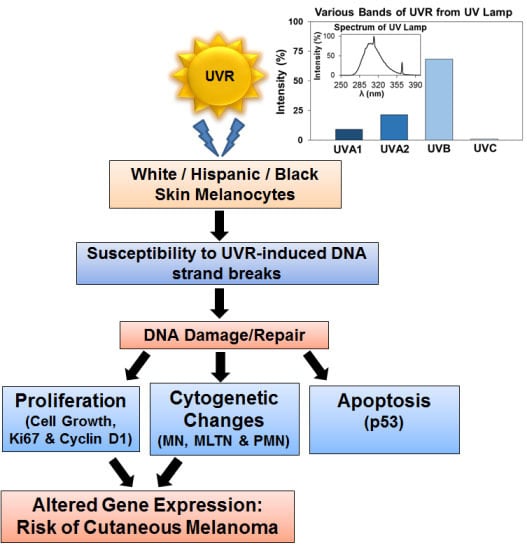
Ultraviolet Radiation-Induced Cytogenetic Damage in White, Hispanic and Black Skin Melanocytes: A Risk for Cutaneous Melanoma
Abstract
:1. Introduction
2. Results
2.1. Melanin in Melanocytes from Three Ethnic Individuals

2.2. Effect of UVR on Growth of Melanocytes in Culture


2.3. Susceptibility of Melanocytes to UVR-Induced Cytogenetic Damage


2.4. Modulation of Melanin Content Post UVR


3. Discussion
4. Experimental Section
4.1. Melanocyte Culture
4.2. Melanocyte Exposure to UVR
4.3. Hematoxylin (H)/Eosin (E) and Immunohistochemical (IHC) Staining
4.4. Fontana-Masson Staining for Melanin
4.5. Melanin Estimation
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dellinger, R.W.; Liu-Smith, F.; Meyskens, F.L. Continuing to illuminate the mechanisms underlying UV-mediated melanomagenesis. J. Photochem. Photobiol. B Biol. 2014, 138, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Jhappan, C.; Noonan, F.P.; Merlino, G. Ultraviolet radiation and cutaneous malignant melanoma. Oncogene 2003, 22, 3099–3112. [Google Scholar] [CrossRef] [PubMed]
- Gray-Schopfer, V.; Wellbrock, C.; Marais, R. Melanoma biology and new targeted therapy. Nature 2007, 445, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Ries, L.; Melbert, D.; Krapcho, M.; Stinchcomb, D.; Howlander, N.; Horner, M.; Mariotto, A.; Miller, B.; Feuer, E.; Altekruse, S. (Eds.) SEER Cancer Statistics Review, 1975–2014; National Cancer Institute: Bethesda, MD, USA. Available online: http://seer. cancer.gov/csr/1975_2004/ (accessed on 7 March 2008).
- Rouhani, P.; Hu, S.; Kirsner, R.S. Melanoma in hispanic and black Americans. Cancer Control 2008, 15, 248–253. [Google Scholar] [PubMed]
- Hu, S.; Parmet, Y.; Allen, G.; Parker, D.F.; Ma, F.; Rouhani, P.; Kirsner, R.S. Disparity in melanoma: A trend analysis of melanoma incidence and stage at diagnosis among whites, Hispanics, and blacks in Florida. Arch. Dermatol. 2009, 145, 1369–1374. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. Available online: http://ci5.iarc.fr/CI5plus/Pages/graph4_sel.aspx (accessed on 14 July 2015).
- D’Ischia, M.; Wakamatsu, K.; Napolitano, A.; Briganti, S.; Garcia-Borron, J.C.; Kovacs, D.; Meredith, P.; Pezzella, A.; Picardo, M.; Sarna, T.; et al. Melanins and melanogenesis: Methods, standards, protocols. Pigment Cell Melanoma Res. 2013, 26, 616–633. [Google Scholar] [CrossRef] [PubMed]
- Jimbow, K.; Quevedo, W.C.; Fitzpatrick, T.B.; Szabo, G. Some aspects of melanin biology: 1950–1975. J. Investig. Dermatol. 1976, 67, 72–89. [Google Scholar] [CrossRef] [PubMed]
- Solano, F. Melanins: Skin Pigments and Much More—Types, Structural Models, Biological Functions, and Formation Routes. New J. Sci. 2014, 2014, 1–28. [Google Scholar] [CrossRef]
- Costin, G.-E.; Hearing, V.J. Human skin pigmentation: Melanocytes modulate skin color in response to stress. FASEB J. 2007, 21, 976–994. [Google Scholar] [CrossRef] [PubMed]
- Agar, N.; Young, A.R. Melanogenesis: A photoprotective response to DNA damage? Mutat. Res. Fundam. Mol. Mech. Mutagen. 2005, 571, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Brash, D.E. Sunlight and the onset of skin cancer. Trends Genet. 1997, 13, 410–414. [Google Scholar] [CrossRef]
- Garland, C.F.; Garland, F.C.; Gorham, E.D. Epidemiologic evidence for different roles of ultraviolet A and B radiation in melanoma mortality rates. Ann. Epidemiol. 2003, 13, 395–404. [Google Scholar] [CrossRef]
- Williams, M.; Ouhtit, A. Towards a better understanding of the molecular mechanisms involved in sunlight-induced melanoma. J. Biomed. Biotechnol. 2005, 2005, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Gandini, S.; Sera, F.; Cattaruzza, M.S.; Pasquini, P.; Abeni, D.; Boyle, P.; Melchi, C.F. Meta-analysis of risk factors for cutaneous melanoma: I. Common and atypical naevi. Eur. J. Cancer 2005, 41, 28–44. [Google Scholar] [CrossRef] [PubMed]
- Tucker, M.A. Is sunlight important to melanoma causation? Cancer Epidemiol. Biomark. Prev. 2008, 17, 467–468. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, G.P.; Besaratinia, A. UV wavelength-dependent DNA damage and human non-melanoma and melanoma skin cancer. Photochem. Photobiol. Sci. 2012, 11, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Kaidbey, K.H.; Agin, P.P.; Sayre, R.M.; Kligman, A.M. Photoprotection by melanin—A comparison of black and Caucasian skin. J. Am. Acad. Dermatol. 1979, 1, 249–260. [Google Scholar] [CrossRef]
- Ortonne, J.-P. Photoprotective properties of skin melanin. Br. J. Dermatol. 2002, 146, S7–S10. [Google Scholar] [CrossRef]
- Wei, Q.; Lee, J.E.; Gershenwald, J.E.; Ross, M.I.; Mansfield, P.F.; Strom, S.S.; Wang, L.-E.; Guo, Z.; Qiao, Y.; Amos, C.I.; et al. Repair of UV light-induced DNA damage and risk of cutaneous malignant melanoma. J. Natl. Cancer Inst. 2003, 95, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Hauser, J.E.; Kadekaro, A.L.; Kavanagh, R.J.; Wakamatsu, K.; Terzieva, S.; Schwemberger, S.; Babcock, G.; Rao, M.B.; Ito, S.; Abdel-Malek, Z.A. Melanin content and MC1R function independently affect UVR-induced DNA damage in cultured human melanocytes. Pigment Cell Res. 2006, 19, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Meyle, K.D.; Guldberg, P. Genetic risk factors for melanoma. Hum. Genet. 2009, 126, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Wan, L.; Hacker, E.; Dai, X.; Lenna, S.; Jimenez-Cervantes, C.; Wang, Y.; Leslie, N.; Xu, G.; Widlund, H.; et al. MC1R is a potent regulator of PTEN after UV exposure in melanocytes. Mol. Cell 2013, 51, 409–422. [Google Scholar] [CrossRef] [PubMed]
- Davies, H.; Bignell, G.R.; Cox, C.; Stephens, P.; Edkins, S.; Clegg, S.; Teague, J.; Woffendin, H.; Garnett, M.J.; Bottomley, W.; et al. Mutations of the BRAF gene in human cancer. Nature 2002, 417, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Platz, A.; Egyhazi, S.; Ringborg, U.; Hansson, J. Human cutaneous melanoma; a review of NRAS and BRAF mutation frequencies in relation to histogenetic subclass and body site. Mol. Oncol. 2008, 1, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Curtin, J.A.; Busam, K.; Pinkel, D.; Bastian, B.C. Somatic activation of KIT in distinct subtypes of melanoma. J. Clin. Oncol. 2006, 24, 4340–4346. [Google Scholar] [CrossRef] [PubMed]
- Puri, N.; Ahmed, S.; Janamanchi, V.; Tretiakova, M.; Zumba, O.; Krausz, T.; Jagadeeswaran, R.; Salgia, R. c-Met is a potentially new therapeutic target for treatment of human melanoma. Clin. Cancer Res. 2007, 13, 2246–2253. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, C.; Ellerhorst, J.A.; Ekmekcioglu, S.; Greene, V.R.; Davies, M.A.; Grimm, E.A. Association of activated c-Met with NRAS-mutated human melanomas: A possible avenue for targeting. Int. J. Cancer 2012, 131, E56–E65. [Google Scholar] [CrossRef] [PubMed]
- Kuluncsics, Z.; Perdiz, D.; Brulay, E.; Muel, B.; Sage, E. Wavelength dependence of ultraviolet-induced DNA damage distribution: Involvement of direct or indirect mechanisms and possible artefacts. J. Photochem. Photobiol. B Biol. 1999, 49, 71–80. [Google Scholar] [CrossRef]
- Korytowski, W.; Pilas, B.; Sarna, T.; Kalyanaraman, B. Photoinduced generation of hydrogen peroxide and hydroxyl radicals in melanins. Photochem. Photobiol. 1987, 45, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Berger, M.; Douki, T.; Morin, B.; Raoul, S.; Ravanat, J.; Spinelli, S. Effects of UV and visible radiation on DNA-final base damage. Biol. Chem. 1997, 378, 1275–1286. [Google Scholar] [PubMed]
- Bennett, D.C. Ultraviolet wavebands and melanoma initiation. Pigment Cell Melanoma Res. 2008, 21, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Mouret, S.; Baudouin, C.; Charveron, M.; Favier, A.; Cadet, J.; Douki, T. Cyclobutane pyrimidine dimers are predominant DNA lesions in whole human skin exposed to UVA radiation. Proc. Natl. Acad. Sci. USA 2006, 103, 13765–13770. [Google Scholar] [CrossRef] [PubMed]
- Premi, S.; Wallisch, S.; Mano, C.M.; Weiner, A.B.; Bacchiocchi, A.; Wakamatsu, K.; Bechara, E.J.H.; Halaban, R.; Douki, T.; Brash, D.E. Chemiexcitation of melanin derivatives induces DNA photoproducts long after UV exposure. Science 2015, 347, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Bradley, M. Double-strand breaks in DNA caused by repair of damage due to ultraviolet light. J. Supramol. Struct. Cell Biochem. 1981, 16, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Emri, G.; Wenczl, E.; van Erp, P.; Jans, J.; Roza, L.; Horkay, I.; Schothorst, A.A. Low doses of UVB or UVA induce chromosomal aberrations in cultured human skin cells. J. Investig. Dermatol. 2000, 115, 435–440. [Google Scholar] [CrossRef] [PubMed]
- De Gruijl, F.R.; van Kranen, H.J.; Mullenders, L.H.F. UV-induced DNA damage, repair, mutations and oncogenic pathways in skin cancer. J. Photochem. Photobiol. B Biol. 2001, 63, 19–27. [Google Scholar] [CrossRef]
- Tadokoro, T.; Kobayashi, N.; Zmudzka, B.Z.; Ito, S.; Wakamatsu, K.; Yamaguchi, Y.; Korossy, K.S. UV-induced DNA damage and melanin content in human skin differing in racial/ethnic origin. FASEB J. 2003, 1177–1179. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, J.; Bredeston, L.; Malanga, G.; Mordoh, J. Role of melanin as a scavenger of active oxygen species. Pigment Cell Res. 1993, 6, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Kvam, E.; Tyrrell, R.M. The role of melanin in the induction of oxidative DNA base damage by ultraviolet A irradiation of DNA or melanoma cells. J. Investig. Dermatol. 1999, 113, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Kvam, E.; Dahle, J. Melanin synthesis may sensitize melanocytes to oxidative DNA damage by ultraviolet A radiation and protect melanocytes from direct DNA damage by ultraviolet B radiation. Pigment Cell Res. 2004, 17, 549–550. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-T.; Choi, B.; Tang, M. Melanocytes are deficient in repair of oxidative DNA damage and UV-induced photoproducts. Proc. Natl. Acad. Sci. USA 2010, 107, 12180–12185. [Google Scholar] [CrossRef] [PubMed]
- Budden, T.; Bowden, N.A. The role of altered nucleotide excision repair and UVB-induced DNA damage in melanomagenesis. Int. J. Mol. Sci. 2013, 14, 1132–1151. [Google Scholar] [CrossRef] [PubMed]
- Nickerson, J.A. Nuclear dreams: The malignant alteration of nuclear architecture. J. Cell. Biochem. 1998, 70, 172–180. [Google Scholar] [CrossRef]
- Zink, D.; Fischer, A.H.; Nickerson, J.A. Nuclear structure in cancer cells. Nat. Rev. Cancer 2004, 4, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Heddle, J.A.; Hite, M.; Kirkhart, B.; Mavournin, K.; MacGregor, J.T.; Newell, G.W.; Salamone, M.F. The induction of micronuclei as a measure of genotoxicity. A report of the U.S. Environmental Protection Agency Gene-Tox Program. Mutat. Res. 1983, 123, 61–118. [Google Scholar] [CrossRef]
- Roser, M.; Bohm, A.; Oldigs, M.; Weichenthal, M.; Reimers, U.; Schmidt-Preuss, U.; Breitbart, E.W.; Rudiger, H.W. Ultraviolet-induced formation of micronuclei and sister chromatid exchange in cultured fibroblasts of patients with cutaneous malignant melanoma. Cancer Genet. Cytogenet. 1989, 41, 129–137. [Google Scholar] [CrossRef]
- Fenech, M.; Kirsch-Volders, M.; Natarajan, A.T.; Surralles, J.; Crott, J.W.; Parry, J.; Norppa, H.; Eastmond, D.A.; Tucker, J.D.; Thomas, P. Molecular mechanisms of micronucleus, nucleoplasmic bridge and nuclear bud formation in mammalian and human cells. Mutagenesis 2011, 26, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Zusman, I.; Kozlenko, M.; Zimber, A. Nuclear polymorphism and nuclear size in precarcinomatous and carcinomatous lesions in rat colon and liver. Cytometry 1991, 12, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Cornforth, M.N.; Goodwin, E.H. Transmission of radiation-induced acentric chromosomal fragments to micronuclei in normal human fibroblasts. Radiat. Res. 1991, 126, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Ma, F.; Collado-Mesa, F.; Kirsner, R.S. UV radiation, latitude, and melanoma in U.S. Hispanics and blacks. Arch. Dermatol. 2004, 140, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Cockburn, M.G.; Zadnick, J.; Deapen, D. Developing epidemic of melanoma in the Hispanic population of California. Cancer 2006, 106, 1162–1168. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.G. Thick melanoma lesions increasing in hispanics. Clin. Rounds 2006, 31, 31. [Google Scholar]
- Pollitt, R.A.; Clarke, C.A.; Swetter, S.M.; Peng, D.H.; Zadnick, J.; Cockburn, M. The expanding melanoma burden in California hispanics: Importance of socioeconomic distribution, histologic subtype, and anatomic location. Cancer 2011, 117, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Clairwood, M.; Ricketts, J.; Grant-kels, J.; Gonsalves, L. Melanoma in skin of color in Connecticut: An analysis of melanoma incidence and stage at diagnosis in non-Hispanic blacks , non-Hispanic whites, and Hispanics. Int. J. Dermatol. 2014, 53, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Jaimes, N.; Oliveria, S.; Halpern, A. A cautionary note on melanoma screening in the Hispanic/Latino population. JAMA Dermatol. 2013, 149, 396–397. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.C.; Eide, M.J.; King, J.; Saraiya, M.; Huang, Y.; Wiggins, C.; Barnholtz-Sloan, J.S.; Martin, N.; Cokkinides, V.; Miller, J.; et al. Racial and ethnic variations in incidence and survival of cutaneous melanoma in the United States, 1999–2006. J. Am. Acad. Dermatol. 2011, 65, S26–S37. [Google Scholar] [CrossRef] [PubMed]
- Scholzen, T.; Gerdes, J. The Ki-67 protein: From the known and the unknown. J. Cell. Physiol. 2000, 182, 311–322. [Google Scholar] [CrossRef]
- Knudsen, K.E.; Diehl, J.A.; Haiman, C.; Knudsen, E.S. Cyclin D1: Polymorphism, aberrant splicing and cancer risk. Oncogene 2006, 25, 1620–1628. [Google Scholar] [CrossRef] [PubMed]
- Marrot, L.; Belaïdi, J.-P.; Jones, C.; Perez, P.; Meunier, J.-R. Molecular responses to stress induced in normal human caucasian melanocytes in culture by exposure to simulated solar UV. Photochem. Photobiol. 2005, 81, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.; Dixon, K.; Medrano, E.E.; Smalara, D.; Im, S.; Mitchell, D.; Babcock, G.; Abdel-malek, Z.A. Comparison of the responses of human melanocytes with different melanin contents to ultraviolet B irradiation. Cancer Res. 1995, 55, 4041–4046. [Google Scholar] [PubMed]
- Medrano, E.E.; Im, S.; Yang, F. Ultraviolet B light induces G1 arrest in human melanocytes by prolonged inhibition of retinoblastoma protein phosphorylation associated with long-term expression of the p21 Waf-1/SDI-1/Cip-1 protein ultraviolet B light Induces G, arrest in human mel. Cancer 1995, 4, 4047–4052. [Google Scholar]
- Wenczl, E.; van der Schans, G.P.; Roza, L.; Kolb, R.M.; Timmerman, A.J.; Smit, N.P.M.; Pavel, S.; Schothorst, A.A. (Pheo)melanin photosensitizes VA-induced DNA damage in cultured human melanocytes. J. Investig. Dermatol. 1998, 111, 678–682. [Google Scholar] [CrossRef] [PubMed]
- Marrot, L.; Belaidi, J.P.; Meunier, J.R.; Perez, P.; Agapakis-Causse, C. The human melanocyte as a particular target for UVA radiation and an endpoint for photoprotection assessment. Photochem. Photobiol. 1999, 69, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Hill, H.Z.; Hill, G.J. UVA, pheomelanin and the carcinogenesis of melanoma. Pigment Cell Res. 2000, 13, S140–S144. [Google Scholar] [CrossRef]
- Besaratinia, A.; Yoon, J.-I.; Schroeder, C.; Bradforth, S.E.; Cockburn, M.; Pfeifer, G.P. Wavelength dependence of ultraviolet radiation-induced DNA damage as determined by laser irradiation suggests that cyclobutane pyrimidine dimers are the principal DNA lesions produced by terrestrial sunlight. FASEB J. 2011, 25, 3079–3091. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, T.B.; Szabo, G. The melanocyte: CYTOLOGY and cytochemistry. J. Investig. Dermatol. 1959, 32, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Toda, K.; Pathak, M.; Parrish, J.A.; Fitzpatrick, T.B.; Quevedo, W.C. Alteration of racial differences in melanosome distribution in human epidermis after exposure to ultraviolet light. Nat. New Biol. 1972, 236, 143–145. [Google Scholar] [CrossRef] [PubMed]
- Sturm, R.A.; Box, N.F.; Ramsay, M. Human pigmentation genetics: The difference is only skin deep. BioEssays 1998, 20, 712–721. [Google Scholar] [CrossRef]
- Brenner, M.; Hearing, V.J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Eller, M.S.; Ostrom, K.; Gilchrest, B.A. DNA damage enhances melanogenesis. Proc. Natl. Acad. Sci. USA 1996, 93, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Eller, M.S.; Gilchrest, B.A. Tanning as part of the eukaryotic SOS response. Pigment Cell Res. 2000, 13, S94–S97. [Google Scholar] [CrossRef]
- Duval, C.; Régnier, M.; Schmidt, R. Distinct melanogenic response of human melanocytes in mono-culture, in co-culture with keratinocytes and in reconstructed epidermis, to UV exposure. Pigment Cell Res. 2001, 14, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Virador, V.M.; Muller, J.; Wu, X.; Abdel-Malek, Z.A.; Yu, Z.X.; Ferrans, V.J.; Kobayashi, N.; Wakamatsu, K.; Ito, S.; Hammer, J.A.; et al. Influence of alpha-melanocyte-stimulating hormone and ultraviolet radiation on the transfer of melanosomes to keratinocytes. FASEB J. 2002, 16, 105–107. [Google Scholar] [PubMed]
- Hall, P.; McKee, P.; Menage, H.D.; Dover, R.; Lane, D.P. High levels of p53 protein in UV-irradiated normal human skin. Oncogene 1993, 8, 203–207. [Google Scholar] [PubMed]
- Yamaguchi, Y.; Coelho, S.G.; Zmudzka, B.Z.; Takahashi, K.; Beer, J.Z.; Hearing, V.J.; Miller, S.A. Cyclobutane pyrimidine dimer formation and p53 production in human skin after repeated UV irradiation. Exp. Dermatol. 2008, 17, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Ullrichs, S.J.; Anderson, C.W.; Mercern, W.E. The p53 Tumor Suppressor Protein, a Modulator of Cell Proliferation. J. Biol. Chem. 1992, 267, 15259–15262. [Google Scholar]
- Bálint, E.E.; Vousden, K.H. Activation and activities of the p53 tumour suppressor protein. Br. J. Cancer 2001, 85, 1813–1823. [Google Scholar] [CrossRef] [PubMed]
- Kastan, M.B.; Onyekwere, O.; Sidransky, D.; Vogelstein, B.; Craig, R.W. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991, 51, 6304–6311. [Google Scholar] [PubMed]
- Eller, M.S.; Maeda, T.; Magnoni, C.; Atwal, D.; Gilchrest, B.A. Enhancement of DNA repair in human skin cells by thymidine dinucleotides: Evidence for a p53-mediated mammalian SOS response. Proc. Natl. Acad. Sci. USA 1997, 94, 12627–12632. [Google Scholar] [CrossRef] [PubMed]
- Diffey, B.L. Sources and measurement of ultraviolet radiation. Methods 2002, 28, 4–13. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dasgupta, A.; Katdare, M. Ultraviolet Radiation-Induced Cytogenetic Damage in White, Hispanic and Black Skin Melanocytes: A Risk for Cutaneous Melanoma. Cancers 2015, 7, 1586-1604. https://doi.org/10.3390/cancers7030852
Dasgupta A, Katdare M. Ultraviolet Radiation-Induced Cytogenetic Damage in White, Hispanic and Black Skin Melanocytes: A Risk for Cutaneous Melanoma. Cancers. 2015; 7(3):1586-1604. https://doi.org/10.3390/cancers7030852
Chicago/Turabian StyleDasgupta, Amrita, and Meena Katdare. 2015. "Ultraviolet Radiation-Induced Cytogenetic Damage in White, Hispanic and Black Skin Melanocytes: A Risk for Cutaneous Melanoma" Cancers 7, no. 3: 1586-1604. https://doi.org/10.3390/cancers7030852
APA StyleDasgupta, A., & Katdare, M. (2015). Ultraviolet Radiation-Induced Cytogenetic Damage in White, Hispanic and Black Skin Melanocytes: A Risk for Cutaneous Melanoma. Cancers, 7(3), 1586-1604. https://doi.org/10.3390/cancers7030852