Metal-Free Enhanced Photocatalytic Activation of Dioxygen by g-C3N4 Doped with Abundant Oxygen-Containing Functional Groups for Selective N-Deethylation of Rhodamine B
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization
2.2. Photocatalytic Activity of O-g-C3N4 for Selective N-Deethylation of RhB
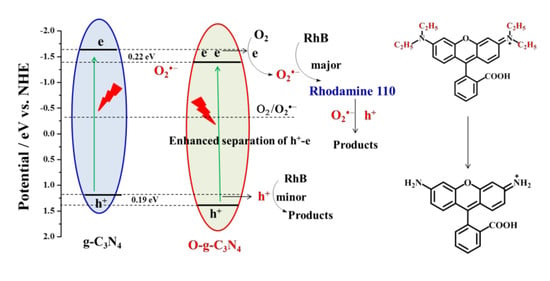
2.3. Radicals Identification and Catalytic Mechanism
2.4. Stability of O-g-C3N4
3. Materials, Experiment and Analysis Methods
3.1. Materials
3.2. Preparation of g-C3N4 and Oxygen Doped g-C3N4 Catalysts
3.3. Characterization
3.4. Photocatalytic Reaction
3.5. Chemical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Konstantinou, I.K.; Albanis, T.A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations: A review. Appl. Catal. B Environ. 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Ruggieri, F.; Di Camillo, D.; Maccarone, L.; Santucci, S.; Lozzi, L. Electrospun Cu-, W- and Fe-doped TiO2 nanofibres for photocatalytic degradation of rhodamine 6G. J. Nanoparticle Res. 2013, 15, 1982–1992. [Google Scholar] [CrossRef]
- Cao, J.; Nie, W.; Huang, L.; Ding, Y.; Lv, K.; Tang, H. Photocatalytic activation of sulfite by nitrogen vacancy modified graphitic carbon nitride for efficient degradation of carbamazepine. Appl. Catal. B Environ. 2019, 241, 18–27. [Google Scholar] [CrossRef]
- Cheng, J.; Hu, Z.; Li, Q.; Li, X.; Fang, S.; Wu, X.; Li, M.; Ding, Y.; Liu, B.; Yang, C. Fabrication of high photoreactive carbon nitride nanosheets by polymerization of amidinourea for hydrogen production. Appl. Catal. B Environ. 2019, 245, 197–206. [Google Scholar] [CrossRef]
- Cheng, J.; Hu, Z.; Lv, K.; Wu, X.; Li, Q.; Li, Y.; Li, X.; Sun, J. Drastic promoting the visible photoreactivity of layered carbon nitride by polymerization of dicyandiamide at high pressure. Appl. Catal. B Environ. 2018, 232, 330–339. [Google Scholar] [CrossRef]
- Mamba, G.; Mishra, A. Graphitic carbon nitride (g-C3N4) nanocomposites: A new and exciting generation of visible light driven photocatalysts for environmental pollution remediation. Appl. Catal. B Environ. 2016, 198, 347–377. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, S.; Chen, S.; Li, D.; Zhang, X.; Shao, W.; Sun, X.; Xie, J.; Zhao, Z.; Zhang, Q.; et al. Enhanced Singlet Oxygen Generation in Oxidized Graphitic Carbon Nitride for Organic Synthesis. Adv. Mater. 2016, 28, 6940–6945. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhou, P.; Zhang, Z.; Yang, C.; Zhang, B.; Deng, K.; Bottle, S.; Zhu, H. Selective oxidation of 5-hydroxymethylfurfural to 2, 5-furandicarboxylic acid using O2 and a photocatalyst of Co-thioporphyrazine bonded to g-C3N4. J. Am. Chem. Soc. 2017, 139, 14775–14782. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Jiao, Y.; Chen, J.; Liu, J.; Liang, J.; Du, A.; Zhang, W.; Zhu, Z.; Smith, S.C.; Jaroniec, M. Nanoporous graphitic-C3N4@carbon metal-free electrocatalysts for highly efficient oxygen reduction. J. Am. Chem. Soc. 2011, 133, 20116–20119. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shen, B.; Hong, Z.; Lin, B.; Gao, B.; Chen, Y. A facile approach to synthesize novel oxygen-doped g-C3N4 with superior visible-light photoreactivity. Chem. Commun. 2012, 48, 12017–12019. [Google Scholar] [CrossRef]
- Zhu, Y.-P.; Ren, T.-Z.; Yuan, Z.-Y. Mesoporous phosphorus-doped g-C3N4 nanostructured flowers with superior photocatalytic hydrogen evolution performance. ACS Appl. Mater. Interfaces 2015, 7, 16850–16856. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, Q.; Liu, B.; Cheng, B.; Ho, W.; Yu, J. Sulfur-doped g-C3N4 with enhanced photocatalytic CO2-reduction performance. Appl. Catal. B Environ. 2015, 176, 44–52. [Google Scholar] [CrossRef]
- Sagara, N.; Kamimura, S.; Tsubota, T.; Ohno, T. Photoelectrochemical CO2 reduction by a p-type boron-doped g-C3N4 electrode under visible light. Appl. Catal. B Environ. 2016, 192, 193–198. [Google Scholar] [CrossRef] [Green Version]
- Cao, J.; Pan, C.; Ding, Y.; Li, W.; Lv, K.; Tang, H. Constructing nitrogen vacancy introduced g-C3N4 pn homojunction for enhanced photocatalytic activity. J. Environ. Chem. Eng. 2019, 7, 102984. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, Y.; Chen, M.; Shi, X.; Zhang, Y.; Cao, J.; Ho, W.; Lee, S.C. Roles of N-Vacancies over Porous g-C3N4 Microtubes during Photocatalytic NOx Removal. ACS Appl. Mater. Interfaces 2019, 11, 10651–10662. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wang, Y.; Zhou, S.; Lin, W.; Kong, Y. A facile one-step synthesis of Fe-doped g-C3N4 nanosheets and their improved visible-light photocatalytic performance. ChemCatChem 2017, 9, 1708–1715. [Google Scholar] [CrossRef] [Green Version]
- Le, S.; Jiang, T.; Zhao, Q.; Liu, X.; Li, Y.; Fang, B.; Gong, M. Cu-doped mesoporous graphitic carbon nitride for enhanced visible-light driven photocatalysis. RSC Adv. 2016, 6, 38811–38819. [Google Scholar] [CrossRef]
- Shi, A.; Li, H.; Yin, S.; Liu, B.; Zhang, J.; Wang, Y. Effect of conjugation degree and delocalized π-system on the photocatalytic activity of single layer g-C3N4. Appl. Catal. B Environ. 2017, 218, 137–146. [Google Scholar] [CrossRef]
- Ma, S.; Zhan, S.; Jia, Y.; Shi, Q.; Zhou, Q. Enhanced disinfection application of Ag-modified g-C3N4 composite under visible light. Appl. Catal. B Environ. 2016, 186, 77–87. [Google Scholar] [CrossRef]
- Hao, R.; Wang, G.; Tang, H.; Sun, L.; Xu, C.; Han, D. Template-free preparation of macro/mesoporous g-C3N4/TiO2 heterojunction photocatalysts with enhanced visible light photocatalytic activity. Appl. Catal. B Environ. 2016, 187, 47–58. [Google Scholar] [CrossRef]
- Sun, Q.; Lv, K.; Zhang, Z.; Li, M.; Li, B. Effect of contact interface between TiO2 and g-C3N4 on the photoreactivity of g-C3N4/TiO2 photocatalyst: (0 0 1) vs (1 0 1) facets of TiO2. Appl. Catal. B Environ. 2015, 164, 420–427. [Google Scholar]
- Cui, L.; Ding, X.; Wang, Y.; Shi, H.; Huang, L.; Zuo, Y.; Kang, S. Facile preparation of Z-scheme WO3/g-C3N4 composite photocatalyst with enhanced photocatalytic performance under visible light. Appl. Surf. Sci. 2017, 391, 202–210. [Google Scholar] [CrossRef]
- Ye, L.; Liu, J.; Jiang, Z.; Peng, T.; Zan, L. Facets coupling of BiOBr-g-C3N4 composite photocatalyst for enhanced visible-light-driven photocatalytic activity. Appl. Catal. B Environ. 2013, 142, 1–7. [Google Scholar] [CrossRef]
- He, Y.; Zhang, L.; Teng, B.; Fan, M. New application of Z-scheme Ag3PO4/g-C3N4 composite in converting CO2 to fuel. Environ. Sci. Technol. 2014, 49, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Zhu, Y.; Yan, Y.; Min, Y.; Fan, J.; Xu, Q. Holey structured graphitic carbon nitride thin sheets with edge oxygen doping via photo-Fenton reaction with enhanced photocatalytic activity. Appl. Catal. B Environ. 2016, 185, 315–321. [Google Scholar] [CrossRef]
- Fu, J.; Zhu, B.; Jiang, C.; Cheng, B.; You, W.; Yu, J. Hierarchical Porous O-Doped g-C3N4 with Enhanced Photocatalytic CO2 Reduction Activity. Small 2017, 13, 1603938. [Google Scholar] [CrossRef]
- Zeng, Y.; Liu, X.; Liu, C.; Wang, L.; Xia, Y.; Zhang, S.; Luo, S.; Pei, Y. Scalable one-step production of porous oxygen-doped g-C3N4 nanorods with effective electron separation for excellent visible-light photocatalytic activity. Appl. Catal. B Environ. 2018, 224, 1–9. [Google Scholar] [CrossRef]
- Huang, Y.H.; Huang, Y.F.; Huang, C.I.; Chen, C.Y. Efficient decolorization of azo dye Reactive Black B involving aromatic fragment degradation in buffered Co2+/PMS oxidative processes with a ppb level dosage of Co2+-catalyst. J. Hazard. Mater. 2009, 170, 1110–1118. [Google Scholar] [CrossRef]
- Yang, S.; Wang, P.; Yang, X.; Shan, L.; Zhang, W.; Shao, X.; Niu, R. Degradation efficiencies of azo dye Acid Orange 7 by the interaction of heat, UV and anions with common oxidants: Persulfate, peroxymonosulfate and hydrogen peroxide. J. Hazard. Mater. 2010, 179, 552–558. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, L.; Shi, R.; Zhu, Y. Chemical exfoliation of graphitic carbon nitride for efficient heterogeneous photocatalysis. J. Mater. Chem. A 2013, 1, 14766–14772. [Google Scholar] [CrossRef]
- Dong, F.; Li, Y.; Wang, Z.; Ho, W.K. Enhanced visible light photocatalytic activity and oxidation ability of porous graphene-like g-C3N4 nanosheets via thermal exfoliation. Appl. Surf. Sci. 2015, 358, 393–403. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, Y.; Li, J.; Wang, Y.; Jiang, G.; Zhao, Z.; Wang, D.; Duan, A.; Liu, J.; Wei, Y. Facile in situ synthesis of graphitic carbon nitride (g-C3N4)-N-TiO2 heterojunction as an efficient photocatalyst for the selective photoreduction of CO2 to CO. Appl. Catal. B Environ. 2014, 158–159, 20–29. [Google Scholar] [CrossRef]
- Yang, L.; Huang, J.; Shi, L.; Cao, L.; Yu, Q.; Jie, Y.; Fei, J.; Ouyang, H.; Ye, J. A surface modification resultant thermally oxidized porous g-C3N4 with enhanced photocatalytic hydrogen production. Appl. Catal. B Environ. 2017, 204, 335–345. [Google Scholar] [CrossRef]
- Cao, S.-W.; Liu, X.-F.; Yuan, Y.-P.; Zhang, Z.-Y.; Liao, Y.-S.; Fang, J.; Loo, S.C.J.; Sum, T.C.; Xue, C. Solar-to-fuels conversion over In2O3/g-C3N4 hybrid photocatalysts. Appl. Catal. B Environ. 2014, 147, 940–946. [Google Scholar] [CrossRef]
- Ye, L.; Chen, S. Fabrication and high visible-light-driven photocurrent response of g-C3N4 film: The role of thiourea. Appl. Surf. Sci. 2016, 389, 1076–1083. [Google Scholar] [CrossRef]
- Yang, F.; Lublow, M.; Orthmann, S.; Merschjann, C.; Tyborski, T.; Rusu, M.; Kubala, S.; Thomas, A.; Arrigo, R.; Hävecker, M. Metal-Free Photocatalytic Graphitic Carbon Nitride on p-Type Chalcopyrite as a Composite Photocathode for Light-Induced Hydrogen Evolution. ChemSusChem 2012, 5, 1227–1232. [Google Scholar] [CrossRef]
- Du, X.; Yi, X.; Wang, P.; Deng, J.; Wang, C.-C. Enhanced photocatalytic Cr(VI) reduction and diclofenac sodium degradation under simulated sunlight irradiation over MIL-100(Fe)/g-C3N4 heterojunctions. Chin. J. Catal. 2019, 40, 70–79. [Google Scholar] [CrossRef]
- Li, X.; Liu, C.; Wu, D.; Li, J.; Huo, P.; Wang, H. Improved charge transfer by size-dependent plasmonic Au on C3N4 for efficient photocatalytic oxidation of RhB and CO2 reduction. Chin. J. Catal. 2019, 40, 928–939. [Google Scholar] [CrossRef]
- Barras, A.; Cordier, S.; Boukherroub, R. Fast photocatalytic degradation of rhodamine B over [Mo6Br8(N3)6]2− cluster units under sun light irradiation. Appl. Catal. B Environ. 2012, 123–124, 1–8. [Google Scholar] [CrossRef]
- Hu, X.; Mohamood, T.; Ma, W.; Chen, C.; Zhao, J. Oxidative decomposition of rhodamine B dye in the presence of VO2+ and/or Pt (IV) under visible light irradiation: N-deethylation, chromophore cleavage, and mineralization. J. Phys. Chem. B 2006, 110, 26012–26018. [Google Scholar] [CrossRef]
- Li, W.; Li, D.; Meng, S.; Chen, W.; Fu, X.; Shao, Y. Novel Approach To Enhance Photosensitized Degradation of Rhodamine B under Visible Light Irradiation by the ZnxCd1-xS/TiO2 Nanocomposites. Environ. Sci. Technol. 2011, 45, 2987–2993. [Google Scholar] [CrossRef] [PubMed]
- Murcia López, S.; Hidalgo, M.C.; Navío, J.A.; Colón, G. Novel Bi2WO6–TiO2 heterostructures for Rhodamine B degradation under sunlike irradiation. J. Hazard. Mater. 2011, 185, 1425–1434. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhao, J.; Hidaka, H. Highly selective deethylation of rhodamine B: Adsorption and photooxidation pathways of the dye on the TiO2/SiO2 composite photocatalyst. Int. J. Photoenergy 2003, 5. [Google Scholar]
- Fu, H.; Zhang, S.; Xu, T.; Zhu, Y.; Chen, J. Photocatalytic Degradation of RhB by Fluorinated Bi2WO6 and Distributions of the Intermediate Products. Environ. Sci. Technol. 2008, 42, 2085–2091. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Takizawa, T.; Honda, K. Photocatalysis through excitation of adsorbates. 1. Highly efficient N-deethylation of rhodamine B adsorbed to cadmium sulfide. J. Phys. Chem. 1977, 81, 1845–1851. [Google Scholar] [CrossRef]
- Li, Z.; Yang, Q.; Chen, C.; Zhang, Z.; Fang, X. Enhanced photocatalytic performance of polymeric C3N4 doped with theobromine composed of an imidazole ring and a pyrimidine ring. Chin. J. Catal. 2019, 40, 875–885. [Google Scholar] [CrossRef]
- Zhang, J.; Mao, X.; Xiao, W.; Zhuang, Y. Photocatalytic degradation of sulfamethazine by graphitic carbon nitride-modified zinc molybdate: Effects of synthesis method on performance, degradation kinetics, and mechanism. Chin. J. Catal. 2017, 38, 2009–2020. [Google Scholar] [CrossRef]
- Wang, F.; Chen, P.; Feng, Y.; Xie, Z.; Liu, Y.; Su, Y.; Zhang, Q.; Wang, Y.; Yao, K.; Lv, W.; et al. Facile synthesis of N-doped carbon dots/g-C3N4 photocatalyst with enhanced visible-light photocatalytic activity for the degradation of indomethacin. Appl. Catal. B Environ. 2017, 207, 103–113. [Google Scholar] [CrossRef]
- Qin, Y.; Ding, Y.; Tang, H. Highly efficient visible-light photocatalytic activity of graphitic carbon nitride prepared from melamine-thiourea molecular composite. J. Environ. Chem. Eng. 2016, 4, 4374–4384. [Google Scholar] [CrossRef]














| Samples | Oxygen (at.%) | Surface Oxygen (at.%) | Eg (eV) | EVB (eV) | ECB (eV) | SBET (m2/g) | RhB Removal (%) | k (min−1) |
|---|---|---|---|---|---|---|---|---|
| g-C3N4 | 2.73 | 1.8 | 2.82 | 1.19 | −1.63 | 14.5 | 55.9 | 0.0032 |
| O-g-C3N4 | 3.16 | 6.9 | 2.79 | 1.38 | −1.41 | 14.7 | 97.8 | 0.079 |
| HPLC Peaks | Retention Time (min) | Corresponding Intermediates of RhB | ESI(-)MS2 m/z | Assigned Substrates |
|---|---|---|---|---|
| RhB | 7.753 |  | 443.2 (100%) 444.2 (31.5%) 445.2 (5.4%) | (RhB-Cl)+ (RhB-Cl+H)+ (RhB-Cl +2H)+ |
| P1 | 6.157 |  | 415.2 (100%) 416.2 (29.3%) 417.2 (4.8%) | P1+ (P1+H)+ (P1+2H)+ |
| P2 | 4.890 |  | 387.2 (100%) 388.2 (27.1%) 389.2 (4.1%) | P2+ (P2+H)+ (P2+2H)+ |
| P3 | 3.640 |  | 387.2 (100%) 388.2 (27.1%) 389.2 (4.1%) | P3+ (P3+H)+ (P3+2H)+ |
| P4 | 3.010 |  | 359.1 (100%) 360.1 (24.9%) 361.1 (3.6%) | P4+ (P4+H)+ (P4+2H)+ |
| P5 | 2.273 |  | 331.1 (100%) 332.1 (22.7%) 333.1 (3.1%) | P5+ (P5+H)+ (P5+2H)+ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Nie, G.; Ding, Y. Metal-Free Enhanced Photocatalytic Activation of Dioxygen by g-C3N4 Doped with Abundant Oxygen-Containing Functional Groups for Selective N-Deethylation of Rhodamine B. Catalysts 2020, 10, 6. https://doi.org/10.3390/catal10010006
Huang J, Nie G, Ding Y. Metal-Free Enhanced Photocatalytic Activation of Dioxygen by g-C3N4 Doped with Abundant Oxygen-Containing Functional Groups for Selective N-Deethylation of Rhodamine B. Catalysts. 2020; 10(1):6. https://doi.org/10.3390/catal10010006
Chicago/Turabian StyleHuang, Jia, Gang Nie, and Yaobin Ding. 2020. "Metal-Free Enhanced Photocatalytic Activation of Dioxygen by g-C3N4 Doped with Abundant Oxygen-Containing Functional Groups for Selective N-Deethylation of Rhodamine B" Catalysts 10, no. 1: 6. https://doi.org/10.3390/catal10010006
APA StyleHuang, J., Nie, G., & Ding, Y. (2020). Metal-Free Enhanced Photocatalytic Activation of Dioxygen by g-C3N4 Doped with Abundant Oxygen-Containing Functional Groups for Selective N-Deethylation of Rhodamine B. Catalysts, 10(1), 6. https://doi.org/10.3390/catal10010006