T-Cadherin Expression in Melanoma Cells Stimulates Stromal Cell Recruitment and Invasion by Regulating the Expression of Chemokines, Integrins and Adhesion Molecules
Abstract
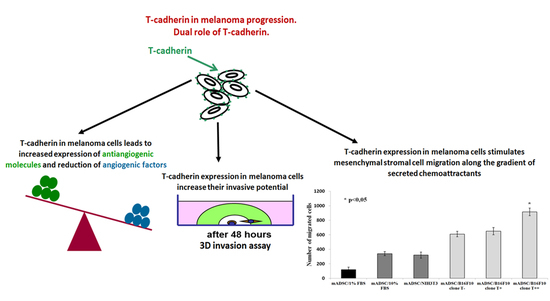
:1. Introduction
2. Results
2.1. Expression of T-cadherin in Mouse Melanoma Cell Clones
2.2. Effect of B16F10 Clones on Mouse Adipose Derived Stromal Cells Migration in Co-Culture Experiments



2.3. Effect of B16F10 Clones on mADSC Proliferation in Co-Culture Experiments

2.4. Effect of T-cadherin Expression on Invasiveness of B16F10 Melanoma Clones in Vitro


2.5. Effect of T-cadherin on Gene Expression

| Fold Regulation Compared to Control Clone | Gene | |
|---|---|---|
| B16F10 Clone T+ | B16F10 Clone T++ | |
| −10 | −11 | Tyrosine kinase receptor 1 (Tie 1) |
| 2 | 2 | Angiopoietin 2 |
| 2 | 3 | Stabilin 1 |
| 4 | 5.5 | Forkhead box F1a (Foxf1a) |
| 5 | 7.8 | Chemokine (C-X-C motif) ligand 10 (CXCL10) |
| 8.7 | 6.3 | Heparanase |
| Fold Regulation Compared to Control Clone | Gene | |
|---|---|---|
| B16F10 Clone T+ | B16F10 Clone T++ | |
| −1,4 | −3.1 | Chemokine (C-C motif) ligand 9 (CCL9) |
| 1.5 | 2 | Chemokine C-C motif receptor-like 1 (CCRL1) |
| 1.8 | 2.5 | Chemokine (C-X-C motif) ligand 11 (CXCL11) |
| 2 | 3.2 | Nuclear factor of kappa light chain gene enhancer in B cells, p105 (Nfkb1) |
| 2.4 | 2.2 | CKLF-like MARVEL transmembrane domain containing 3 (CMTM3) |
| 3.3 | 2.4 | Chemokine (C-C motif) ligand 5 (CCL5) |
| 2.9 | 15.3 | Chemokine (C-X-C motif) ligand 10 (CXCL10) |
| 5.7 | 17.6 | Chemokine C-X-C motif receptor 7 (CXCR7) |

| Fold Regulation Compared to Control Clone | Gene | |
|---|---|---|
| B16F10 Clone T+ | B16F10 Clone T++ | |
| −2.3 | −2.1 | A distintegrin-like and metallopeptidase (reprolysin type) with thrombospondin type 1 motif, 5 (Adamts 5) |
| 1 | 3.3 | Integrin alpha E, epithelial-associated |
| 1.1 | 2.3 | Integrin beta 3 |
| 1.2 | 3.9 | Fibronectin 1 |
| 1.3 | 2.6 | Integrin alpha 5 (fibronectin receptor alpha) |
| 1.3 | 3.2 | Matrix metallopeptidase 14 (MMP14) |
| 1.8 | 2.5 | Integrin alpha V |
| 2.3 | 2 | Transforming growth factor, beta induced (TGFbi) |
| 4 | 15 | Cadherin 1 |

3. Discussion
4. Materials and Methods
4.1. Cell Culture and Generation of Stable Cell Lines
4.2. Preparation of Tumor Conditioned Media
4.3. Western Blot
4.4. Quantitative RT PCR
| Gene | Annealing Temperature | Forward 5′-3′ | Reverse 5′-3′ |
|---|---|---|---|
| GAPDH | 60 | GACCCCTTCATTGACCTCAACTAC | TGGTGGTGCAGGATGCATTGCTGA |
| β-actin | 61 | AGTGTGACGTTGACATCCGTA | GCCAGAGCAGTAATCTCCTTCT |
| T-cadherin | 60 | TTCAGCAGAAAGTGTTCCATAT | GTGCATGGACGAACAGAGT |
4.5. RT PCR Array Assay
4.6. Immunofluorescence and Confocal Imaging
4.7. Three-Dimension Invasion Assay in Vitro
4.8. TranswellTM Co-Culture Experiments
4.9. Statistics
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wheelock, M.J.; Johnson, K.R. Cadherins as modulators of cellular phenotype. Annu. Rev. Cell Dev. Biol. 2003, 19, 207–235. [Google Scholar] [CrossRef] [PubMed]
- Ranscht, B.; Dours-Zimmermann, M.T. T-cadherin, a novel cadherin cell adhesion molecule in the nervous system lacks the conserved cytoplasmic region. Neuron 1991, 7, 391–402. [Google Scholar] [CrossRef]
- Philippova, M.P.; Bochkov, V.N.; Stambolsky, D.V.; Tkachuk, V.A.; Resink, T.J. T-cadherin and signal-transducing molecules co-localize in caveolin-rich membrane domain of vascular smooth muscle cells. FEBS Lett. 1998, 429, 207–210. [Google Scholar] [CrossRef]
- Philippova, M.; Ivanov, D.; Tkachuk, V.; Erne, P.; Resink, T.J. Polarisation of T-cadherin to the leading edge of migrating vascular cells in vitro: A function in vascular cell motility? Histochem. Cell Biol. 2003, 120, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Andreeva, A.V.; Kutuzov, M.A. Cadherin 13 in cancer. Genes Chromosomes Cancer 2010, 49, 775–790. [Google Scholar] [CrossRef] [PubMed]
- Pfaff, D.; Philippova, M.; Kyriakakis, E.; Maslova, K.; Rupp, K.; Buechner, S.A.; Lezzi, G.; Spagnoli, G.C.; Erne, P.; Resink, T.J. Paradoxical effects of T-cadherin on squamous cell carcinoma: Up- and down-regulation increase xenograft growth by distinct mechanisms. J. Pathol. 2011, 225, 512–524. [Google Scholar] [CrossRef] [PubMed]
- Rubina, K.; Sysoeva, V.; Semina, E.; Kalinina, N.; Yurlova, E.; Khlebnikova, A.; Molochkov, V. Malignant transformation in skin is associated with the loss of T-cadherin expression in human keratinocytes and heterogeneity in T-cadherin expression in tumor vasculature. In Tumor Angiogenesis; Sophia, R., Ed.; INTECH: Rijeka, Croatia, 2012; pp. 135–166. [Google Scholar]
- Kuphal, S.; Martyn, A.C.; Pedley, J.; Crowther, L.M.; Bonazzi, V.F.; Parsons, P.G.; Bosserhoff, A.K.; Hayward, N.K.; Boyle, G.M. H-cadherin expression reduces invasion of malignant melanoma. Pigment Cell Melanoma Res. 2009, 22, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Matsuyoshi, N.; Liang, S.B.; Takeuchi, T.; Ohtsuki, Y.; Miyachi, Y. Expression of T-cadherin in basal keratinocytes of skin. J. Investig. Dermatol. 2002, 118, 1080–1084. [Google Scholar] [CrossRef] [PubMed]
- Rubina, K.A.; Yurlova, E.I.; Sysoeva, V.Y.; Semina, E.V.; Kalinina, N.I.; Poliakov, A.A.; Mikhaylova, I.N.; Andronova, N.V.; Treshalina, H.M. T-cadherin stimulates melanoma cell proliferation and mesenchymal stromal cell recruitment, but inhibits angiogenesis in a mouse melanoma model. In Research Direction in Tumor Angiogenesis; Chai, J., Ed.; INTECH: Rijeka, Croatia, 2013; pp. 143–174. [Google Scholar]
- Rubina, K.; Kalinina, N.; Potekhina, A.; Efimenko, A.; Semina, E.; Poliakov, A.; Wilkinson, D.G.; Parfyonova, Y.; Tkachuk, V. T-cadherin suppresses angiogenesis in vivo by inhibiting migration of endothelial cells. Angiogenesis 2007, 10, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Yurlova, E.I.; Rubina, K.A.; Sysoeva, V.Y.; Sharonov, G.V.; Semina, E.V.; Parfenova, E.V.; Tkachuk, V.A. T-cadherin suppresses the cell proliferation of mouse melanoma B16F10 and tumor angiogenesis in the model of the chorioallantoic membrane. Ontogenez 2010, 41, 261–270. [Google Scholar] [CrossRef]
- Rubina, K.; Kalinina, N.; Efimenko, A.; Lopatina, T.; Melikhova, V.; Tsokolaeva, Z.; Sysoeva, V.; Tkachuk, V.; Parfyonova, Y. Adipose stromal cells stimulate angiogenesis via promoting progenitor cell differentiation, secretion of angiogenic factors and enhancing vessel maturation. Tissue Eng. A 2009, 15, 2039–2050. [Google Scholar] [CrossRef] [PubMed]
- Spaeth, E.L.; Dembinski, J.L.; Sasser, A.K.; Watson, K.; Klopp, A.; Hall, B.; Andreeff, M.; Marini, F. Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PLoS ONE 2009, 4, e4992. [Google Scholar] [CrossRef] [PubMed]
- Strieter, R.M.; Kunkel, S.L.; Arenberg, D.A.; Burdick, M.D.; Polverini, P.J. Interferon gamma-inducible protein 10 (IP-10), a member of the C-X-C chemokine family, is an inhibitor of angiogenesis. BBRC 1995, 210, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Sonveaux, P.; Liu, S.; Zhao, Y.; Mi, J.; Clary, B.M.; Li, C.Y.; Kontos, C.D.; Dewhirst, M.W. Systemic overexpression of angiopoietin-2 promotes tumor microvessel regression and inhibits angiogenesis and tumor growth. Cancer Res. 2007, 67, 3835–3844. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Folkman, J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996, 86, 353–364. [Google Scholar] [CrossRef]
- Philippova, M.; Banfi, A.; Ivanov, D.; Gianni-Barrera, R.; Allenspach, R.; Erne, P.; Resink, T. Atypical GPI-anchoredT-cadherin stimulates angiogenesis in vitro and in vivo. ATVB 2006, 26, 2222–2230. [Google Scholar] [CrossRef]
- Belloni, D.; Scabini, S.; Foglieni, C.; Veschini, L.; Giazzon, A.; Colombo, B.; Fulgenzi, A.; Helle, K.B.; Ferrero, M.E.; Corti, A.; et al. The vasostatin-I fragment of chromogranin A inhibits VEGF-induced endothelial cell proliferation and migration. FASEB J. 2007, 21, 3052–3062. [Google Scholar] [CrossRef] [PubMed]
- Holash, J.; Maisonpierre, P.C.; Compton, D.; Boland, P.; Alexander, C.R.; Zagzag, D.; Yancopoulos, G.D.; Wiegand, S.J. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science 1999, 284, 1994–1998. [Google Scholar] [CrossRef] [PubMed]
- Leker, R.R.; Toth, Z.E.; Shahar, T.; Cassiani-Ingoni, R.; Szalayova, I.; Key, S.; Bratincsák, A.; Mezey, E. Transforming growth factor alpha induces angiogenesis and neurogenesis following stroke. Neuroscience 2009, 163, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.N.; Tozawa, Y.; Deutsch, U.; Wolburg-Buchholz, K.; Fujiwara, Y.; Gendron-Maguire, M.; Gridley, T.; Wolburg, H.; Risau, W.; Qin, Y. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature 1995, 376, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, F.; D’Alessio, S.; Fibbi, G.; Del Rosso, M.; Calorini, L. Cytokine-dependent invasiveness in B16 murine melanoma cells: Role of uPA system and MMP-9. Oncol. Rep. 2006, 15, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Liotta, L.A. Tumor invasion and metastases—Role of the extracellular matrix: Rhoads Memorial Award lecture. Cancer Res. 1986, 46, 1–7. [Google Scholar] [PubMed]
- Makrilia, N.; Kollias, A.; Manolopoulos, L.; Syrigos, K. Cell adhesion molecules: Role and clinical significance in cancer. Cancer Investig. 2009, 27, 1023–1037. [Google Scholar] [CrossRef] [PubMed]
- Kuphal, S.; Bauer, R.; Bosserhoff, A.K. Integrin signaling in malignant melanoma. Cancer Metastasis Rev. 2005, 24, 195–222. [Google Scholar] [CrossRef] [PubMed]
- Khatib, A.M.; Nip, J.; Fallavollita, L.; Lehmann, M.; Jensen, G.; Brodt, P. Regulation of urokinase plasminogen activator/plasmin-mediated invasion of melanoma cells by the integrin vitronectin receptor alphaVbeta3. Int. J. Cancer 2001, 91, 300–308. [Google Scholar] [CrossRef]
- Oikawa, Y.; Hansson, J.; Sasaki, T.; Rousselle, P.; Domogatskaya, A.; Rodin, S.; Tryggvason, K.; Patarroyo, M. Melanoma cells produce multiple laminin isoforms and strongly migrate on α5 laminin(s) via several integrin receptors. Exp. Cell Res. 2011, 317, 1119–1133. [Google Scholar] [CrossRef] [PubMed]
- Gaggioli, C.; Robert, G.; Bertolotto, C.; Bailet, O.; Abbe, P.; Spadafora, A.; Bahadoran, P.; Ortonne, J.P.; Baron, V.; Ballotti, R.; et al. Tumor-derived fibronectin is involved in melanoma cell invasion and regulated by V600E B-Raf signaling pathway. J. Investig. Dermatol. 2007, 127, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, O.; Goteri, G.; Lucarini, G.; Filosa, A.; Pieramici, T.; Rubini, C.; Biagini, G.; Offidani, A. Potential role of CCL27 and CCR10 expression in melanoma progression and immune escape. Eur. J. Cancer 2006, 42, 1181–1187. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Nannuru, K.C.; Sadanandam, A.; Varney, M.L.; Singh, R.K. CXCR1 and CXCR2 enhances human melanoma tumourigenesis, growth and invasion. Br. J. Cancer 2009, 100, 1638–1646. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubina, K.A.; Surkova, E.I.; Semina, E.V.; Sysoeva, V.Y.; Kalinina, N.I.; Poliakov, A.A.; Treshalina, H.M.; Tkachuk, V.A. T-Cadherin Expression in Melanoma Cells Stimulates Stromal Cell Recruitment and Invasion by Regulating the Expression of Chemokines, Integrins and Adhesion Molecules. Cancers 2015, 7, 1349-1370. https://doi.org/10.3390/cancers7030840
Rubina KA, Surkova EI, Semina EV, Sysoeva VY, Kalinina NI, Poliakov AA, Treshalina HM, Tkachuk VA. T-Cadherin Expression in Melanoma Cells Stimulates Stromal Cell Recruitment and Invasion by Regulating the Expression of Chemokines, Integrins and Adhesion Molecules. Cancers. 2015; 7(3):1349-1370. https://doi.org/10.3390/cancers7030840
Chicago/Turabian StyleRubina, Kseniya A., Ekaterina I. Surkova, Ekaterina V. Semina, Veronika Y. Sysoeva, Natalia I. Kalinina, Alexei A. Poliakov, Helena M. Treshalina, and Vsevolod A. Tkachuk. 2015. "T-Cadherin Expression in Melanoma Cells Stimulates Stromal Cell Recruitment and Invasion by Regulating the Expression of Chemokines, Integrins and Adhesion Molecules" Cancers 7, no. 3: 1349-1370. https://doi.org/10.3390/cancers7030840
APA StyleRubina, K. A., Surkova, E. I., Semina, E. V., Sysoeva, V. Y., Kalinina, N. I., Poliakov, A. A., Treshalina, H. M., & Tkachuk, V. A. (2015). T-Cadherin Expression in Melanoma Cells Stimulates Stromal Cell Recruitment and Invasion by Regulating the Expression of Chemokines, Integrins and Adhesion Molecules. Cancers, 7(3), 1349-1370. https://doi.org/10.3390/cancers7030840