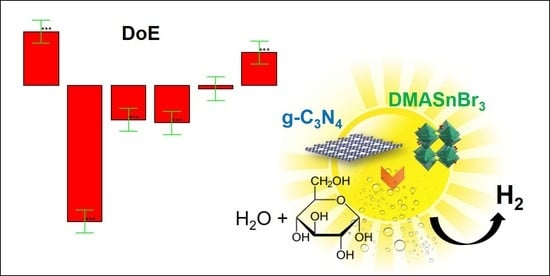
Carbon Nitride-Perovskite Composites: Evaluation and Optimization of Photocatalytic Hydrogen Evolution in Saccharides Aqueous Solution
Abstract
:1. Introduction
2. Results and Discussion
2.1. Comparison between DMASnBr3/g-C3N4 and PEA2SnBr4/g-C3N4 after Chemometric Optimization
2.2. Further Investigation of DMASnBr3/g-C3N4
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wen, J.; Xie, J.; Chen, X.; Li, X. A review on g-C3N4-based photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Nasir, M.S.; Yang, G.; Ayub, I.; Wang, S.; Wang, L.; Wang, X.; Yan, W.; Peng, S.; Ramakarishna, S. Recent development in graphitic carbon nitride based photocatalysis for hydrogen generation. Appl. Catal. B 2019, 257, 117855. [Google Scholar] [CrossRef]
- Lam, S.S.; Nguyen, V.-H.; Dinh, M.T.N.; Khieu, D.Q.; La, D.D.; Nguyen, H.T.; Vo, D.V.N.; Xia, C.; Varma, R.S.; Shokouhimehr, M.; et al. Mainstrem avenues for boosting graphitic carbon nitride efficiency: Towards enhanced solar light-driven photocatalytic hydrogen production and environmental remediation. J. Mater. Chem. A 2020, 8, 10571–10603. [Google Scholar] [CrossRef]
- Speltini, A.; Scalabrini, A.; Maraschi, F.; Sturini, M.; Pisanu, A.; Malavasi, L.; Profumo, A. Improved photocatalytic H2 production assisted by aqueous glucose biomass by oxidized g-C3N4. Int. J. Hydrogen Energy 2018, 43, 14925–14933. [Google Scholar] [CrossRef]
- Speltini, A.; Gualco, F.; Maraschi, F.; Sturini, M.; Dondi, D.; Malavasi, L.; Profumo, A. Photocatalytic hydrogen evolution assisted by aqueous (waste)biomass under simulated solar light: Oxidized g-C3N4 vs. P25 titanium dioxide. Int. J. Hydrogen Energy 2019, 44, 4072–4078. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Xia, Y.; Xun, M.; Chen, H.; Liu, X.; Yin, X. Metal-free carbon quantum dots implant graphitic carbon nitride: Enhanced photocatalytic dye wastewater purification with simultaneous hydrogen production. Int. J. Mol. Sci. 2020, 21, 1052. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Pradhan, B.; Hofkens, J.; Roeffaers, M.B.; Steele, J.A. Solar-driven metal halide perovskite photocatalysis: Design, stability, and performance. ACS Energy Lett. 2020, 5, 1107–1123. [Google Scholar] [CrossRef]
- Romani, L.; Malavasi, L. Solar-driven hydrogen generation by metal halide perovskites: Materials, approaches, and mechanistic view. ACS Omega 2020, (in press). [Google Scholar] [CrossRef]
- Stanks, S.D.; Snaith, H.L. Metal-halide perovskites for photovoltaic and light-emitting devices. Nat. Nanotec. 2015, 10, 391–402. [Google Scholar]
- Pu, Y.-C.; Fan, H.-C.; Liu, T.-W.; Chen, J.-W. Methylamine lead bromide perovskite/protonated graphitic carbon nitride nanocomposites: Interfacial charge carrier dynamics and photocatalysis. J. Mater. Chem. A 2017, 5, 25438–25449. [Google Scholar] [CrossRef]
- Li, Z.; Wu, S.; Zhang, J.; Yuan, Y.; Wang, Z.; Zhu, Z. Improving photovoltaic performance using perovskite/surface-modified graphitic carbon nitride heterojunction. Sol. RRL 2020, 4, 1900413. [Google Scholar] [CrossRef]
- Romani, L.; Bala, A.; Kumar, V.; Speltini, A.; Milella, A.; Fracassi, F.; Listorti, A.; Profumo, A.; Malavasi, L. PEA2SnBr4: A water-stable lead-free two-dimensional perovskite and demonstration of its use as co-catalyst in hydrogen photogeneration and organic-dye degradation. J. Mater. Chem. C 2020, 8, 9189–9194. [Google Scholar] [CrossRef]
- Romani, L.; Speltini., A.; Ambrosio, F.; Mosconi, E.; Profumo, A.; Marelli, M.; Margadonna, S.; Milella, A.; Fracassi, F.; Listorti, A.; et al. Water-stable DMASnBr3 lead-free perovskite for effective solar-driven photocatalysis. Angew. Chem. Int. Ed. 2020. [Google Scholar] [CrossRef]
- Pisanu, A.; Speltini, A.; Quadrelli, P.; Drera, G.; Sangaletti, L.; Malavasi, L. Enhanced air-stability of Sn-based hybrid perovskites induced by dimethylammonium (DMA): Synthesis, characterization, aging and hydrogen photogeneration of the MA1-xDMAxSnBr3 system. J. Mater. Chem. C 2019, 7, 7020–7026. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Fu, X.; Wang, C.; Ni, M.; Leung, M.K.H.; Wang, X.; Fu, X. Hydrogen production over titania based photocatalysts. ChemSusChem 2010, 3, 681–694. [Google Scholar] [CrossRef]
- Silva, C.G.; Sampaio, M.J.; Marques, R.R.N.; Ferreira, L.A.; Tavares, P.B.; Silva, A.M.T.; Faria, J.L. Photocatalytic production of hydrogen from methanol and saccharides using carbon nanotube-TiO2 catalysts. Appl. Catal. B Environ. 2015, 178, 82–90. [Google Scholar] [CrossRef] [Green Version]
- Fu, X.; Long, J.; Wang, X.; Leung, D.Y.C.; Ding, Z.; Wu, L.; Zhang, Z.; Li, Z.; Fu, X. Photocatalytic reforming of biomass: A systematic study of hydrogen evolution from glucose solution. Int. J. Hydrogen Energy 2008, 33, 6484–6491. [Google Scholar] [CrossRef]
- Kisch, H. On the problem of comparing rates or apparent quantum yields in heterogeneous photocatalysis. Angew. Chem. Int. Ed. 2010, 49, 9588–9589. [Google Scholar] [CrossRef] [PubMed]
- Speltini, A.; Sturini, M.; Maraschi, F.; Dondi, D.; Serra, A.; Profumo, A.; Buttafava, A.; Albini, A. Swine sewage as sacrificial biomass for photocatalytic hydrogen gas production: Explorative study. Int. J. Hydrogen Energy 2014, 39, 11433–11440. [Google Scholar] [CrossRef]
- Speltini, A.; Sturini, M.; Maraschi, F.; Dondi, D.; Fisogni, G.; Annovazzi, E.; Profumo, A.; Buttafava, A. Evaluation of UV-A and solar light photocatalytic hydrogen gas evolution from olive mill wastewater. Int. J. Hydrogen Energy 2015, 40, 4303–4310. [Google Scholar] [CrossRef]
- Baranowski, M.; Plochocka, P. Excitons in metal-halide perovskites. Adv. Energy Mater. 2020, 10, 1903659. [Google Scholar] [CrossRef]
- Bowker, M. Photocatalytic hydrogen production and oxygenate photoreforming. Catal. Lett. 2012, 142, 923–929. [Google Scholar] [CrossRef]
- Zhang, J.-H.; Wei, M.-J.; Wei, Z.-W.; Pan, M.; Su, C.-Y. Ultrathin graphitic carbon nitride nanosheets for photocatalytic hydrogen evolution. ACS Appl. Nano Mater. 2020, 3, 1010–1018. [Google Scholar] [CrossRef]
- Kennedy, J.; Bahruji, H.; Bowker, M.; Davies, P.R.; Bouleghlimat, E.; Issarapanacheewin, S. Hydrogen generation by photocatalytic reforming of potential biofuels: Polyols, cyclic alcohols, and saccharides. J. Photochem. Photobiol. Chem. 2018, 356, 451–456. [Google Scholar] [CrossRef]
- Jiang, X.; Manawan, M.; Feng, T.; Qian, R.; Zhao, T.; Zhou, G.; Kong, F.; Wang, Q.; Dai, S.; Pan, J.H. Anatase and rutile in evonik aeroxide P25: Heterojunctioned or individual nanoparticles? Catal. Today 2018, 300, 12–17. [Google Scholar] [CrossRef]
- Li, K.L.; Xia, L.X.; Li, J.; Pang, J.; Cao, G.Y.; Xi, Z.W. Salt-assisted acid hydrolysis of starch to D-glucose under microwave irradiation. Carbohydr. Res. 2001, 331, 9–12. [Google Scholar]
- Wang, D.; Hou, F.; Ma, X.; Chen, W.; Yan, L.; Ding, T.; Ye, X.; Liu, D. Study on the mechanism of ultrasound-accelerated enzymatic hydrolysis of starch: Analysis of ultrasound effect on different objects. Int. J. Biol. Macromol. 2020, 148, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Speltini, A.; Sturini, M.; Dondi, D.; Annovazzi, E.; Maraschi, F.; Caratto, V.; Profumo, A.; Buttafava, A. Sunlight-promoted photocatalytic hydrogen gas evolution from water-suspended cellulose: Systematic study. Photochem. Photobiol. Sci. 2014, 13, 1410–1419. [Google Scholar] [CrossRef] [Green Version]
- Pisanu, A.; Speltini, A.; Vigani, B.; Ferrari, F.; Mannini, M.; Calisi, N.; Cortigiani, B.; Caneschi, A.; Quadrelli, P.; Profumo, A.; et al. Enhanced hydrogen photogeneration by bulk g-C3N4 through a simple and efficient oxidation route. Dalton Trans. 2018, 47, 6772–6778. [Google Scholar] [CrossRef]
- Sun, H.; Zhou, X.; Zhang, H.; Tu, W. An efficient exfoliation method to obtain graphitic carbon nitride nanosheets with superior visible-light photocatalytic activity. Int. J. Hydrogen Energy 2017, 42, 7930–7937. [Google Scholar] [CrossRef]





| Variable | Level Codes | |
|---|---|---|
| −1 | +1 | |
| Glucose concentration (M), x1 | 0.025 | 0.2 |
| Catalyst amount (g L−1), x2 | 0.5 | 2 |
| Pt loading (wt%), x3 | 0.5 | 3 |
| Exp | Glucose Concentration (M), x1 | Catalyst Amount (g L−1), x2 | Pt Loading (wt%), x3 | DMASnBr3/g-C3N4 HER (µmoles g−1 h−1) | PEA2SnBr4/g-C3N4 HER (µmoles g−1 h−1) |
|---|---|---|---|---|---|
| 1 | 0.2 | 2 | 3 | 143 | 27 |
| 2 | 0.025 | 2 | 3 | 128 | 14 |
| 3 | 0.2 | 0.5 | 3 | 696 | 99 |
| 4 | 0.025 | 0.5 | 3 | 341 | 100 |
| 5 | 0.2 | 2 | 0.5 | 194 | 147 |
| 6 | 0.025 | 2 | 0.5 | 92 | 43 |
| 7 | 0.2 | 0.5 | 0.5 | 925 | 191 |
| 8 | 0.025 | 0.5 | 0.5 | 606 | 188 |
| Sample | HER (µmoles g−1 h−1) 1 |
|---|---|
| water + 0.5 g L−1 catalyst | 12 |
| water + 0.5 g L−1 catalyst + 0.5 wt% Pt | 62 |
| 0.2 M glucose + 0.5 g L−1 catalyst | 142 |
| 0.2 M glucose + 0.5 g L−1 catalyst + 0.5 wt% Pt | 925 |
| water | n.q. |
| 0.2 M glucose | n.q. |
| Sample | AQY | TON | HER (µmoles g−1 h−1) |
|---|---|---|---|
| distilled water | 0.1 | 202 | 62 |
| aqueous glucose | 2.0 | 3007 | 925 |
| aqueous starch | 0.3 | 473 | 146 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Speltini, A.; Romani, L.; Dondi, D.; Malavasi, L.; Profumo, A. Carbon Nitride-Perovskite Composites: Evaluation and Optimization of Photocatalytic Hydrogen Evolution in Saccharides Aqueous Solution. Catalysts 2020, 10, 1259. https://doi.org/10.3390/catal10111259
Speltini A, Romani L, Dondi D, Malavasi L, Profumo A. Carbon Nitride-Perovskite Composites: Evaluation and Optimization of Photocatalytic Hydrogen Evolution in Saccharides Aqueous Solution. Catalysts. 2020; 10(11):1259. https://doi.org/10.3390/catal10111259
Chicago/Turabian StyleSpeltini, Andrea, Lidia Romani, Daniele Dondi, Lorenzo Malavasi, and Antonella Profumo. 2020. "Carbon Nitride-Perovskite Composites: Evaluation and Optimization of Photocatalytic Hydrogen Evolution in Saccharides Aqueous Solution" Catalysts 10, no. 11: 1259. https://doi.org/10.3390/catal10111259
APA StyleSpeltini, A., Romani, L., Dondi, D., Malavasi, L., & Profumo, A. (2020). Carbon Nitride-Perovskite Composites: Evaluation and Optimization of Photocatalytic Hydrogen Evolution in Saccharides Aqueous Solution. Catalysts, 10(11), 1259. https://doi.org/10.3390/catal10111259