | 0–18 (8.0) | CT | Netherlands | [32] |
| Funato M et al., 2018 | Retrospective | 16 (9 M, 7 F) | 1–18 (7.2) | HSCT | Japan | [33] |
| Gandemer V et al., 2007 | RCT | 145 (93 M, 52 F) | 5–18 (11.6) | CT | France | [34] |
| Gobbo M et al., 2018 | RCT | 101 (54 M, 47 F) | 3–18 (11.9) | CT | Italy | [35] |
| Gutiérrez-Vargas R et al., 2020 | Quasi-experimental | 49 (29 M, 20 F) | 8–16 (11.1 ± 2.7) | CT | Mexico | [36] |
| Immonen E et al., 2020 | RCT | 45 (25 M, 20 F) | 2–18 (6.5) | CT | Finland | [37] |
| Kamsvåg T et al., 2020 | RCT | 49 (26 M, 23 F) | 4–17 (11.3 ± 3.8) | HSCT | Sweden | [38] |
| Khurana H et al., 2013 | RCT | 72 (57 M, 15 F) | 6–15 (9.3 ± 2.6) | CT | India | [39] |
| Kobya HB et al., 2016 | Quasi-experimental | 76 (38 M, 38 F) | 6–18 (10.9 ± 4.1) | CT | Turkey | [40] |
| Lauritano D et al., 2014 | Case-control study | 40 (21 M, 19 F) | 7–16 (11.0) | RT | Italy | [41] |
| Lucchese A et al., 2016 | RCT | 54 (26 M, 28 F) | 7–16 (11.0) | HSCT | Italy | [42] |
| Medeiros-Filho JB et al., 2017 | RCT | 15 (14 M, 1 F) | 3–16 (9.5) | CT and CT + RT | Brazil | [43] |
| Morris J et al., 2016 | Clinical trial | 27 (15 M, 12 F) | 1–16 (8.5) | CT + RT | United States | [44] |
| Mubaraki S et al., 2020 | RCT | 45 (20 M, 25 F) | 7–10 (7.7 ± 3.1) | HSCT | Saudi Arabia | [45] |
| Nunes LFM et al., 2020 | Retrospective | 148 (82 M, 66 F) | 1–17 (9.2 ± 4.6) | HSCT | Brazil | [46] |
| Pinto LP et al., 2006 | Clinical trial | 33 (n.a.) | 2–15 (8.5) | CT | Brazil | [47] |
| Prakash S et al., 2020 | RCT | 44 (35 M, 9 F) | 8–18 (11.5 ± 2.9) | CT | India | [48] |
| Raphael MF et al., 2014 | RCT | 29 (19 M, 10 F) | 4–18 (11.3 ± 3.9) | CT or HSCT | Netherlands | [49] |
| Rathe M et al., 2020 | RCT | 62 (32 M, 30 F) | 1–18 (5) | CT | Denmark | [50] |
| Reyad D et al., 2022 | RCT | 44 (21 M, 23 F) | 2–14 (7.4 ± 2.5) | CT | Egypt | [51] |
| Sato A et al., 2006 | Retrospective | 24 (19 M, 5 F) | 2–16 (7.0) | CT | Japan | [52] |
| Shah D et al., 2023 | RCT | 90 (n.a.) | 3–18 (6.0) | CT | India | [53] |
| Shahrabi M et al., 2022 | RCT | 60 (n.a.) | 4–18 (11.9 ± 5.5) | HSCT | Iran | [54] |
| Soares ADS et al., 2021 | RCT | 60 (n.a.) | 6–12 (9.0) | CT | Brazil | [55] |
| Soto M et al., 2015 | Clinical trial | 24 (17 M, 7 F) | 2–16 (7.9) | HSCT | Brazil | [56] |
| Sung L et al., 2007 | RCT | 16 (10 M, 6 F) | 6–18 (12.7) | CT | Canada | [57] |
| Vitale M et al., 2017 | RCT | 16 (n.a.) | 3–18 (10.5) | CT or HSCT | Italy | [58] |
| Widjaja NA et al., 2020 | RCT | 48 (31 M, 17 F) | 1–18 (6.3) | CT | Indonesia | [59] |
| Author and Publication Year | Oral Mucositis Treatment Type | Oral Mucositis Treatment Duration (Days) | Oral Mucositis Outcome | Ref. |
|---|---|---|---|---|
| Abdulrhman M et al., 2012 | Honey | 10 | Decreased duration | [21] |
| Mixture of honey, olive oil–propolis extract, and beeswax | 10 | Decreased duration | ||
| Alkhouli M et al., 2021 | 70% Aloe vera | (n.a.) | Decreased severity | [22] |
| Alkhouli M et al., 2019 | Olive oil | (n.a.) | Decreased incidence and decreased severity | [23] |
| Amadori F et al., 2016 | LLLT | 4 | Decreased pain | [24] |
| Badr LK et al., 2023 | Manuka honey | 7 | Decreased severity and decreased pain | [25] |
| Olive oil | 7 | Decreased pain | ||
| Bardellini E et al., 2016 | Mucosyte® mouthwash | 8 | Decreased severity and decreased pain | [26] |
| Bostanabad MA et al., 2018 | Satureja hortensis extract mucoadhesive gel of 1% | 5 | Decreased pain | [27] |
| Chang YH et al., 2017 | Parenteral glutamine | 3 | Decreased incidence and decreased severity | [28] |
| Costa EM et al., 2003 | 0.12% Chlorhexidine | 10 | Decreased incidence and decreased severity | [29] |
| Cruz LB et al., 2007 | LLLT | 5 | No effect | [30] |
| de Castro JFL et al., 2013 | LLLT | 5 | Decreased incidence and decreased severity | [31] |
| de Koning BA et al., 2007 | TGF-β2 | (n.a.) | No effect | [32] |
| Funato M et al., 2018 | Polaprezinc sodium alginate suspension | (n.a.) | Decreased incidence | [33] |
| Gandemer V et al., 2007 | Chewing gum | 3 | No effect | [34] |
| Gobbo M et al., 2018 | LLLT | 4 | Decreased severity and decreased pain | [35] |
| Gutiérrez-Vargas R et al., 2020 | Zinc gluconate | (n.a.) | Decreased severity, decreased duration and decreased pain | [36] |
| Immonen E et al., 2020 | Calcium phosphate rinse (Caphosol®) | 7 | No effect | [37] |
| Kamsvåg T et al., 2020 | Oral cryotherapy | 13 | No effect | [38] |
| Khurana H et al., 2013 | Vitamin E | 7 | Decreased severity | [39] |
| Pycnogenol | 7 | Decreased severity | ||
| Kobya HB et al., 2016 | Honey | 21 | Decreased severity and decreased duration | [40] |
| Lauritano D et al., 2014 | Palifermin | 21 | Decreased severity | [41] |
| Lucchese A et al., 2016 | Palifermin | 3–6 | Decreased incidence, decreased severity and decreased duration | [42] |
| Medeiros-Filho JB et al., 2017 | LLLT and PCT | 8 | Decreased severity | [43] |
| Morris J et al., 2016 | Palifermin | 6 | Decreased incidence | [44] |
| Mubaraki S et al., 2020 | Calcium phosphate rinse | (n.a.) | No effect | [45] |
| Nunes LFM et al., 2020 | LLLT | (n.a.) | Decreased incidence and decreased severity | [46] |
| Pinto LP et al., 2006 | 0.12% Chlorhexidine | 10 | Decreased incidence | [47] |
| Prakash S et al., 2020 | Ketamine mouthwash | (n.a.) | No effect | [48] |
| Raphael MF et al., 2014 | Calcium phosphate rinse (Caphosol®) | (n.a.) | No effect | [49] |
| Rathe M et al., 2020 | Bovine colostrum | 29 | Decreased severity | [50] |
| Reyad F et al., 2022 | LLLT | 4 | Decreased severity | [51] |
| Sato A et al., 2006 | Oral cryotherapy and propantheline | (n.a.) | Decreased incidence and decreased severity | [52] |
| Shah D et al., 2023 | Zinc gluconate syrup | 14 | No effect | [53] |
| Shahrabi M et al., 2022 | Mucosamin® oral spray | 14 | Decreased incidence, decreased severity and decreased duration | [54] |
| Soares ADS et al., 2021 | 3% Andiroba (Carapa guianensis) orabase | 11 | Decreased severity and decreased pain | [55] |
| Soto M et al., 2015 | LLLT | 22 | Decreased incidence and decreased severity | [56] |
| Sung L et al., 2007 | Vitamin E | 14 | No effect | [57] |
| Vitale M et al., 2017 | HPLT | 4 | Decreased severity and decreased pain | [58] |
| Widjaja NA et al., 2020 | Oral glutamine | 14 | Decreased incidence and decreased severity | [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braguês, R.; Marvão, M.F.; Correia, P.; Silva, R.M. Oral Mucositis Management in Children under Cancer Treatment: A Systematic Review. Cancers 2024, 16, 1548. https://doi.org/10.3390/cancers16081548
Braguês R, Marvão MF, Correia P, Silva RM. Oral Mucositis Management in Children under Cancer Treatment: A Systematic Review. Cancers. 2024; 16(8):1548. https://doi.org/10.3390/cancers16081548
Chicago/Turabian StyleBraguês, Ricardo, Maria Francisca Marvão, Patrícia Correia, and Raquel M. Silva. 2024. "Oral Mucositis Management in Children under Cancer Treatment: A Systematic Review" Cancers 16, no. 8: 1548. https://doi.org/10.3390/cancers16081548
APA StyleBraguês, R., Marvão, M. F., Correia, P., & Silva, R. M. (2024). Oral Mucositis Management in Children under Cancer Treatment: A Systematic Review. Cancers, 16(8), 1548. https://doi.org/10.3390/cancers16081548