Recent Advances in the Synthesis of Sulfides, Sulfoxides and Sulfones via C-S Bond Construction from Non-Halide Substrates
Abstract
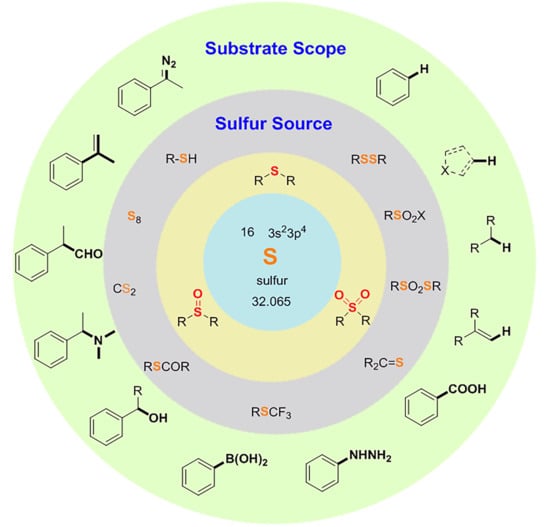
:1. Introduction
2. Synthesis of Sulfides via C-S Bond Construction
2.1. Disulfide as Sulfenylation Source
2.1.1. C(sp3)-S Bond Formation with Disulfide
2.1.2. C(sp2)-S Bond Formation with Disulfide
2.2. Thiol as Sulfenylation Source
2.2.1. C(sp3)-S Bond Formation with Thiol
2.2.2. C(sp2)-S Bond Formation with Thiol
2.3. Elemental Sulfur as Sulfenylation Source
2.4. Sulfonyl Derivative as the Sulfenylation Source
2.5. Other Sulfenylation Sources
2.6. Trifluoromethylthio Reagents as Sulfenylation Sources
3. Synthesis of Sulfoxides via C-S Bond Construction
4. Synthesis of Sulfones via C-S Bond Construction
5. Conclusions and Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Cremlyn, R.J. An Introduction to Organosulfur Chemistry; John Wiley and Sons: Chichester, UK, 1996. [Google Scholar]
- Mampuys, P.; McElroy, C.R.; Clark, J.H.; Orru, R.V.A.; Maes, B.U.W. Thiosulfonates as emerging reactants: Synthesis and applications. Adv. Synth. Catal. 2020, 362, 3–64. [Google Scholar] [CrossRef] [Green Version]
- Parcell, S. Sulfur in human nutrition and applications in medicine. Altern. Med. Rev. 2002, 7, 22–44. [Google Scholar] [PubMed]
- Feng, M.; Tang, B.; Liang, S.H.; Jiang, X. Sulfur containing scaffolds in drugs: Synthesis and application in medicinal chemistry. Curr. Top. Med. Chem. 2016, 16, 1200–1216. [Google Scholar] [CrossRef]
- Devendar, P.; Yang, G.-F. Sulfur-containing agrochemicals. Top. Curr. Chem. 2017, 375, 82. [Google Scholar] [CrossRef]
- Li, X.; Ma, W.; Li, H.; Zhang, Q.; Liu, H. Sulfur-functionalized metal-organic frameworks: Synthesis and applications as advanced adsorbents. Coord. Chem. Rev. 2020, 408, 213191. [Google Scholar] [CrossRef]
- Ferro, C.T.B.; Dos Santos, B.F.; da Silva, C.D.G. Review of the syntheses and activities of some sulfur-containing drugs. Curr. Org. Synth. 2020, 17, 192–210. [Google Scholar] [CrossRef]
- Wang, N.; Saidhareddy, P.; Jiang, X. Construction of sulfur-containing moieties in the total synthesis of natural products. Nat. Prod. Rep. 2020, 37, 246–275. [Google Scholar] [CrossRef]
- Ley, S.V.; Thomas, A.W. Modern synthetic methods for copper-mediated C(aryl)-O, C(aryl)-N, and C(aryl)-S bond formation. Angew. Chem. Int. Ed. 2003, 42, 5400–5449. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, S.; Xiao, Y. Transition-metal-catalyzed synthesis of phenols and aryl thiols. Beilstein J. Org. Chem. 2017, 13, 589–611. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Fajer, A.N.; Yessimbekov, Z.; Kazemi, M.; Mohammadi, M. Diaryl sulfides synthesis: Copper catalysts in C-S bond formation. J. Sulfur Chem. 2019, 40, 451–468. [Google Scholar] [CrossRef]
- Shen, C.; Zhang, P.; Sun, Q.; Bai, S.; Hor, T.S.A.; Liu, X. Recent advances in C-S bond formation via C-H bond functionalization and decarboxylation. Chem. Soc. Rev. 2015, 44, 291–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.-F.; Basha, R.S.; Badsara, S.S. Engineered C-S bond construction. Top. Curr. Chem. 2018, 376, 25. [Google Scholar] [CrossRef] [PubMed]
- Arisawa, M.; Yamaguchi, M. Rhodium-catalyzed synthesis of organosulfur compounds involving S-S bond cleavage of disulfides and sulfur. Molecules 2020, 25, 3595. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.; Miel, H.; Ring, A.; Slattery, C.N.; Maguire, A.R.; McKervey, M.A. Modern organic synthesis with α-diazocarbonyl compounds. Chem. Rev. 2015, 115, 9981–10080. [Google Scholar] [CrossRef]
- Ciszewski, Ł.W.; Rybicka-Jasińska, K.; Gryko, D. Recent developments in photochemical reactions of diazo compounds. Org. Biomol. Chem. 2019, 17, 432–448. [Google Scholar] [CrossRef]
- Khanal, H.D.; Kim, S.H.; Lee, Y.R. Rhodium(ii)-catalyzed direct sulfenylation of diazooxindoles with disulfides. RSC Adv. 2016, 6, 58501–58510. [Google Scholar] [CrossRef]
- Zhang, Z.; Kong, P.; Wang, X.-C. An approach for synthesis of dithioacetals by CuI-catalyzed C-S coupling reaction of N-tosylhydrazone with disulfides. ChemistrySelect 2018, 3, 5667–5669. [Google Scholar] [CrossRef]
- Song, F.; Gou, T.; Wang, B.-Q.; Shi, Z.-J. Catalytic activations of unstrained C-C bond involving organometallic intermediates. Chem. Soc. Rev. 2018, 47, 7078–7115. [Google Scholar] [CrossRef]
- Wu, X.; Zhu, C. Recent advances in radical-mediated C-C bond fragmentation of non-strained molecules. Chin. J. Chem. 2019, 37, 171–182. [Google Scholar] [CrossRef]
- Wu, X.; Wang, Y. Metal-free S-methylation of diaryl disulfides with di-tert-butyl peroxide. Tetrahedron Lett. 2018, 59, 1240–1243. [Google Scholar] [CrossRef]
- Smaligo, A.J.; Kwon, O. Dealkenylative thiylation of C(sp3)-C(sp2) Bonds. Org. Lett. 2019, 21, 8592–8597. [Google Scholar] [CrossRef] [PubMed]
- Shahidzadeh, E.S.; Nowrouzi, N.; Abbasi, M. Utilizing 2-phenylpropanal as coupling partner for C-S bond formation via sequential thioarylation and decarbonylation process: A novel strategy for the synthesis of aryl alkyl sulfides. Appl. Organomet. Chem. 2019, 33, e5211. [Google Scholar] [CrossRef]
- Qin, Y.; Han, Y.; Tang, Y.; Wei, J.; Yang, M. A general method for site-selective Csp3–S bond formation via cooperative catalysis. Chem. Sci. 2020, 11, 1276–1282. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Yang, F.; Yu, Z.; Tang, X.; Wu, Y.; Ma, C.; Meng, Q. Copper(I)-catalyzed sulfenylation of 1,3-dicarbonyl substrates with disulfides under mild conditions. Synlett 2019, 30, 2181–2184. [Google Scholar]
- An, R.; Liao, L.; Liu, X.; Song, S.; Zhao, X. Acid-catalyzed oxidative cleavage of S-S and Se-Se bonds with DEAD: Efficient access to sulfides and selenides. Org. Chem. Front. 2018, 5, 3557–3561. [Google Scholar] [CrossRef]
- Liu, Z.; Quyang, K.; Yang, N. The thiolation of pentafluorobenzene with disulfides by C-H, C-F bond activation and C-S bond formation. Org. Biomol. Chem. 2018, 16, 988–992. [Google Scholar] [CrossRef]
- Noikham, M.; Yotphan, S. Copper-catalyzed regioselective direct C-H thiolation and thiocyanation of uracils. Eur. J. Org. Chem. 2019, 2019, 2759–2766. [Google Scholar] [CrossRef]
- Zheng, C.; Lu, F.; Lu, H.; Xin, J.; Deng, Y.; Yang, D.; Wang, S.; Huang, Z.; Gao, M.; Lei, A. Copper-catalyzed selective radical-radical cross-coupling for C-S bond formation: An access to α-alkylthionitriles. Chem. Commun. 2018, 54, 5574–5577. [Google Scholar] [CrossRef]
- Chand, S.; Pandey, A.K.; Singh, R.; Kumar, S.; Singh, K.N. Eosin-Y-catalyzed photoredox C-S bond formation: Easy access to thioethers. Chem. Asian J. 2019, 14, 4712–4716. [Google Scholar] [CrossRef]
- Yi, X.; Feng, J.; Huang, F.; Baell, J.B. Metal-free C-C, C-O, C-S and C-N bond formation enabled by SBA-15 supported TFMSA. Chem. Commun. 2020, 56, 1243–1246. [Google Scholar] [CrossRef]
- Jiang, W.; Li, N.; Zhou, L.; Zeng, Q. Copper-catalyzed stereospecific C-S coupling reaction of enantioenriched tertiary benzylic amines via in situ activation with methyl triflate. ACS Catal. 2018, 8, 9899–9906. [Google Scholar] [CrossRef]
- Kolb, M. Ketene dithioacetals in organic synthesis: Recent developments. Synthesis 1990, 1990, 171–190. [Google Scholar] [CrossRef]
- Zou, J.; Wang, Y.; Huang, L.; Jiang, Y.; Chen, J.; Zhu, L.; Yang, Y.; Feng, Y.; Peng, X.; Wang, Z. One pot preparation of α-dithioacetal/α-diselenoacetal amides via a dual-C-S/C-Se bond formation and C-C bond cleavage cascade of 3-oxo-butanamides. Org. Chem. Front. 2018, 5, 2317–2321. [Google Scholar] [CrossRef]
- Basha, R.S.; Chen, C.-W.; Reddy, D.M.; Lee, C.-F. Iodine-mediated direct generation of o-quinone methides at room temperature: A facile protocol for the synthesis of ortho-hydroxybenzyl thioethers. Chem. Asian J. 2018, 13, 2475–2483. [Google Scholar] [CrossRef]
- Roy, D.; Panda, G. A dehydrative arylation and thiolation of tertiary alcohols catalyzed by in situ generated triflic acid—Viable protocol for C-C and C-S bond formation. Tetrahedron 2018, 74, 6270–6277. [Google Scholar] [CrossRef]
- Hu, B.; Zhang, Q.; Zhao, S.; Wang, Y.; Xu, L.; Yan, S.; Yu, F. Direct oxidative disulfenylation/cyclization of 2′-hydroxyacetophenones with thiophenols for the synthesis of 2,2-dithio-benzofuran-3(2H)-ones. Adv. Synth. Catal. 2019, 361, 49–54. [Google Scholar] [CrossRef]
- Li, C.; Li, J.; Tan, C.; Wu, W.; Jiang, H. DDQ-mediated regioselective C-S bond formation: Efficient access to allylic sulfides. Org. Chem. Front. 2018, 5, 3158–3162. [Google Scholar] [CrossRef]
- Huang, L.-S.; Han, D.-Y.; Xu, D.-Z. Iron-catalyzed cross-dehydrogenative coupling of oxindoles with thiols/selenols for direct C(sp3)−S/Se bond formation. Adv. Synth. Catal. 2019, 361, 4016–4021. [Google Scholar] [CrossRef]
- Benoit, E.; Bueno, B.; Choiniere, C.; Gagnon, A. First use of an organobismuth reagent in C(sp3)–S bond formation: Access to aryl cyclopropyl sulfides via copper-catalyzed S–cyclopropylation of thiophenols using tricyclopropylbismuth. J. Organomet. Chem. 2019, 893, 72–77. [Google Scholar] [CrossRef]
- Tian, J.; Yuan, S.; Xiao, F.; Huang, H.; Deng, G.-J. Concise synthesis of N-thiomethyl benzoimidazoles through base-promoted sequential multicomponent assembly. RSC Adv. 2019, 9, 30570–30574. [Google Scholar] [CrossRef] [Green Version]
- Zou, J.-X.; Jiang, Y.; Lei, S.; Yin, G.-F.; Hu, X.-L.; Zhao, Q.-Y.; Wang, Z. Synthesis of α-arylthioacetones using TEMPO as the C 3 synthon via a reaction cascade of sequential oxidation, skeletal rearrangement and C-S bond formation. Org. Biomol. Chem. 2019, 17, 2341–2345. [Google Scholar] [CrossRef] [PubMed]
- Hosseinian, A.; Nezhad, P.D.K.; Ahmadi, S.; Rahmani, Z.; Monfared, A. A walk around the decarboxylative C-S cross-coupling reactions. J. Sulfur Chem. 2019, 40, 88–112. [Google Scholar] [CrossRef]
- Li, M.; Hoover, J.M. Aerobic copper-catalyzed decarboxylative thiolation. Chem. Commun. 2016, 52, 8733–8736. [Google Scholar] [CrossRef] [PubMed]
- Jafarpour, F.; Asadpour, M.; Azizzade, M.; Ghasemi, M.; Rajai-Daryasarei, S. An iodide-mediated transition-metal-free strategy towards unsymmetrical diaryl sulfides via arylhydrazines and thiols. Synthesis 2020, 52, 727–734. [Google Scholar] [CrossRef]
- Moghaddam, F.M.; Pourkaveh, R.; Gholamtajari, M. Nano CoCuFe2O4-catalyzed coupling reaction of arylboronic acid with amines and thiols: An atom-economic and ligand-free route to access unsymmetrical amines and sulfides. Appl. Organomet. Chem. 2018, 32, e4568. [Google Scholar] [CrossRef]
- Singh, D.; Kumar, G.; Dheer, D.; Jyoti; Kushwaha, M.; Ahmed, Q.N.; Shankar, R. BCl3-mediated C-N, C-S, and C-O bond formation of imidazo[1,2-a]pyridine benzylic ethers. ACS Omega 2019, 4, 4530–4539. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, P.; Nandi, A.K.; Chhetri, G.; Das, S. Generation of ArS- and ArSe-substituted 4-quinolone derivatives using sodium iodide as an inducer. J. Org. Chem. 2018, 83, 12411–12419. [Google Scholar] [CrossRef]
- Choudhuri, K.; Maiti, S.; Mal, P. Iodine(III) enabled dehydrogenative aryl C-S coupling by in situ generated sulfenium ion. Adv. Synth. Catal. 2019, 361, 1092–1101. [Google Scholar] [CrossRef]
- Huang, X.; Chen, Y.; Zhen, S.; Song, L.; Gao, M.; Zhang, P.; Li, H.; Yuan, B.; Yang, G. Cobalt-catalyzed aerobic cross-dehydrogenative coupling of C-H and thiols in water for C-S formation. J. Org. Chem. 2018, 83, 7331–7340. [Google Scholar] [CrossRef]
- Yuan, Y.; Cao, Y.; Qiao, J.; Lin, Y.; Jiang, X.; Weng, Y.; Tang, S.; Lei, A. Electrochemical oxidative C-H sulfenylation of imidazopyridines with hydrogen evolution. Chin. J. Chem. 2019, 37, 49–52. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Deng, G.-J.; Huang, H. Recent advances in sulfur-containing heterocycle formation via direct C-H sulfuration with elemental sulfur. Synlett 2020, 31. [Google Scholar] [CrossRef]
- Semwal, R.; Ravi, C.; Saxena, S.; Adimurthy, S. Copper-catalyzed multicomponent reactions (MCRs) for disulfenylation of imidazo[1,2-a]pyridines using elemental sulfur and arylhalides and intramolecular cyclization of haloimidazo[1,2-a]pyridines. J. Org. Chem. 2019, 84, 14151–14160. [Google Scholar] [CrossRef] [PubMed]
- Atashkar, B.; Rostami, A.; Rostami, A.; Zolfigol, M.A. NiFe2O4 as a magnetically recoverable nanocatalyst for odourless C-S bond formation via the cleavage of C-O bond in the presence of S8 under mild and green conditions. Appl. Organomet. Chem. 2019, 33, e4691. [Google Scholar] [CrossRef]
- Khakyzadeh, V.; Rostami, A.; Veisi, H.; Shaghasemi, B.S.; Reimhult, E.; Luque, R.; Xia, Y.; Darvishi, S. Direct C-S bond formation via C-O bond activation of phenols in a crossover Pd/Cu dual-metal catalysis system. Org. Biomol. Chem. 2019, 17, 4491–4497. [Google Scholar] [CrossRef]
- Ghosh, A.; Lecomte, M.; Kim-Lee, S.-H.; Radosevich, A.T. Organophosphorus-catalyzed deoxygenation of sulfonyl chlorides: Electrophilic (fluoroalkyl)sulfenylation by PIII/PV = O redox cycling. Angew. Chem. Int. Ed. 2019, 58, 2864–2869. [Google Scholar] [CrossRef]
- Huang, K.; Yang, M.; Lai, X.-J.; Hu, X.; Qiu, G.; Liu, J.-B. CuI-catalyzed direct synthesis of diaryl thioethers from arylboronic acids and arylsulfonyl chlorides. Appl. Organometal. Chem. 2019, 33, e5385. [Google Scholar]
- Zhao, F.; Tan, Q.; Wang, D.; Chen, J.; Deng, G.-J. Efficient C-S bond formation by direct functionalization of C(sp3)−H bond adjacent to heteroatoms under metal-free conditions. Adv. Synth. Catal. 2019, 361, 4075–4081. [Google Scholar] [CrossRef]
- Guo, W.-S.; Gong, H.; Zhang, Y.-A.; Wen, L.-R.; Li, M. Fast construction of 1,3-benzothiazepines by direct intramolecular dehydrogenative C-S bond formation of thioamides under metal-free conditions. Org. Lett. 2018, 20, 6394–6397. [Google Scholar] [CrossRef]
- Ishitobi, K.; Muto, K.; Yamaguchi, J. Pd-catalyzed alkenyl thioether synthesis from thioesters and N-tosylhydrazones. ACS Catal. 2019, 9, 11685–11690. [Google Scholar] [CrossRef]
- Pandey, A.K.; Chand, S.; Singh, R.; Kumar, S.; Singh, K.N. Iodine-catalyzed synthesis of 3-arylthioindoles employing a 1-aryltriazene/CS 2 combination as a new sulfenylation source. ACS Omega 2020, 5, 7627–7635. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Zhang, M.; Lin, Q.; Weng, Z. [(bpy)CuSCF3]: A Practical and Efficient Reagent for the Construction of C-SCF3 Bonds. Synlett 2020, 31. [Google Scholar] [CrossRef]
- Honeker, R.; Garza-Sanchez, R.A.; Hopkinson, M.N.; Glorius, F. Visible-light-promoted trifluoromethylthiolation of styrenes by dual photoredox/halide catalysis. Chem. Eur. J. 2016, 22, 4395–4399. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shan, C.C.; Tung, C.-H.; Xu, Z. Dual gold and photoredox catalysis: Visible light-mediated intermolecular atom transfer thiosulfonylation of alkenes. Chem. Sci. 2017, 8, 2610–2615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dagousset, G.; Simon, C.; Anselmi, E.; Tuccio, B.; Billard, T.; Magnier, E. Generation of the SCF 3 radical by photoredox catalysis: Intra- and intermolecular carbotrifluoromethylthiolation of alkenes. Chem. Eur. J. 2017, 23, 4282–4286. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yang, B.; Wei, A.; Sheng, J.; Tian, M.; Li, Q.; Lu, K. Silver-mediated radical aryltrifluoromethylthiolation of activated alkenes by S-trifluoromethyl 4-methylbenzenesulfonothioate. Tetrahedron Lett. 2018, 59, 1719–1722. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Li, Z.; Bolm, C. Transition-metal-free arylations of in-situ generated sulfenates with diaryliodonium salts. Org. Lett. 2018, 20, 7104–7106. [Google Scholar] [CrossRef]
- Shi, W.; Miao, T.; Li, Y.; Li, P.; Wang, L. Selective synthesis of diaryl sulfoxides and m-arylthio sulfones from arylsulfinic acids and arenes via BF 3-promoted C-S bond formation. Org. Lett. 2018, 20, 4416–4420. [Google Scholar] [CrossRef]
- Zhao, J.-L.; Guo, S.-H.; Qiu, J.; Gou, X.-F.; Hua, C.-W.; Chen, B. Iron(III) phthalocyanine-chloride-catalyzed synthesis of sulfones from sulfonylhydrazones. Tetrahedron Lett. 2016, 57, 2375–2378. [Google Scholar] [CrossRef]
- Deng, L.; Kleij, A.W.; Yang, W. Diversity-orientated stereoselective synthesis through Pd-catalyzed switchable decarboxylative C-N/C-S bond formation in allylic surrogates. Chem. Eur. J. 2018, 24, 19156–19161. [Google Scholar] [CrossRef]
- Wang, F.; Xu, P.; Wang, S.-Y.; Ji, S.-J. Cu(II)/Ag(I)-catalyzed cascade reaction of sulfonylhydrazone with anthranils: Synthesis of 2-aryl-3-sulfonyl substituted quinoline derivatives. Org. Lett. 2018, 20, 2204–2207. [Google Scholar] [CrossRef]
- Zhu, X.-Y.; Han, Y.-P.; Li, M.; Li, X.-S.; Liang, Y.-M. Copper-catalyzed radical sulfonylation of N-propargylindoles with concomitant 1,2-aryl migration. Adv. Synth. Catal. 2018, 360, 3460–3465. [Google Scholar] [CrossRef]
- Liu, T.; Liu, J.; Xia, S.; Meng, J.; Shen, X.; Zhu, X.; Chen, W.; Sun, C.; Cheng, F. Catalyst-free 1,6-conjugate addition/aromatization/sulfonylation of para-quinone methides: Facile access to diarylmethyl sulfones. ACS Omega 2018, 3, 1409–1415. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.-X.; Wang, R.-N.; Huang, H.-L.; Gong, S.-W.; Li, Q.-L.; Zhang, S.-L.; Ma, C.-L.; Li, C.-Z.; Du, J.-Y. Lewis acid-catalyzed tandem cyclization of in situ generated o-quinone methides and arylsulfonyl hydrazides for a one-pot entry to 3-sulfonylbenzofurans. Org. Chem. Front. 2019, 6, 3929–3933. [Google Scholar] [CrossRef]
- Pagire, S.K.; Hossain, A.; Reiser, O. Temperature controlled selective C-S or C-C bond formation: Photocatalytic sulfonylation versus arylation of unactivated heterocycles utilizing aryl sulfonyl chlorides. Org. Lett. 2018, 20, 648–651. [Google Scholar] [CrossRef]
- Kuchukulla, R.R.; Li, F.; He, Z.; Zhou, L.; Zeng, Q. Synthesis of sultams and cyclic N-sulfonyl ketimines via iron-catalyzed intramolecular aliphatic C-H amidation. Green Chem. 2019, 21, 5808–5812. [Google Scholar] [CrossRef]
- Johnson, T.C.; Elbert, B.L.; Farley, A.J.M.; Gorman, T.W.; Genicot, C.; Lallemand, B.; Pasau, P.; Flasz, J.; Castro, J.L.; MacCoss, M.; et al. Direct sulfonylation of anilines mediated by visible light. Chem. Sci. 2018, 9, 629–633. [Google Scholar] [CrossRef] [Green Version]
- Nikl, J.; Ravelli, D.; Schollmeyer, D.; Waldvogel, S.R. Straightforward electrochemical sulfonylation of arenes and aniline derivatives using sodium sulfinates. ChemElectroChem 2019, 6, 4450–4455. [Google Scholar] [CrossRef] [Green Version]
- Rao, C.; Mai, S.; Song, Q. Rh(ii)/phosphine-cocatalyzed synthesis of dithioketal derivatives from diazo compounds through simultaneous construction of two different C-S bonds. Chem. Commun. 2018, 54, 5964–5967. [Google Scholar] [CrossRef]
- Reddy, R.J.; Waheed, M.; Krishna, G.R. Phenylboronic acid-catalyzed tandem construction of S-S and C-S bonds: A new method for the synthesis of benzyl disulfanylsulfone derivatives from S-benzyl thiosulfonates. Org. Biomol. Chem. 2020, 18, 3243–3248. [Google Scholar] [CrossRef]



























































Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Ding, H.; Pu, X.; Qian, Z.; Xiao, Y. Recent Advances in the Synthesis of Sulfides, Sulfoxides and Sulfones via C-S Bond Construction from Non-Halide Substrates. Catalysts 2020, 10, 1339. https://doi.org/10.3390/catal10111339
Zhang R, Ding H, Pu X, Qian Z, Xiao Y. Recent Advances in the Synthesis of Sulfides, Sulfoxides and Sulfones via C-S Bond Construction from Non-Halide Substrates. Catalysts. 2020; 10(11):1339. https://doi.org/10.3390/catal10111339
Chicago/Turabian StyleZhang, Rui, Huaiwei Ding, Xiangling Pu, Zhiping Qian, and Yan Xiao. 2020. "Recent Advances in the Synthesis of Sulfides, Sulfoxides and Sulfones via C-S Bond Construction from Non-Halide Substrates" Catalysts 10, no. 11: 1339. https://doi.org/10.3390/catal10111339
APA StyleZhang, R., Ding, H., Pu, X., Qian, Z., & Xiao, Y. (2020). Recent Advances in the Synthesis of Sulfides, Sulfoxides and Sulfones via C-S Bond Construction from Non-Halide Substrates. Catalysts, 10(11), 1339. https://doi.org/10.3390/catal10111339