Additive-Free Baeyer–Villiger Oxidation of Cyclic Ketone Catalyzed by Carboxylic-Functionalized Poly(Ionic Liquids) and Polyoxometalate Ionic Self-Assemblies
Abstract
:1. Introduction
2. Results and Discussion
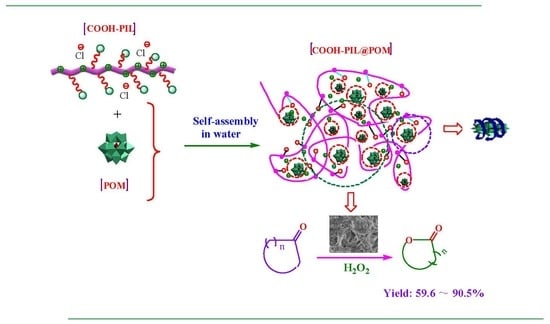
2.1. Synthesis and Characterization of Two Ionic Self-Assemblies
2.2. Catalytic Performance in Additive-Free BV Oxidation
2.3. Catalyst Reusability
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Preparation of Ionic Liquid Monomer (COOH-PIL)
3.3. Preparation of Ionic Self-Assemblies
3.4. BV Oxidation of Cyclic Ketones
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Badu, R.P.; Oconnor, K.; Seeram, R. Current progress on bio-based polymers and their future trends. Prog. Biomate. 2013, 2, 8. [Google Scholar]
- Zhu, Y.; Romain, C.; Williams, C.K. Sustainable polymers from renewable resources. Nature 2016, 540, 352–362. [Google Scholar] [CrossRef]
- Hillmyer, M.A.; Tolman, W.B. Aliphatic polyester block polymers: Renewable, degradable, and sustainable. Acc. Chem. Res. 2014, 47, 2390–2396. [Google Scholar] [CrossRef]
- Hillmyer, A.C.; Tolman, W.B. Aliphatic polyesters: Synthesis, properties and applications. Adv. Polym. Sci. 2002, 157, 1–40. [Google Scholar]
- Tenbrink, G.J.; Arends, I.W.C.E.; Sheldon, R.A. The-Baeyer-Villiger reaction: New developments toward greener procedures. Chem. Rev. 2004, 104, 4105–4124. [Google Scholar] [CrossRef] [PubMed]
- Sitko, M.; Szelwicka, A.; Wojewodka, A.; Skwarek, A.; Tadasiewicz, D.; Schimmelpfennig, L.; Dziuba, K.; Witczakc, M.M.; Chrobok, A. Perdecanoic acid as a safe and stable mediumchain peracid for Baeyer–Villiger oxidation of cyclic ketones to lactones. RSC Adv. 2019, 9, 30012–30018. [Google Scholar] [CrossRef] [Green Version]
- Corma, A.; Navarro, M.T.; Renz, M. Lewis acidic Sn (IV) centers-grafted onto MCM-41 as catalytic sites for the Baeyer–Villiger oxidation with hydrogen peroxide. J. Catal. 2003, 219, 242–246. [Google Scholar] [CrossRef]
- Chen, T.; Wang, B.; Li, Y.; Liu, L.; Qiu, S. Hydrothermal synthesis of tin contain mesoporous silicas and their catalytic performance over Baeyer–Villiger oxidation of cyclohexanone to ε-carpolactone: Comparison of Sn/MCM-41 and Sn/SBA-15. J. Porrous Mater. 2015, 22, 949–957. [Google Scholar] [CrossRef]
- Mandai, K.; Hanata, M.; Mitsudo, K.; Mandai, H.; Hashimoto, H.; Takada, J. Bateriogenic iron oxide as an effective catalyst for Baeyer-Villiger oxidation with molecular oxygen and benzaldehyde. Tetrahedron 2015, 71, 9403–9407. [Google Scholar] [CrossRef]
- Llamas, R.; Jimenez-Sanchidrian, C.; Ruiz, J.R. Environmentally friendly Baeyer-Villiger oxidation with H2O2/nitrile over Mg(OH)2 and MgO. Appl. Catal. B Environ. 2007, 72, 18–25. [Google Scholar] [CrossRef]
- Thomas, J.M.; Raja, R. Catalytically active centres in porous oxides: Design and performance of highly selective news catalysts. Chem. Commun. 2001, 8, 675–687. [Google Scholar] [CrossRef]
- Fischer, J.; Holderich, W.F. Baeyer–Villiger-oxidation of cyclopentanone with aqueous hydrogen by acid heterogeneous catalysis. Appl. Catal. A Gen. 1999, 180, 435–443. [Google Scholar] [CrossRef]
- Xing, W.Z.; Ma, Q.G.; Peng, X.H. Silica/A153-SO3H: An efficient catalyst for the Baeyer-Villiger oxidation of cyclic ketones with hydrogen peroxide. C. R. Chim. 2015, 18, 581–585. [Google Scholar] [CrossRef]
- Chen, S.Y.; Zhou, X.T.; Wang, J.Z.; Luo, R.C.; Luo, Q.J.; Yu, L.J.; Ji, H.B. Promoting the aerobic Baeyer-Villiger oxidation of ketone over carboxylic multi-walled carbon namotubes. Mol. Catal. 2017, 438, 152–157. [Google Scholar] [CrossRef]
- Wei, Y.L.; Yang, Z.W.; Zhao, G.H.; Yang, W. Synthesis and catalytic properties of macroporpus SiO2-coated CNT-sieve-composite supported 12-tungstophosph-oric acid catalysts with dual pore structure for Baeyer-Villiger oxidation of cyclic ketones under microwave irradition. J. Catal. 2019, 371, 196–206. [Google Scholar] [CrossRef]
- Yuan, J.; Mecerreyes, D.; Antonietti, M. Poly(ionic liquid)s: An updaye. Prog. Polym. Sci. 2014, 38, 1009. [Google Scholar] [CrossRef]
- Leng, Y.; Zhao, J.; Jiang, P.; Wang, J. Amphiphilic polyoxometalate-paired polymer coated Fe3O4: Magnetically recyclable catalyst for epoxidation of bio-derived olefins with H2O2. ACS Appl. Mater. Interfaces 2014, 6, 5947–5954. [Google Scholar] [CrossRef]
- Adhikary, S.D.; Tiwari, A.; Nagaiah, T.C.; Manda, D. Stabilization of cobalt-poly-oxometalate over poly(ionic liquids) compsiites for efficient electrocatalytic water oxidation. ACS Appl. Mater. Interfaces 2018, 10, 38872–38879. [Google Scholar] [CrossRef]
- Fafiee, E.; Shahebrahimi, S. Organic–inorganic hybrid polyionic liquid based polyoxomatalate as nano porous material for selective oxidation of sulfides. J. Mole. Struct. 2017, 1139, 255–263. [Google Scholar]
- Kim, D.W.; Chi, D.Y. Polymer-supported ionic liquids: Imidazolium salts as catalysts for nucleophilic substitution reaction including fluorinations. Angew. Chem. Int. Ed. 2004, 43, 483–485. [Google Scholar] [CrossRef]
- Parvulescu, C.I.; Hardacre, C. Catalysis in ionic liquids. Chem. Rev. 2007, 107, 2615–2665. [Google Scholar] [CrossRef]
- Tao, D.J.; Liu, F.J.; Wang, L.; Jiang, L.L. A green and efficient hydration of alkynes catalyzed by hierarchically porous poly(ionic liquid)s. Appl. Catal. A Gen. 2018, 564, 56–63. [Google Scholar] [CrossRef]
- Jin, M.J.; Taher, A.; Kang, H.J.; Choi, M.; Ryoo, R. Palladium acetate immobiliz-ed in a hierarchical MFI zeolite-supported ionic liquid: A highly active and recyclable catalyst for Suzuki reaction in water. Green Chem. 2009, 11, 309–313. [Google Scholar] [CrossRef]






| Entry | Catalyst | Reaction Conditions | Conversion (%) | Selectivity (%) | Yield (%) |
|---|---|---|---|---|---|
| 1 | blank | 5 mmol ketone, H2O2 15 mmol, DCE 15 mL, 60 °C,4 h | - | - | - |
| 2 | H3PW12O40 | 5 mmol ketone, catalyst 0.0025 mmol, H2O2 15 mmol, DCE 15 mL, 60 °C, 4 h | 23 | 67 | 15.4 |
| 3 | H4SiW12O40 | 5 mmol ketone, catalyst 0.0025 mmol, H2O2 15 mmol, DCE 15 mL, 60 °C, 4 h | 12 | 84 | 10.8 |
| 4 | COOH@PIL | 5 mmol ketone, catalyst 0.0025 mmol, H2O2 15 mmol, DCE 15 mL, 60 °C, 4 h | 10.2 | 82 | 8.4 a |
| 5 | COOH-PIL@P-POM | 5 mmol ketone, catalyst 0.0025 mmol, H2O2 15 mmol, DCE 15 mL, 60 °C, 4 h | 83.4 | 93 | 77.6 a |
| 6 | COOH-PIL@Si-POM | 5 mmol ketone, catalyst 0.0025 mmol, H2O2 15 mmol, DCE 15 mL, 60 °C, 4 h | 68.5 | 90 | 61.6 a |
| 7 | COOH-PIL@P-POM | 5 mmol ketone, catalyst 0.0025 mmol, H2O2 15 mmol, DCE 15 mL, 60 °C, 4 h | 78.6 | 92 | 72.3 b |
| 8 | COOH-PIL@P-POM | 5 mmol ketone, catalyst 0.0025 mmol, H2O2 15 mmol, DCE 15 mL, 60 °C, 4 h | 87.3 | 85 | 74.2 c |
| Entry | Catalyst | Ketone | Lactone | Yield a (%) |
|---|---|---|---|---|
| 1 | COOH-PIL@ P-POM |  |  | 86.7 |
| 2 | COOH-PIL@Si-POM | 80.3 | ||
| 3 | COOH-PIL@P-POM |  |  | 85.2 |
| 4 | COOH-PIL@Si-POM | 78.4 | ||
| 5 | COOH-PIL@P-POM |  |  | 90.5 |
| 6 | COOH-PIL@Si-POM | 81.3 | ||
| 7 | COOH-PIL@P-POM |  |  | 76.2 |
| 8 | COOH-PIL@Si-POM | 60.8 | ||
| 9 | COOH-PIL@P-POM |  |  | 75.7 |
| 10 | COOH-PIL@Si-POM | 59.6 | ||
| 11 | COOH-PIL@P-POM |  |  | 75.8 |
| 12 | COOH-PIL@Si-POM | 60.2 | ||
| 13 | COOH-PIL@P-POM |  |  | 7.2 |
| 14 | COOH-PIL@Si-POM | 3.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Xue, H.; Lin, Q.; Yu, A. Additive-Free Baeyer–Villiger Oxidation of Cyclic Ketone Catalyzed by Carboxylic-Functionalized Poly(Ionic Liquids) and Polyoxometalate Ionic Self-Assemblies. Catalysts 2020, 10, 127. https://doi.org/10.3390/catal10010127
Li X, Xue H, Lin Q, Yu A. Additive-Free Baeyer–Villiger Oxidation of Cyclic Ketone Catalyzed by Carboxylic-Functionalized Poly(Ionic Liquids) and Polyoxometalate Ionic Self-Assemblies. Catalysts. 2020; 10(1):127. https://doi.org/10.3390/catal10010127
Chicago/Turabian StyleLi, Xinzhong, Hanyu Xue, Qi Lin, and Aimin Yu. 2020. "Additive-Free Baeyer–Villiger Oxidation of Cyclic Ketone Catalyzed by Carboxylic-Functionalized Poly(Ionic Liquids) and Polyoxometalate Ionic Self-Assemblies" Catalysts 10, no. 1: 127. https://doi.org/10.3390/catal10010127
APA StyleLi, X., Xue, H., Lin, Q., & Yu, A. (2020). Additive-Free Baeyer–Villiger Oxidation of Cyclic Ketone Catalyzed by Carboxylic-Functionalized Poly(Ionic Liquids) and Polyoxometalate Ionic Self-Assemblies. Catalysts, 10(1), 127. https://doi.org/10.3390/catal10010127