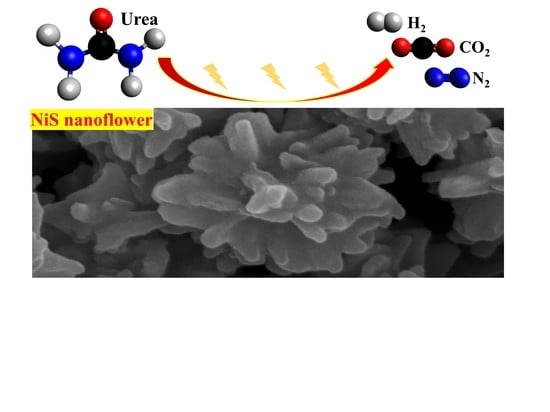
Nanostructured β−NiS Catalyst for Enhanced and Stable Electro−oxidation of Urea
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterizations of Materials
2.2. Electrochemical Oxidation of Urea
3. Experimental
3.1. Preparation of Materials
3.2. Characterizations
3.3. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mazloomi, K.; Gomes, C. Hydrogen as an energy carrier: Prospects and challenges. Renew. Sustain. Energy Rev. 2012, 16, 3024–3033. [Google Scholar] [CrossRef]
- Rollinson, A.N.; Jones, J.; Dupont, V.; Twigg, M.V. Urea as a hydrogen carrier: A perspective on its potential for safe, sustainable and long-term energy supply. Energy Environ. Sci. 2011, 4, 1216–1224. [Google Scholar] [CrossRef] [Green Version]
- Hamonts, K.; Balaine, N.; Moltchanova, E.; Beare, M.; Thomas, S.; Wakelin, S.A.; O’Callaghan, M.; Condron, L.M.; Clough, T.J. Influence of soil bulk density and matric potential on microbial dynamics, inorganic N transformations, N2O and N2 fluxes following urea deposition. Soil Biol. Biochem. 2013, 65, 1–11. [Google Scholar] [CrossRef]
- Lan, R.; Tao, S.; Irvine, J.T.S. A direct urea fuel cell—Power from fertiliser and waste. Energy Environ. Sci. 2010, 3, 438–441. [Google Scholar] [CrossRef] [Green Version]
- Boggs, B.K.; King, R.L.; Botte, G.G. Urea electrolysis: Direct hydrogen production from urine. Chem. Commun. 2009, 32, 4859–4861. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wu, Z.; Tao, S. Urea-Based Fuel Cells and Electrocatalysts for Urea Oxidation. Energy Technol. 2016, 4, 1329–1337. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, R.; Tripathy, N.; Hahn, Y.-B. Highly stable urea sensor based on ZnO nanorods directly grown on Ag/glass electrodes. Sens. Actuators B Chem. 2014, 194, 290–295. [Google Scholar] [CrossRef]
- Simka, W.; Piotrowski, J.; Robak, A.; Nawrat, G. Electrochemical treatment of aqueous solutions containing urea. J. Appl. Electrochem. 2009, 39, 1137–1143. [Google Scholar] [CrossRef]
- Carlesi Jara, C.; Di Giulio, S.; Fino, D.; Spinelli, P. Combined direct and indirect electroxidation of urea containing water. J. Appl. Electrochem. 2008, 38, 915–922. [Google Scholar] [CrossRef]
- King, R.L.; Botte, G.G. Investigation of multi-metal catalysts for stable hydrogen production via urea electrolysis. J. Power Sources 2011, 196, 9579–9584. [Google Scholar] [CrossRef]
- Amstutz, V.; Katsaounis, A.; Kapalka, A.; Comninellis, C.; Udert, K.M. Effects of carbonate on the electrolytic removal of ammonia and urea from urine with thermally prepared IrO2 electrodes. J. Appl. Electrochem. 2012, 42, 787–795. [Google Scholar] [CrossRef]
- Simka, W.; Piotrowski, J.; Nawrat, G. Influence of anode material on electrochemical decomposition of urea. Electrochim. Acta 2007, 52, 5696–5703. [Google Scholar] [CrossRef]
- Xia, L.; Liao, Y.; Qing, Y.; Xu, H.; Gao, Z.; Li, W.; Wu, Y. In Situ Growth of Porous Ultrathin Ni(OH)2 Nanostructures on Nickel Foam: An Efficient and Durable Catalysts for Urea Electrolysis. ACS Appl. Energy Mater. 2020, 3, 2996–3004. [Google Scholar] [CrossRef]
- Ji, R.-Y.; Chan, D.-S.; Jow, J.-J.; Wu, M.-S. Formation of open-ended nickel hydroxide nanotubes on three-dimensional nickel framework for enhanced urea electrolysis. Electrochem. Commun. 2013, 29, 21–24. [Google Scholar] [CrossRef]
- Wu, M.-S.; Ji, R.-Y.; Zheng, Y.-R. Nickel hydroxide electrode with a monolayer of nanocup arrays as an effective electrocatalyst for enhanced electrolysis of urea. Electrochim. Acta 2014, 144, 194–199. [Google Scholar] [CrossRef]
- Wu, M.-S.; Lin, G.-W.; Yang, R.-S. Hydrothermal growth of vertically-aligned ordered mesoporous nickel oxide nanosheets on three-dimensional nickel framework for electrocatalytic oxidation of urea in alkaline medium. J. Power Sources 2014, 272, 711–718. [Google Scholar] [CrossRef]
- Vedharathinam, V.; Botte, G.G. Direct evidence of the mechanism for the electro-oxidation of urea on Ni(OH)2 catalyst in alkaline medium. Electrochim. Acta 2013, 108, 660–665. [Google Scholar] [CrossRef]
- Vedharathinam, V.; Botte, G.G. Understanding the electro-catalytic oxidation mechanism of urea on nickel electrodes in alkaline medium. Electrochim. Acta 2012, 81, 292–300. [Google Scholar] [CrossRef]
- Wang, D.; Botte, G.G. In Situ X-Ray Diffraction Study of Urea Electrolysis on Nickel Catalysts. ECS Electrochem. Lett. 2014, 3, H29–H32. [Google Scholar] [CrossRef]
- Ouyang, C.; Wang, X.; Wang, C.; Zhang, X.; Wu, J.; Ma, Z.; Dou, S.; Wang, S. Hierarchically Porous Ni3S2 Nanorod Array Foam as Highly Efficient Electrocatalyst for Hydrogen Evolution Reaction and Oxygen Evolution Reaction. Electrochim. Acta 2015, 174, 297–301. [Google Scholar] [CrossRef]
- Jiang, N.; Tang, Q.; Sheng, M.; You, B.; Jiang, D.-E.; Sun, Y. Nickel sulfides for electrocatalytic hydrogen evolution under alkaline conditions: A case study of crystalline NiS, NiS2, and Ni3S2 nanoparticles. Catal. Sci. Technol. 2016, 6, 1077–1084. [Google Scholar] [CrossRef]
- Sivanantham, A.; Ganesan, P.; Shanmugam, S. Hierarchical NiCo2S4 Nanowire Arrays Supported on Ni Foam: An Efficient and Durable Bifunctional Electrocatalyst for Oxygen and Hydrogen Evolution Reactions. Adv. Funct. Mater. 2016, 26, 4661–4672. [Google Scholar] [CrossRef]
- Tang, C.; Cheng, N.; Pu, Z.; Xing, W.; Sun, X. NiSe Nanowire Film Supported on Nickel Foam: An Efficient and Stable 3D Bifunctional Electrode for Full Water Splitting. Angew. Chem. Int. Ed. Engl. 2015, 54, 9351–9355. [Google Scholar] [CrossRef]
- Zhou, H.; Yu, F.; Liu, Y.; Sun, J.; Zhu, Z.; He, R.; Bao, J.; Goddard, W.A.; Chen, S.; Ren, Z. Outstanding hydrogen evolution reaction catalyzed by porous nickel diselenide electrocatalysts. Energy Environ. Sci. 2017, 10, 1487–1492. [Google Scholar] [CrossRef] [Green Version]
- You, B.; Jiang, N.; Sheng, M.; Bhushan, M.W.; Sun, Y. Hierarchically Porous Urchin-Like Ni2P Superstructures Supported on Nickel Foam as Efficient Bifunctional Electrocatalysts for Overall Water Splitting. ACS Catal. 2015, 6, 714–721. [Google Scholar] [CrossRef]
- Tang, C.; Zhang, R.; Lu, W.; Wang, Z.; Liu, D.; Hao, S.; Du, G.; Asiri, A.M.; Sun, X. Energy-Saving Electrolytic Hydrogen Generation: Ni2P Nanoarray as a High-Performance Non-Noble-Metal Electrocatalyst. Angew. Chem. Int. Ed. Engl. 2017, 56, 842–846. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, D.; Zhang, L.; Fan, L.; Zhang, X.; Hu, S. Nanostructured Nickel Nitride with Reduced Graphene Oxide Composite Bifunctional Electrocatalysts for an Efficient Water-Urea Splitting. Nanomaterials 2019, 9, 1583. [Google Scholar] [CrossRef] [Green Version]
- Feng, L.L.; Yu, G.; Wu, Y.; Li, G.D.; Li, H.; Sun, Y.; Asefa, T.; Chen, W.; Zou, X. High-index faceted Ni3S2 nanosheet arrays as highly active and ultrastable electrocatalysts for water splitting. J. Am. Chem. Soc. 2015, 137, 14023–14026. [Google Scholar] [CrossRef]
- Zhu, X.; Dou, X.; Dai, J.; An, X.; Guo, Y.; Zhang, L.; Tao, S.; Zhao, J.; Chu, W.; Zeng, X.C.; et al. Metallic Nickel Hydroxide Nanosheets Give Superior Electrocatalytic Oxidation of Urea for Fuel Cells. Angew. Chem. Int. Ed. Engl. 2016, 55, 12465–12469. [Google Scholar] [CrossRef]
- Liu, M.; Jiao, Y.; Zhan, S.; Wang, H. Ni3S2 nanowires supported on Ni foam as efficient bifunctional electrocatalyst for urea-assisted electrolytic hydrogen production. Catal. Today 2020, 355, 596–601. [Google Scholar] [CrossRef]
- Yang, J.; Duan, X.; Qin, Q.; Zheng, W. Solvothermal synthesis of hierarchical flower-like β-NiS with excellent electrochemical performance for supercapacitors. J. Mater. Chem. A 2013, 1, 7880–7884. [Google Scholar] [CrossRef]
- Faure, C.; Delmas, C.; Fouassier, M. Characterization of a turbostratic α-nickel hydroxide quantitatively obtained from an NiSO4 solution. J. Power Sources 1991, 35, 279–290. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, J.; Zhao, Y.; Zhang, L.; Guo, D.; Xia, D. One-pot synthesis of porous nickel cobalt sulphides: Tuning the composition for superior pseudocapacitance. J. Mater. Chem. A 2015, 3, 428–437. [Google Scholar] [CrossRef]
- Guan, B.; Li, Y.; Yin, B.; Liu, K.; Wang, D.; Zhang, H.; Cheng, C. Synthesis of hierarchical NiS microflowers for high performance asymmetric supercapacitor. Chem. Eng. J. 2017, 308, 1165–1173. [Google Scholar] [CrossRef]
- Jing, F.; Lv, Q.; Xiao, J.; Wang, Q.; Wang, S. Highly active and dual-function self-supported multiphase NiS–NiS2–Ni3S2/NF electrodes for overall water splitting. J. Mater. Chem. A 2018, 6, 14207–14214. [Google Scholar] [CrossRef]
- Yuan, C.; Gao, B.; Su, L.; Chen, L.; Zhang, X. Electrochemically Induced Phase Transformation and Charge-Storage Mechanism of Amorphous CoSx Nanoparticles Prepared by Interface-Hydrothermal Method.pdf. J. Electrochem. Soc. 2009, 156, A199–A203. [Google Scholar] [CrossRef]
- Tao, D.P.; Richardson, P.E.; Luttrell, G.H.; Yoon, R.H. Electrochemical studies of pyrite oxidation and reduction using freshly-fractured electrodes and rotating ring-disc electrodes. Electrochim. Acta 2003, 48, 3615–3623. [Google Scholar] [CrossRef]
- Li, W.; Wang, S.; Xin, L.; Wu, M.; Lou, X. Single-crystal β-NiS nanorod arrays with a hollow-structured Ni3S2 framework for supercapacitor applications. J. Mater. Chem. A 2016, 4, 7700–7709. [Google Scholar] [CrossRef]
- Forslund, R.P.; Mefford, J.T.; Hardin, W.G.; Alexander, C.T.; Johnston, K.P.; Stevenson, K.J. Nanostructured LaNiO3 Perovskite Electrocatalyst for Enhanced Urea Oxidation. ACS Catal. 2016, 6, 5044–5051. [Google Scholar] [CrossRef]
- Ding, R.; Qi, L.; Jia, M.; Wang, H. Facile synthesis of mesoporous spinel NiCo2O4 nanostructures as highly efficient electrocatalysts for urea electro-oxidation. Nanoscale 2014, 6, 1369–1376. [Google Scholar] [CrossRef]
- Tong, Y.; Chen, P.; Zhang, M.; Zhou, T.; Zhang, L.; Chu, W.; Wu, C.; Xie, Y. Oxygen Vacancies Confined in Nickel Molybdenum Oxide Porous Nanosheets for Promoted Electrocatalytic Urea Oxidation. ACS Catal. 2017, 8, 1–7. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, T.-H.; Lin, Y.-C.; Hou, B.-W.; Liang, W.-Y. Nanostructured β−NiS Catalyst for Enhanced and Stable Electro−oxidation of Urea. Catalysts 2020, 10, 1280. https://doi.org/10.3390/catal10111280
Wu T-H, Lin Y-C, Hou B-W, Liang W-Y. Nanostructured β−NiS Catalyst for Enhanced and Stable Electro−oxidation of Urea. Catalysts. 2020; 10(11):1280. https://doi.org/10.3390/catal10111280
Chicago/Turabian StyleWu, Tzu-Ho, Yan-Cheng Lin, Bo-Wei Hou, and Wei-Yuan Liang. 2020. "Nanostructured β−NiS Catalyst for Enhanced and Stable Electro−oxidation of Urea" Catalysts 10, no. 11: 1280. https://doi.org/10.3390/catal10111280
APA StyleWu, T. -H., Lin, Y. -C., Hou, B. -W., & Liang, W. -Y. (2020). Nanostructured β−NiS Catalyst for Enhanced and Stable Electro−oxidation of Urea. Catalysts, 10(11), 1280. https://doi.org/10.3390/catal10111280