Effect of KCoMoS2 Catalyst Structures on the Catalytic Performance of Higher Alcohols Synthesis via CO Hydrogenation
Abstract
:1. Introduction
2. Results and Discussion
2.1. XRD and BET Results
2.2. TEM Results
2.3. HAADF-STEM-DES Results
2.4. XPS Results
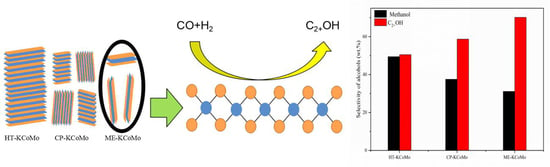
2.5. HAS Performances of the Catalysts
3. Experimental
3.1. Catalyst Preparation
3.1.1. Reverse Microemulsion Method
3.1.2. Traditional Coprecipitation Method
3.1.3. Hydrothermal Synthesis Method
3.2. Characterization
3.3. Catalyst Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Spivey, J.J.; Egbebi, A. Heterogeneous catalytic synthesis of ethanol from biomass-derived syngas. Chem. Soc. Rev. 2007, 36, 1514–1528. [Google Scholar] [CrossRef]
- Subramani, V.; Gangwal, S.K. A review of recent literature to search for an efficient catalytic process for the conversion of syngas to ethanol. Energ. Fuel. 2008, 22, 814–839. [Google Scholar] [CrossRef]
- Von Blottnitz, H.; Curran, M.A. A review of assessments conducted on bio-ethanol as a transportation fuel from a net energy, greenhouse gas, and environmental life cycle perspective. J. Clean. Prod. 2007, 15, 607–619. [Google Scholar] [CrossRef]
- Surisetty, V.R.; Dalai, A.K.; Kozinski, J. Alcohols as alternative fuels: An overview. Appl. Catal. A Gen. 2001, 404, 1–11. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Zhang, M.; Zhang, W.; Zhang, M.; Xie, H.; Wu, Y.; Tan, Y. The support effects on the direct conversion of syngas to higher alcohol synthesis over copper-based catalysts. Catalysts 2019, 9, 199. [Google Scholar] [CrossRef] [Green Version]
- Heracleous, E.; Liakakou, E.T.; Lappas, A.A.; Lemonidou, A.A. Investigation of K-promoted Cu-Zn-Al, Cu-X-Al and Cu-Zn-X (X= Cr, Mn) catalysts for carbon monoxide hydrogenation to higher alcohols. Appl. Catal. A Gen. 2013, 455, 145–154. [Google Scholar] [CrossRef]
- Guo, S.; Liu, G.; Han, T.; Zhang, Z.; Liu, Y. K-Modulated Co nanoparticles trapped in La-Ga-O as superior catalysts for higher alcohols synthesis from syngas. Catalysts 2019, 9, 218. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Duan, J.; Zhang, Q.; Li, Y.; Fang, K. Preparation, Structural Characteristics, and Catalytic Performance of Cu–Co Alloy Supported on Mn–Al Oxide for Higher Alcohol Synthesis via Syngas. Ind. Eng. Chem. Res. 2018, 57, 14957–14966. [Google Scholar] [CrossRef]
- Yu, J.; Mao, D.; Han, L.; Guo, Q.; Lu, G. CO hydrogenation over Fe-promoted Rh–Mn–Li/SiO2 catalyst: The effect of sequences for introducing the Fe promoter. Fuel Process. Technol. 2013, 112, 100–105. [Google Scholar] [CrossRef]
- Chen, W.; Ding, Y.; Xue, F.; Song, X.; Ning, L. Highly efficient β-SiC-supported 0.5% Rh-based catalyst for CO hydrogenation to C2 oxygenates. Catal. Commun. 2016, 85, 44–47. [Google Scholar] [CrossRef]
- Luan, X.; Yong, J.; Dai, X.; Zhang, X.; Qiao, H.; Yang, Y.; Huang, X. Tungsten-doped molybdenum sulfide with dominant double-layer structure on mixed MgAl oxide for higher alcohol synthesis in CO hydrogenation. Ind. Eng. Chem. Res. 2018, 57, 10170–10179. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Y.; Gao, S.; Fang, H.; Liang, X.; Yuan, Y. Synthesis of higher alcohols by CO hydrogenation on a K-promoted Ni–Mo catalyst derived from Ni–Mo phyllosilicate. Catal. Sci. Technol. 2018, 8, 4219–4228. [Google Scholar] [CrossRef]
- Christensen, J.M.; Mortensen, P.M.; Trane, R.; Jensen, P.A.; Jensen, A.D. Effects of H2S and process conditions in the synthesis of mixed alcohols from syngas over alkali promoted cobalt-molybdenum sulfide. Appl. Catal. A Gen. 2009, 366, 29–43. [Google Scholar] [CrossRef]
- Herman, R.G. Advances in catalytic synthesis and utilization of higher alcohols. Catal. Today 2000, 55, 233–245. [Google Scholar] [CrossRef]
- Koizumi, N.; Murai, K.; Ozaki, T.; Yamada, M. Development of sulfur tolerant catalysts for the synthesis of high quality transportation fuels. Catal. Today 2004, 89, 465–478. [Google Scholar] [CrossRef]
- Bao, J.; Fu, Y.; Sun, Z.; Gao, C. A highly active K-Co-Mo/C catalyst for mixed alcohol synthesis from CO + H2. Chem. Commun. 2003, 746–747. [Google Scholar] [CrossRef]
- Liu, J.; Wang, E.; Lv, J.; Li, Z.; Wang, B.; Ma, X.; Qin, S.; Sun, Q. Investigation of sulfur-resistant, highly active unsupported MoS2 catalysts for synthetic natural gas production from CO methanation. Fuel Process. Technol. 2013, 110, 249–257. [Google Scholar] [CrossRef]
- Zaman, S.; Smith, K.J. A review of molybdenum catalysts for synthesis gas conversion to alcohols: Catalysts, mechanisms and kinetics. Catal. Rev. 2012, 54, 41–132. [Google Scholar] [CrossRef]
- Andersson, R.; Boutonnet, M.; Järås, S. Correlation patterns and effect of syngas conversion level for product selectivity to alcohols and hydrocarbons over molybdenum sulfide based catalysts. Appl. Catal. A Gen. 2012, 417, 119–128. [Google Scholar] [CrossRef]
- Stevens, R.R.; Conway, M.M. Mixed alcohols production from syngas. U.S. Patent 4 831 060, 16 May 1989. [Google Scholar]
- Surisetty, V.R.; Eswaramoorthi, I.; Dalai, A.K. Comparative study of higher alcohols synthesis over alumina and activated carbon-supported alkali-modified MoS2 catalysts promoted with group VIII metals. Fuel 2012, 96, 77–84. [Google Scholar] [CrossRef]
- Jaramillo, T.F.; Jørgensen, K.P.; Bonde, J.; Nielsen, J.H.; Horch, S.; Chorkendorff, I. Identification of active edge sites for electrochemical H2 evolution from MoS2 nanocatalysts. Science 2007, 317, 100–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daage, M.; Chianelli, R.R. Structure-function relations in molybdenum sulfide catalysts: The “rim-edge” model. J. Catal. 1994, 149, 414–427. [Google Scholar] [CrossRef]
- Morrill, M.R.; Thao, N.T.; Shou, H.; Davis, R.J.; Barton, D.G.; Ferrari, D.; Agrawal, P.K.; Jones, C.W. Origins of unusual alcohol selectivities over mixed MgAl oxide-supported K/MoS2 catalysts for higher alcohol synthesis from syngas. ACS Catal. 2013, 3, 1665–1675. [Google Scholar] [CrossRef]
- Claure, M.T.; Chai, S.H.; Dai, S.; Unocic, K.A.; Alamgir, F.M.; Agrawal, P.K.; Jones, C.W. Tuning of higher alcohol selectivity and productivity in CO hydrogenation reactions over K/MoS2 domains supported on mesoporous activated carbon and mixed MgAl oxide. J. Catal. 2015, 324, 88–97. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhao, L.; Liu, X.; Zeng, C.; Fang, K. Preparation of KNiMo-based catalysts by using non-thermal plasma and their catalytic performance in the synthesis of higher alcohols from syngas. J. Fuel Chem. Technol. 2019, 47, 513–521. [Google Scholar] [CrossRef]
- Chianelli, R.R.; Berhault, G.; Torres, B. Unsupported transition metal sulfide catalysts: 100 years of science and application. Catal. Today 2009, 147, 275–286. [Google Scholar] [CrossRef]
- Okamoto, Y.; Shimokawa, T.; Imanaka, T.; Teranishi, S. X-ray photoelectron spectroscopy study of cobalt-molybdenum binary oxide catalysts. J. Catal. 1979, 57, 153–166. [Google Scholar] [CrossRef]
- Li, H.; Zhang, W.; Wang, Y.; Shui, M.; Sun, S.; Bao, J.; Gao, C. Nanosheet-structured K–Co–MoS2 catalyst for the higher alcohol synthesis from syngas: Synthesis and activation. J. Energy Chem. 2019, 30, 57–62. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Li, P.; Liu, X.; Liu, T.; Jiang, Z.; Li, C. Morphology-performance relation of (Co)MoS2 catalysts in the hydrodesulfurization of FCC gasoline. Appl. Catal. A Gen. 2018, 556, 20–28. [Google Scholar] [CrossRef]
- Chiang, S.J.; Liaw, B.J.; Chen, Y.Z. Preparation of NiB nanoparticles in water-in-oil microemulsions and their catalysis during hydrogenation of carbonyl and olefinic groups. Appl. Catal. A Gen. 2007, 319, 144–152. [Google Scholar] [CrossRef]
- Konarova, M.; Tang, F.; Chen, J.; Wang, G.; Rudolph, V.; Beltramini, J. Nano-and Microscale Engineering of the Molybdenum Disulfide-Based Catalysts for Syngas to Ethanol Conversion. ChemCatChem 2014, 6, 2394–2402. [Google Scholar] [CrossRef]
- Liu, M.; Li, X.; Xu, Z.; Li, B.; Chen, L.; Shan, N. Synthesis of chain-like MoS2 nanoparticles in W/O reverse microemulsion and application in photo catalysis. Chin. Sci. Bull. 2012, 57, 3862–3866. [Google Scholar] [CrossRef] [Green Version]
- Wei, B.; Wang, L.; Miao, Q.; Yuan, Y.; Dong, P.; Vajtai, R.; Fei, W. Fabrication of manganese oxide/three-dimensional reduced graphene oxide composites as the supercapacitors by a reverse microemulsion method. Carbon 2015, 85, 249–260. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, M.; Zhu, C.; Mu, X.; Zhang, K.; Zhong, L.; Fang, K.; Wu, M. Fabrication of Highly Stable SiO2 Encapsulated Multiple CuFe Nanoparticles for Higher Alcohols Synthesis via CO Hydrogenation. Catal. Lett. 2018, 148, 1080–1092. [Google Scholar] [CrossRef]
- París, R.S.; Montes, V.; Boutonnet, M.; Järås, S. Higher alcohol synthesis over nickel-modified alkali-doped molybdenum sulfide catalysts prepared by conventional coprecipitation and coprecipitation in microemulsions. Catal. Today 2015, 258, 294–303. [Google Scholar] [CrossRef]
- Iranmahboob, J.; Hill, D.O. Alcohol synthesis from syngas over K2CO3/CoS/MoS2 on activated carbon. Catal. Lett. 2002, 78, 49–55. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.; Zhang, G.; Xu, T.; Wan, P.; Sun, X. A metallic CoS2 nanopyramid array grown on 3D carbon fiber paper as an excellent electrocatalyst for hydrogen evolution. J. Mater. Chem. A 2015, 3, 6306–6310. [Google Scholar] [CrossRef]
- Zhu, L.; Susac, D.; Teo, M.; Wong, K.C.; Wong, P.C.; Parsons, R.R.; Bizzotto, D.; Mitchella, K.A.R.; Campbell, S.A. Investigation of CoS2-based thin films as model catalysts for the oxygen reduction reaction. J. Catal. 2008, 258, 235–242. [Google Scholar] [CrossRef]
- Dai, X.; Du, K.; Li, Z.; Liu, M.; Ma, Y.; Sun, H.; Zhang, X.; Yang, Y. Co-doped MoS2 nanosheets with the dominant CoMoS phase coated on carbon as an excellent electrocatalyst for hydrogen evolution. ACS Appl. Mater. Inter. 2015, 7, 27242–27253. [Google Scholar] [CrossRef]
- Nikulshin, P.A.; Ishutenko, D.I.; Mozhaev, A.A.; Maslakov, K.I.; Pimerzin, A.A. Effects of composition and morphology of active phase of CoMo/Al2O3 catalysts prepared using Co2Mo10–heteropolyacid and chelating agents on their catalytic properties in HDS and HYD reactions. J. Catal. 2014, 312, 152–169. [Google Scholar] [CrossRef]
- Alstrup, I.; Chorkendorff, I.; Candia, R.; Clausen, B.S.; Topsøe, H. A combined X-Ray photoelectron and Mössbauer emission spectroscopy study of the state of cobalt in sulfided, supported, and unsupported Co-Mo catalysts. J. Catal. 1982, 7, 397–409. [Google Scholar] [CrossRef]
- Ferrari, D.; Budroni, G.; Bisson, L.; Rane, N.J.; Dickie, B.D.; Kang, J.H.; Rozeveld, S.J. Effect of potassium addition method on MoS2 performance for the syngas to alcohol reaction. Appl. Catal. A Gen. 2013, 462, 302–309. [Google Scholar] [CrossRef]
- Topsøe, N.Y.; Topsøe, H. Characterization of the structures and active sites in sulfided Co-MoAl2O3 and Ni-MoAl2O3 catalysts by NO chemisorption. J. Catal. 1983, 84, 386–401. [Google Scholar] [CrossRef]
- Dong, K.; Ma, X.; Zhang, H.; Lin, G. Novel MWCNT-support for Co-Mo sulfide catalyst in HDS of thiophene and HDN of pyrrole. J. Nat. Gas. Chem. 2006, 15, 28–37. [Google Scholar] [CrossRef]






| Catalyst | Particle Size 1/nm | Surface Area 2/(m2·g−1) | Average Pore Size 2/(nm) | Pore Volume 2/(cm3·g−1) |
|---|---|---|---|---|
| HT-KCoMo | 33.39 | 11.00 | 9.83 | 0.07 |
| CP-KCoMo | 18.24 | 32.70 | 10.69 | 0.15 |
| ME-KCoMo | 14.70 | 37.26 | 4.79 | 0.08 |
| Catalyst | Surface Atomic Ratio |
|---|---|
| Mo:S:Co | |
| ME-KCoMo | 1:2.16:0.46 |
| CP-KCoMo | 1:2.22:0.81 |
| HT-KCoMo | 1:2.14:0.25 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, N.; Mu, X.; Zhao, L.; Fang, K. Effect of KCoMoS2 Catalyst Structures on the Catalytic Performance of Higher Alcohols Synthesis via CO Hydrogenation. Catalysts 2020, 10, 151. https://doi.org/10.3390/catal10020151
Qin N, Mu X, Zhao L, Fang K. Effect of KCoMoS2 Catalyst Structures on the Catalytic Performance of Higher Alcohols Synthesis via CO Hydrogenation. Catalysts. 2020; 10(2):151. https://doi.org/10.3390/catal10020151
Chicago/Turabian StyleQin, Niannian, Xiaoliang Mu, Lu Zhao, and Kegong Fang. 2020. "Effect of KCoMoS2 Catalyst Structures on the Catalytic Performance of Higher Alcohols Synthesis via CO Hydrogenation" Catalysts 10, no. 2: 151. https://doi.org/10.3390/catal10020151
APA StyleQin, N., Mu, X., Zhao, L., & Fang, K. (2020). Effect of KCoMoS2 Catalyst Structures on the Catalytic Performance of Higher Alcohols Synthesis via CO Hydrogenation. Catalysts, 10(2), 151. https://doi.org/10.3390/catal10020151