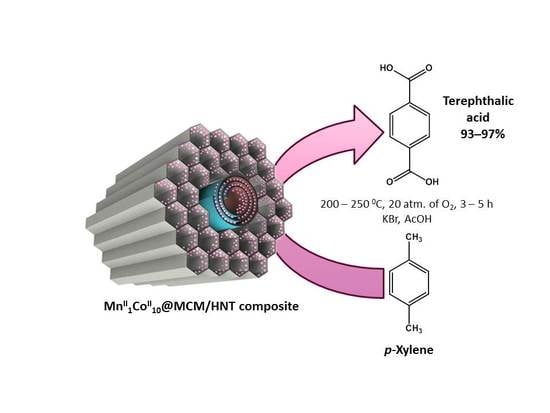
Manganese and Cobalt Doped Hierarchical Mesoporous Halloysite-Based Catalysts for Selective Oxidation of p-Xylene to Terephthalic Acid
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Synthesis and Characterization of Hierarchical Mesoporous MCM-41/HNT Composite, Doped with Mn and Co
2.2. Oxidation of p-Xylene in the Presence of Hierarchical Mesoporous MCM-41/HNT Composite Doped with Mn and Co
3. Experimental
3.1. Chemicals
3.2. Analyses and Instrumentations
3.3. The Synthesis of Mn/Co-Containing Oxidation Catalyst Based on the MCM-41/HNT Composite
- ωMn (XFS): 0.15%.
- ωCo (XFS): 1.29%.
3.4. Protocol for the Catalytic Experiments
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sheehan, R.J. Terephthalic acid, dimethyl terephthalate, and isophthalic acid. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2002. [Google Scholar]
- Nazimok, V.F.; Ovchinnikov, V.I.; Potekhin, V.M. Liquid-Phase Oxidation of Alkyl Aromatic Hydrocarbons; Chemistry: Moscow, Russia, 1987; p. 240. (In Russian) [Google Scholar]
- Li, M.; Niu, F.; Zuo, X.; Metelski, P.D.; Busch, D.H.; Subramaniam, B. A spray reactor concept for catalytic oxidation of p-xylenetoproduce high-purity terephthalicacid. Chem. Eng. Sci. 2013, 104, 93–102. [Google Scholar] [CrossRef] [Green Version]
- Partenheimer, W. Methodology and scope of metal/bromide autoxidation of hydrocarbons. Catal. Today 1995, 23, 69–158. [Google Scholar] [CrossRef]
- Saffer, A.; Barker, R.S. Preparation of Aromatic Polycarboxylic Acids. U.S. Patent 2833816 A, 6 May 1958. [Google Scholar]
- Saffer, A.; Barker, R.S. Oxidation Chemical Process. U.S. Patent 3089906 A, 14 May 1963. [Google Scholar]
- Karakhanov, E.A.; Maksimov, A.L.; Zolotukhina, A.V.; Vinokurov, V.A. Oxidation of p-xylene. A review. Russ. J. Appl. Chem. 2018, 91, 707–727. [Google Scholar] [CrossRef]
- Tomás, R.A.F.; Bordado, J.C.M.; Gomes, J.F.P. p-xylene oxidation to terephthalic acid: A literature review oriented toward process optimization and development. Chem. Rev. 2013, 113, 7421–7469. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, Y.; Yamashita, G.; Tokashiki, M.; Yamaji, T. New Oxidation Process for Production of Terephthalic Acid from p-Xylene. Ind. Eng. Chem. 1970, 62, 38–42. [Google Scholar] [CrossRef]
- Fadzil, N.A.M.; Rahim, M.H.A.; Maniam, G.P. A brief review of para-xylene oxidation to terephthalic acid as a model of primary C–H bond activation. Chin. J. Catal. 2014, 35, 1641–1652. [Google Scholar] [CrossRef]
- Zuo, X.; Subramaniam, B.; Busch, D.H. Liquid-phase oxidation of toluene and p-toluic acid under mild conditions: Synergistic effects of cobalt, zirconium, ketones, and carbon dioxide. Ind. Eng. Chem. Res. 2008, 47, 546–552. [Google Scholar] [CrossRef]
- Sun, W.; Yi, P.; Zhao, L.; Zhou, X. Simplified free-radical reaction kinetics for p-xylene oxidation to terephthalic acid. Chem. Eng. Technol. 2008, 31, 1402–1409. [Google Scholar] [CrossRef]
- Zuo, X.; Niu, F.; Snavely, K.; Subramaniam, B.; Busch, D.H. Liquid phase oxidation of p-xylene to terephthalic acid at medium-high temperatures: Multiple benefits of CO2-expanded liquids. Green Chem. 2010, 12, 260–267. [Google Scholar] [CrossRef] [Green Version]
- Hronec, M.; Hrabě, Z. Liquid-phase oxidation of p-xylene catalyzed by metal oxides. Ind. Eng. Chem. Prod. Res. Develop. 1986, 25, 257–261. [Google Scholar] [CrossRef]
- Li, Y.; Duan, D.; Wu, M.; Li, J.; Yan, Z.; Wang, W.; Zi, G.; Wang, J. One-step synthesis of 2,5-dihydroxyterephthalic acid by the oxidation of p-xylene over M-MCM-41 (M = Fe, Fe/Cu, Cu) catalysts. Chem. Eng. J. 2016, 306, 777–783. [Google Scholar] [CrossRef]
- Chavan, S.A.; Srinivas, D.; Ratnasamy, P. Selective oxidation of para-xylene to terephthalic acid by μ3-oxo-bridged Co/Mn cluster complexes encapsulated in zeolite–Y. J. Catal. 2001, 204, 409–419. [Google Scholar] [CrossRef]
- Lvov, Y.; Wang, W.; Zhang, L.; Fakhrullin, R. Halloysite clay nanotubes for loading and sustained release of functional compounds. Adv. Mater. 2016, 28, 1227–1250. [Google Scholar] [CrossRef] [PubMed]
- Vinokurov, V.A.; Stavitskaya, A.V.; Chudakov, Y.A.; Ivanov, E.V.; Shrestha, L.K.; Ariga, K.; Darrat, Y.A.; Lvov, Y.M. Formation of metal clusters in halloysite clay nanotubes. Sci. Technol. Adv. Mater. 2017, 18, 147–151. [Google Scholar] [CrossRef]
- Vinokurov, V.A.; Stavitskaya, A.V.; Glotov, A.P.; Novikov, A.A.; Zolotukhina, A.V.; Kotelev, M.S.; Gushchin, P.A.; Ivanov, E.V.; Darrat, Y.; Lvov, Y.M. Nanoparticles formed onto/into halloysite clay tubules: Architectural synthesis and applications. Chem. Rec. 2018, 18, 858–867. [Google Scholar] [CrossRef]
- Vinokurov, V.; Glotov, A.; Chudakov, Y.; Stavitskaya, A.; Ivanov, E.; Gushchin, P.; Zolotukhina, A.; Maximov, A.; Karakhanov, E.; Lvov, Y. Core/shell ruthenium–halloysite nanocatalysts for hydrogenation of phenol. Ind. Eng. Chem. Res. 2017, 56, 14043–14052. [Google Scholar] [CrossRef]
- Glotov, A.; Stavitskaya, A.; Chudakov, Y.; Ivanov, E.; Huang, W.; Vinokurov, V.; Zolotukhina, A.; Maximov, A.; Karakhanov, E.; Lvov, Y. Mesoporous metal catalysts templated on clay nanotubes. Bull. Chem. Soc. Jpn. 2019, 92, 61–69. [Google Scholar] [CrossRef]
- Glotov, A.; Stytsenko, V.; Artemova, M.; Kotelev, M.; Ivanov, E.; Gushchin, P.; Vinokurov, V. Hydroconversion of aromatic hydrocarbons over bimetallic catalysts. Catalysts 2019, 9, 38. [Google Scholar] [CrossRef] [Green Version]
- Glotov, A.P.; Stavitskaya, A.V.; Chudakov, Y.A.; Artemova, M.I.; Smirnova, E.M.; Demikhova, N.R.; Shabalina, T.N.; Gureev, A.A.; Vinokurov, V.A. Nanostructured ruthenium catalysts in hydrogenation of aromatic compounds. Petrol. Chem. 2018, 58, 1221–1226. [Google Scholar] [CrossRef]
- Glotov, A.; Levshakov, N.; Stavitskaya, A.; Artemova, M.; Gushchin, P.; Ivanov, E.; Vinokurov, V.; Lvov, Y. Templated self-assembly of ordered mesoporous silica on clay nanotubes. Chem. Commun. 2019, 55, 5507–5510. [Google Scholar] [CrossRef]
- Lvov, Y.; Panchal, A.; Fu, Y.; Fakhrullin, R.; Kryuchkova, M.; Batasheva, S.; Stavitskaya, A.; Glotov, A.; Vinokurov, V. Interfacial self-assembly in halloysite nanotube composites. Langmuir 2019, 35, 8646–8865. [Google Scholar] [CrossRef] [PubMed]
- Glotov, A.; Levshakov, N.; Vutolkina, A.; Lysenko, S.; Karakhanov, E.; Vinokurov, V. Aluminosilicates supported La-containing sulfur reduction additives for FCC catalyst: Correlation between activity, support structure and acidity. Catal. Today 2019, 329, 135–141. [Google Scholar] [CrossRef]
- Glotov, A.P.; Levshakov, N.S.; Vutolkina, A.V.; Lysenko, S.V.; Gushchin, P.A.; Vinokurov, V.A. Bimetallic sulfur reduction additives based on alumosilicate of Al-MCM-41 type for cracking catalysts: Desulfurazing activity vs. ratio of components in a support. Russ. J. Appl. Chem. 2019, 92, 562–568. [Google Scholar] [CrossRef]
- Karakhanov, E.; Akopyan, A.; Golubev, O.; Anisimov, A.; Glotov, A.; Vutolkina, A.; Maximov, A. Alkali earth catalysts based on mesoporous MCM-41 and Al-SBA-15 for sulfone removal from middle distillates. ACS Omega 2019, 4, 12736–12744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghiaci, M.; Mostajeran, M.; Gil, A. Synthesis and characterization of Co–Mn nanoparticles immobilized on a modified bentonite and its application for oxidation of p-xylene to terephthalic acid. Ind. Eng. Chem. Res. 2012, 51, 15821–15831. [Google Scholar] [CrossRef]
- Carver, J.C.; Schweitzer, G.K.; Carlson, T.A. Use of X-Ray Photoelectron Spectroscopy to Study Bonding in Cr, Mn, Fe, and Co Compounds. J. Chem. Phys. 1972, 57, 973–981. [Google Scholar] [CrossRef]
- Franzen, H.F.; Umana, M.X.; McCreary, J.R.; Thorn, R.J. XPS spectra of some transition metal and alkaline earth monochalcogenides. J. Solid State Chem. 1976, 18, 363–368. [Google Scholar] [CrossRef]
- Tan, B.J.; Klabunde, K.J.; Sherwood, P.M.A. XPS studies of solvated metal atom dispersed (SMAD) catalysts. Evidence for layered cobalt-manganese particles on alumina and silica. J. Am. Chem. Soc. 1991, 113, 855–861. [Google Scholar] [CrossRef]
- McIntyre, N.S.; Cook, M.G. X-ray photoelectron studies on some oxides and hydroxides of cobalt, nickel, and copper. Anal. Chem. 1975, 47, 2208–2213. [Google Scholar] [CrossRef]
- Mc Intyre, N.S.; Johnston, D.D.; Coatsworth, L.L.; Davidson, R.D.; Brown, J.R. X-ray photoelectron spectroscopic studies of thin film oxides of cobalt and molybdenum. Surf. Interface Anal. 1990, 15, 265–272. [Google Scholar] [CrossRef]
- Kim, K.S. X-ray-photoelectron spectroscopic studies of the electronic structure of CoO. Phys. Rev. B 1975, 11, 2177–2185. [Google Scholar] [CrossRef]
- Umezawa, Y.; Reilley, C.N. Effect of argon ion bombardment on metal complexes and oxides studied by x-ray photoelectron spectroscopy. Anal. Chem. 1978, 50, 1290–1295. [Google Scholar] [CrossRef]
- Oku, M. X-ray photoelectron spectra of KMnO4 and K2MnO4 fractured in situ. J. Electron Spectrosc. Relat. Phenom. 1995, 74, 135–148. [Google Scholar] [CrossRef]
- Brown, D.G.; Weser, U. XPS spectra of spin-triplet cobalt (III) complexes. Z. Nat. B 1979, 34, 1468–1470. [Google Scholar] [CrossRef] [Green Version]
- Nefedov, V.I.; Baranovskii, I.B.; Molodkin, A.K.; Omuralieva, V.O. X-ray photoelectron study of cobalt compounds. Russ. J. Inorg. Chem. 1973, 18, 1295. (In Russian) [Google Scholar]
- Nefedov, V.I.; Gati, D.; Dzhurinskii, B.F.; Sergushin, N.P.; Salyn, Y.V. X-ray photoelectron study of several elements oxides. Russ. J. Inorg. Chem. 1975, 20, 2307. (In Russian) [Google Scholar]
- Nefedov, V.I.; Firsov, M.N.; Shaplygin, I.S. Electronic structures of MRhO2, MRh2O4, RhMO4 and Rh2MO6 on the basis of X-ray spectroscopy and ESCA data. J. Electron Spectrosc. Relat. Phenom. 1982, 26, 65–78. [Google Scholar] [CrossRef]
- Molinaro, F.S.; Little, R.G.; Ibers, J.A. Oxygen binding to a model for the active site in cobalt-substituted hemoglobin. J. Am. Chem. Soc. 1977, 99, 5628–5632. [Google Scholar] [CrossRef]
- Emara, A.A.A.; Ali, A.M.; El-Asmy, A.F.; Ragab, E.-S.M. Investigation of the oxygen affinity of manganese (II), cobalt (II) and nickel (II) complexes with some tetradentate Schiff bases. J. Saudi Chem. Soc. 2011, 18, 762–773. [Google Scholar] [CrossRef] [Green Version]
- Beamson, G.; Briggs, D. High Resolution XPS of Organic Polymers: The Scienta ESCA300 Database; John Wiley & Sons: Chichester, UK, 1992; 295p. [Google Scholar]
- Briggs, D.; Beamson, G. Primary and secondary oxygen-induced C 1s binding energy shifts in X-ray photoelectron spectroscopy of polymers. Anal. Chem. 1992, 64, 1729–1736. [Google Scholar] [CrossRef]
- Yuan, H.; Fang, X.; Ma, Q.; Mao, J.; Chen, K.; Chen, Z.; Li, H. New mechanistic insight into the aerobic oxidation of methylaromatic compounds catalyzed by Co–Mn–Br and its applications. J. Catal. 2016, 339, 284–291. [Google Scholar] [CrossRef]
- Wang, Q.; Li, X.; Wang, L.; Cheng, Y.; Xie, G. Effect of water content on the kinetics of p-xylene liquid-phase catalytic oxidation to terephthalic acid. Ind. Eng. Chem. Res. 2005, 44, 4518–4522. [Google Scholar] [CrossRef]
- Li, M.; Niu, F.; Busch, D.H.; Subramaniam, B. Kinetic investigations of p-xylene oxidation to terephthalic acid with a Co/Mn/Br catalyst in a homogeneous liquid phase. Ind. Eng. Chem. Res. 2014, 53, 9017–9026. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, X.; Wang, L.; Wang, Q. Optimum ratio of Co/Mn in the liquid-phase catalytic oxidation of p-xylene to terephthalic acid. Ind. Eng. Chem. Res. 2006, 45, 4156–4162. [Google Scholar] [CrossRef]
- Li, K.; Li, S. CoBr2-MnBr2 containing catalysts for catalytic oxidation of p-xylene to terephthalic acid. Appl. Catal. A Gen. 2008, 340, 271–277. [Google Scholar] [CrossRef]
- Liu, H.; Chen, G.; Jiang, H.; Li, Y.; Luque, R. From alkyl aromatics to aromatic esters: Efficient and selective C–H activation promoted by a bimetallic heterogeneous catalyst. ChemSusChem 2012, 5, 1892–1896. [Google Scholar] [CrossRef] [PubMed]
- Kesavan, L.; Tiruvalam, R.; Ab Rahim, M.H.; bin Saiman, M.I.; Enache, D.I.; Jenkins, R.L.; Dimitratos, N.; Lopez-Sanchez, J.A.; Taylor, S.H.; Knight, D.W.; et al. Solvent-free oxidation of primary carbon-hydrogen bonds in toluene using Au-Pd alloy nanoparticles. Science 2011, 331, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Tbibitt, J.M.; Gong, W.H.; Schammel, W.P.; Hepfer, R.P.; Adamian, V.; Brugge, S.P.; Metelski, P.D.; Zhou, C. Process for the Production of Aromatic Carboxylic Acids in Water. WO Patent 2007133976 A2, 22 November 2007. [Google Scholar]
- Tashiro, Y.; Iwahama, T.; Sakaguchi, S.; Ishii, Y. A new strategy for the preparation of terephthalic acid by the aerobic oxidation of p-xylene using N-hydroxyphthalimide as a catalyst. Adv. Synth. Catal. 2001, 343, 220–225. [Google Scholar] [CrossRef]
- Saha, B.; Koshino, N.; Espenson, J.H. N-hydroxyphthalimides and metal cocatalysts for the autoxidation of p-xylene to terephthalic acid. J. Phys. Chem. A 2004, 108, 425–431. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, X.Y.; Wang, Q.B.; Tan, Z.; Guo, C.C.; Deng, W.; Liu, Z.G.; Zhang, H.F. The preparation of terephthalic acid by solvent-free oxidation of p-xylene with air over T(p-Cl)PPMnCl and Co(OAc)2. Chin. Chem. Lett. 2011, 22, 135–138. [Google Scholar] [CrossRef]






| Mn (wt. %) | Co (wt. %) | Surface Concentration by XPS, at. % | Mn Valency States at Mn 2p3/2 Line, at. % | Co Valency States at Co 2p3/2 Line, at. % | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mn | Co | Si | Al | C | N | O | MnO (eV) | [MnO4] Bound (eV) | CoO (eV) | [CoO6] Bound (eV) | ||
| 0.15 | 1.29 | <0.1 | 0.7 | 25.9 | 2.5 | 5.8 | 0.6 | 64.2 | 79.8 (642.5) | 20.2 (646.9) | 15.3 (780.0) | 84.7 (782.5) |
| Entry | Catalyst | Conv., % | Product Yields, % mol. | TOF, h−1 | ||||
|---|---|---|---|---|---|---|---|---|
| pTald | pTac | 4-HMBA | 4-CBA | TPA | ||||
| 1 | Mn(OAc)2/Co(OAc)2 | 98.0 | 0.15 | 0.95 | 1.2 | 0.5 | 95.2 | 37.5 |
| 2 | MnII1CoII10@MCM-41/HNT | 99.0 | 0.2 | 1.0 | 4.0 | - | 93.8 | 142.5 |
| Entry | Catalyst | P(O2), atm | KBr | Conv., % | Product Yields, % mol. | TOF, h−1 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| pTald | pTac | TPAld 2 | 4-HMBA | 4-CBA | TPA | ||||||
| 1 | Mn(OAc)2/Co(OAc)2 | 5 | Yes | 89.1 | 11.7 | 49.8 | x | 12.6 | 11.8 | 3.2 | 19.0 |
| 2 | MnII1CoII10@MCM-41/HNT | 5 | Yes | 87.9 | 14.1 | 62.8 | 0.25 | 6.2 | 4.1 | 0.4 | 63.2 |
| 3 | MnII1CoII10@MCM-41/HNT | 20 | No | 2.2 | 1.1 | 0.4 | - | 0.1 | 0.1 | 0.5 | 1.6 |
| Entry. | T, °C | t, h | Conv., % | Product Yields, % mol. | TOF, h−1 | ||||
|---|---|---|---|---|---|---|---|---|---|
| pTald | pTac | 4-HMBA | 4-CBA | TPA | |||||
| 1 | 150 | 5 | 36.8 | 28.8 | 5.2 | 1.9 | 0.1 | 0.8 | 15.7 |
| 2 | 150 | 3 | 25.9 | 21.1 | 2.5 | 1.8 | - | 0.5 | |
| 3 | 150 | 1 | 10.2 | 8.0 | 0.5 | 1.5 | - | 0.2 | |
| 4 | 200 | 5 | 99.8 | 0.1 | 0.2 | 2.3 | - | 97.2 | 401.7 |
| 5 | 200 | 3 | 99.0 | 0.2 | 1.0 | 4.0 | - | 93.8 | |
| 6 | 200 | 1 | 98.8 | 1.9 | 11.7 | 0.2 | - | 85.0 | |
| 7 | 250 | 5 | 100 | - | - | 0.5 | - | 99.5 | 424.8 |
| 8 | 250 | 3 | 99.9 | - | 0.5 | 1.8 | - | 97.6 | |
| 9 | 250 | 1 | 99.5 | - | 4.5 | 2.1 | - | 92.9 | |
| Entry | Mn (wt. %) | Co (wt. %) | Reaction Conditions |
|---|---|---|---|
| 1 | 0.15 | 1.29 | Initial catalyst |
| 2 | 0.15 | 0.22 | 150 °C, 20 atm. of O2, 3 h |
| 3 | 0.03 | 0.13 | 200 °C, 20 atm. of O2, 3 h |
| 4 | 0.04 | 0.09 | 250 °C, 20 atm. of O2, 3 h |
| 5 | 0.01 | 0.10 | 200 °C, 20 atm. of O2, 5 h |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karakhanov, E.; Maximov, A.; Zolotukhina, A.; Vinokurov, V.; Ivanov, E.; Glotov, A. Manganese and Cobalt Doped Hierarchical Mesoporous Halloysite-Based Catalysts for Selective Oxidation of p-Xylene to Terephthalic Acid. Catalysts 2020, 10, 7. https://doi.org/10.3390/catal10010007
Karakhanov E, Maximov A, Zolotukhina A, Vinokurov V, Ivanov E, Glotov A. Manganese and Cobalt Doped Hierarchical Mesoporous Halloysite-Based Catalysts for Selective Oxidation of p-Xylene to Terephthalic Acid. Catalysts. 2020; 10(1):7. https://doi.org/10.3390/catal10010007
Chicago/Turabian StyleKarakhanov, Eduard, Anton Maximov, Anna Zolotukhina, Vladimir Vinokurov, Evgenii Ivanov, and Aleksandr Glotov. 2020. "Manganese and Cobalt Doped Hierarchical Mesoporous Halloysite-Based Catalysts for Selective Oxidation of p-Xylene to Terephthalic Acid" Catalysts 10, no. 1: 7. https://doi.org/10.3390/catal10010007
APA StyleKarakhanov, E., Maximov, A., Zolotukhina, A., Vinokurov, V., Ivanov, E., & Glotov, A. (2020). Manganese and Cobalt Doped Hierarchical Mesoporous Halloysite-Based Catalysts for Selective Oxidation of p-Xylene to Terephthalic Acid. Catalysts, 10(1), 7. https://doi.org/10.3390/catal10010007