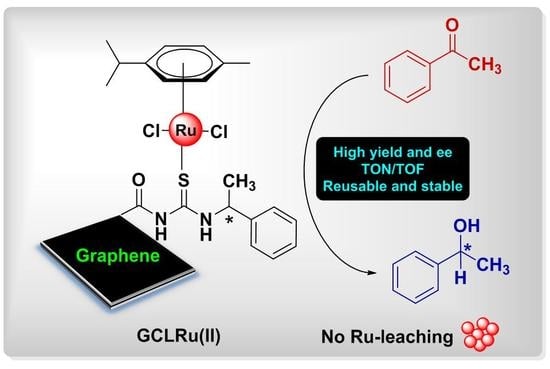
Stepwise Construction of Ru(II)Center Containing Chiral Thiourea Ligand on Graphene Oxide: First Efficient, Reusable, and Stable Catalyst for Asymmetric Transfer Hydrogenation of Ketones
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of G-CLRu(II)
2.2. Asymmetric Transfer Hydrogenation of Ketones
2.3. Recovery, Reusability, and Stability
3. Experimental Section
3.1. Materials
3.2. Preparation of G-CLRu(II) Catalyst
3.3. Characterization of G-CLRu(II)
3.4. Procedure for G-CLRu(II)-Mediated Asymmetric Transfer Hydrogenation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, Y.Y.; Yu, S.L.; Shen, W.Y.; Gao, J.X. Iron-, cobalt-, and nickel-catalyzed asymmetric transfer hydrogenation and asymmetric hydrogenation of ketones. Acc. Chem. Res. 2015, 48, 2587–2598. [Google Scholar] [CrossRef]
- Zuo, W.; Lough, A.J.; Li, Y.F.; Morris, R.H. Amine (imine) diphosphine iron catalysts for asymmetric transfer hydrogenation of ketones and imines. Science 2013, 342, 1080–1083. [Google Scholar] [CrossRef] [Green Version]
- Ikariya, T.; Blacker, A.J. Asymmetric transfer hydrogenation of ketones with bifunctional transition metal-based molecular catalysts. Acc. Chem. Res. 2007, 40, 1300–1308. [Google Scholar] [CrossRef] [PubMed]
- Gladiali, S.; Alberico, E. Asymmetric transfer hydrogenation: Chiral ligands and applications. Chem. Soc. Rev. 2006, 35, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.Y.; Xiao, H.; Chen, X.H.; Gong, L.Z. Consecutive intramolecular hydroamination/asymmetric transfer hydrogenation under relay catalysis of an achiral gold complex/chiral bronsted acid binary system. J. Am. Chem. Soc. 2009, 131, 9182–9183. [Google Scholar] [CrossRef] [PubMed]
- He, Y.M.; Fan, Q.H. Phosphine-free chiral metal catalysts for highly effective asymmetric catalytic hydrogenation. Org. Biomol. Chem. 2010, 8, 2497–2504. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wu, J.; Wang, F.; Liao, J.; Zhang, H.; Lian, C.; Zhu, J.; Deng, J. Asymmetric transfer hydrogenation of ketones and imines with novel water-soluble chiral diamine as ligand in neat water. Green Chem. 2007, 9, 23–25. [Google Scholar] [CrossRef]
- Baratta, W.; Da Ros, P.; Del Zotto, A.; Sechi, A.; Zangrando, E.; Rigo, P. Cyclometalated Ruthenium(II) Complexes as Highly Active Transfer Hydrogenation Catalysts. Angew Chem. Int. Ed. 2004, 43, 3584–3588. [Google Scholar] [CrossRef]
- Sheeba, M.M.; Muthu Tamizh, M.; Farrugia, L.J.; Endo, A.; Karvembu, R. Chiral (η6-p-Cymene)ruthenium(II) Complexes Containing Monodentate Acylthiourea Ligands for Efficient Asymmetric Transfer Hydrogenation of Ketones. Organometallics 2014, 33, 540–550. [Google Scholar] [CrossRef]
- Collis, A.E.; Horvath, I.T. Heterogenization of homogeneous catalytic systems. Catal. Sci. Technol. 2011, 1, 912–919. [Google Scholar] [CrossRef]
- Mihalcik, D.J.; Lin, W. Mesoporous silica nanosphere supported ruthenium catalysts for asymmetric hydrogenation. Angew Chem. Int. Ed. 2008, 47, 6229–6232. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, G.; Gu, H.; Huang, T.; Zhang, Y.; Li, H. Magnetically recoverable SiO2-coated Fe3O4 nanoparticles: A new platform for asymmetric transfer hydrogenation of aromatic ketones in aqueous medium. Chem. Commun. 2011, 47, 2583–2585. [Google Scholar] [CrossRef] [PubMed]
- Babin, M.; Clement, R.; Gagnon, J.; Fontaine, F.G. Homogeneous asymmetric transfer hydrogenation of ketones using a ruthenium catalyst anchored on chitosan: Natural chirality at work. New J. Chem. 2012, 36, 1548–1551. [Google Scholar] [CrossRef] [Green Version]
- Tang, S.; Jin, R.; Zhang, H.; Yao, H.; Zhuang, J.; Liu, G.; Li, H. Recoverable organorhodium-functionalized polyhedral oligomeric silsesquioxane: A bifunctional heterogeneous catalyst for asymmetric transfer hydrogenation of aromatic ketones in aqueous medium. Chem. Commun. 2012, 48, 6286–6288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonnenberg, J.F.; Coombs, N.; Dube, P.A.; Morris, R.H. Iron nanoparticles catalyzing the asymmetric transfer hydrogenation of ketones. J. Am. Chem. Soc. 2012, 134, 5893–5899. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.; Xing, M.; Zhang, J. Recent advances in three-dimensional graphene based materials for catalysis applications. Chem. Soc. Rev. 2018, 47, 2165–2216. [Google Scholar] [CrossRef]
- Schaetz, A.; Zeltner, M.; Stark, W.J. Carbon modifications and surfaces for catalytic organic transformations. ACS Catal. 2012, 2, 1267–1284. [Google Scholar] [CrossRef]
- Garrido-Barros, P.; Gimbert-Surinnach, C.; Moonshiram, D.; Picon, A.; Monge, P.; Batista, V.S.; Llobet, A. Electronic π-delocalization boosts catalytic water oxidation by Cu (II) molecular catalysts heterogenized on graphene sheets. J. Am. Chem. Soc. 2017, 139, 12907–12910. [Google Scholar] [CrossRef]
- Kumar, S.; Khatri, O.P.; Cordier, S.; Boukherroub, R.; Jain, S.L. Graphene Oxide Supported Molybdenum Cluster: First Heterogenized Homogeneous Catalyst for the Synthesis of Dimethylcarbonate from CO2 and Methanol. Chem. Eur. J. 2015, 21, 3488–3494. [Google Scholar] [CrossRef]
- Zhao, Q.; Bai, C.; Zhang, W.; Li, Y.; Zhang, G.; Zhang, F.; Fan, X. Catalytic epoxidation of olefins with graphene oxide supported copper (Salen) complex. Ind. Eng. Chem. Res. 2014, 53, 4232–4238. [Google Scholar] [CrossRef]
- Li, Z.; Wu, S.; Ding, H.; Zheng, D.; Hu, J.; Wang, X.; Huo, Q.; Guan, J.; Kan, Q. Immobilized Cu (II) and Co (II) salen complexes on graphene oxide and their catalytic activity for aerobic epoxidation of styrene. New J. Chem. 2013, 37, 1561–1568. [Google Scholar] [CrossRef]
- Su, H.; Wu, S.; Li, Z.; Huo, Q.; Guan, J.; Kan, Q. Co (II), Fe (III) or VO (II) Schiff base metal complexes immobilized on graphene oxide for styrene epoxidation. Appl. Organomet. Chem. 2015, 29, 462–467. [Google Scholar] [CrossRef]
- Shylesh, S.; Jia, M.; Thiel, W.R. Recent Progress in the Heterogenization of Complexes for Single-Site Epoxidation Catalysis. Eur. J. Inorg. Chem. 2010, 2010, 4395–4410. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, P.; Deb, A.; Maiti, D.; Jain, S.L. Graphene oxide grafted with iridium complex as a superior heterogeneous catalyst for chemical fixation of carbon dioxide to dimethylformamide. Carbon 2016, 100, 632–640. [Google Scholar] [CrossRef]
- Sabater, S.; Mata, J.A.; Peris, E. Catalyst enhancement and recyclability by immobilization of metal complexes onto graphene surface by noncovalent interactions. ACS Catal. 2014, 4, 2038–2047. [Google Scholar] [CrossRef]
- Movahed, S.K.; Esmatpoursalmani, R.; Bazgir, A. N-Heterocyclic carbene palladium complex supported on ionic liquid-modified graphene oxide as an efficient and recyclable catalyst for Suzuki reaction. RSC Adv. 2014, 4, 14586–14591. [Google Scholar] [CrossRef]
- Yang, Q.; Pan, X.; Clarke, K.; Li, K. Covalent functionalization of graphene with polysaccharides. Ind. Eng. Chem. Res. 2011, 51, 310–317. [Google Scholar] [CrossRef]
- Sheeba, M.M.; Tamizh, M.M.; Bhuvanesh, N.S.; Karvembu, R. Water soluble Ru (II)–p-cymene complexes of chiral aroylthiourea ligands derived from unprotected D/L-alanine as proficient catalysts for asymmetric transfer hydrogenation of ketones. Appl. Organomet. Chem. 2019, 33, 4667. [Google Scholar] [CrossRef]
- Mariconda, A.; Longo, P.; Agovino, A.; Guadagno, L.; Sorrentino, A.; Raimondo, M. Synthesis of ruthenium catalysts functionalized graphene oxide for self-healing applications. Polymer 2015, 69, 330–342. [Google Scholar] [CrossRef]
- Fang, M.; Wang, K.; Lu, H.; Yang, Y.; Nutt, S. Covalent polymer functionalization of graphene nanosheets and mechanical properties of composites. J. Mater. Chem. 2009, 19, 7098–7105. [Google Scholar] [CrossRef]
- Sheeba, M.M.; Preethi, S.; Nijamudheen, A.; Tamizh, M.M.; Datta, A.; Farrugia, L.J.; Karvembu, R. Half-sandwich Ru (η6-C6H6) complexes with chiral aroylthioureas for enhanced asymmetric transfer hydrogenation of ketones–experimental and theoretical studies. Catal. Sci. Technol. 2015, 5, 4790–4799. [Google Scholar] [CrossRef]
- Deng, D.; Xiao, L.; Chung, I.M.; Kim, I.S.; Gopiraman, M. Industrial-quality graphene oxide switched highly efficient metal-and solvent-free synthesis of β-ketoenamines under feasible conditions. ACS Sustain. Chem. Eng. 2017, 5, 1253–1259. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, T.; Abney, C.W.; Li, Z.; Lin, W. Graphene-Immobilized Monomeric Bipyridine-Mx+ (Mx+ = Fe3+, Co2+, Ni2+, or Cu2+) Complexes for Electrocatalytic Water Oxidation. ACS Appl. Mater. Interfaces 2014, 6, 18475–18479. [Google Scholar] [CrossRef] [PubMed]
- Sheeba, M.M.; Tamizh, M.M.; Babu, S.G.; Bhuvanesh, N.S.; Karvembu, R. Ru (II)-p-cymene complexes containing esters of chiral D/L-phenylalanine derived aroylthiourea ligands for enantioselective reduction of pro-chiral ketones. RSC Adv. 2016, 6, 68494–68503. [Google Scholar] [CrossRef]
- San Tan, S.; Yanagisawa, S.; Inagaki, K.; Kassim, M.B.; Morikawa, Y. Experimental and computational studies on ruthenium (ii) bis-diimine complexes of N, N′-chelate ligands: The origin of changes in absorption spectra upon oxidation and reduction. Phys. Chem. Chem. Phys. 2019, 21, 7973–7988. [Google Scholar] [CrossRef]
- Ando, T.; Kamigaito, M.; Sawamoto, M. Catalytic activities of ruthenium (II) complexes in transition-metal-mediated living radical polymerization: Polymerization, model reaction, and cyclic voltammetry. Macromolecules 2000, 33, 5825–5829. [Google Scholar] [CrossRef]
- Gopiraman, M.; Saravanamoorthy, S.; Deng, D.; Ilangovan, A.; Kim, I.S.; Chung, I.M. Facile Mechanochemical Synthesis of Nickel/Graphene Oxide Nanocomposites with Unique and Tunable Morphology: Applications in Heterogeneous Catalysis and Supercapacitors. Catalysts 2019, 9, 486. [Google Scholar] [CrossRef] [Green Version]
- Gopiraman, M.; Ganesh Babu, S.; Khatri, Z.; Kai, W.; Kim, Y.A.; Endo, M.; Karvembu, R.; Kim, I.S. Dry synthesis of easily tunable nano ruthenium supported on graphene: Novel nanocatalysts for aerial oxidation of alcohols and transfer hydrogenation of ketones. J. Phys. Chem. C 2013, 117, 23582–23596. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, F.; Pan, Y.; Jin, L.; Liu, B.; Mao, Y.; Huang, J. Multiwall-carbon-nanotube/cellulose composite fibers with enhanced mechanical and electrical properties by cellulose grafting. RSC Adv. 2018, 8, 5678–5684. [Google Scholar] [CrossRef] [Green Version]
- You, X.; Zou, G.; Ye, Q.; Zhang, Q.; He, P. Ruthenium (II) complex-sensitized solid-state polymerization of diacetylene in the visible light region. J. Mater. Chem. 2008, 18, 4704–4711. [Google Scholar] [CrossRef]
- Liu, Z.; Ou, J.; Wang, H.; You, X.; Ye, M. Synthesis and characterization of hydrazide-linked and amide-linked organic polymers. ACS Appl. Mater. Interfaces 2016, 8, 32060–32067. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Moon, J.M.; Choi, J.; Hwang, H.; Shim, Y.B. Magnetic force assisted electrochemical sensor for the detection of thrombin with aptamer-antibody sandwich formation. Biosens. Bioelectron. 2018, 117, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Varughese, B.; Chellamma, S.; Lieberman, M. XPS study of self-assembly of ruthenium dimers [((acac) 2Ru) 2bptz]0,+ on hydrophobic and hydrophilic SAMs. Langmuir 2002, 18, 7964–7970. [Google Scholar] [CrossRef]
- Agnes, C.; Arnault, J.C.; Omnes, F.; Jousselme, B.; Billon, M.; Bidan, G.; Mailley, P. XPS study of ruthenium tris-bipyridine electrografted from diazonium salt derivative on microcrystalline boron doped diamond. Phys. Chem. Chem. Phys. 2009, 11, 11647–11654. [Google Scholar] [CrossRef]
- Morgan, D.J. Resolving ruthenium: XPS studies of common ruthenium materials. Surf. Interface Anal. 2015, 47, 1072–1079. [Google Scholar] [CrossRef]
- Gao, F.; Jin, R.; Zhang, D.; Liang, Q.; Ye, Q.; Liu, G. Flower-like mesoporous silica: A bifunctionalized catalyst for rhodium-catalyzed asymmetric transfer hydrogenation of aromatic ketones in aqueous medium. Green Chem. 2013, 15, 2208–2214. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Han, D.; Gao, Q.; Li, C. Asymmetric transfer hydrogenation using recoverable ruthenium catalyst immobilized into magnetic mesoporous silica. J. Mol. Catal. A Chem. 2009, 298, 31–35. [Google Scholar] [CrossRef]
- Sandee, A.J.; Petra, D.G.; Reek, J.N.; Kamer, P.C.; van Leeuwen, P.W. Solid-phase synthesis of homogeneous ruthenium catalysts on silica for the continuous asymmetric transfer hydrogenation reaction. Chem. Eur. J. 2001, 7, 1202–1208. [Google Scholar] [CrossRef]
- Jiang, H.Y.; Yang, C.F.; Li, C.; Fu, H.Y.; Chen, H.; Li, R.X.; Li, X.J. Heterogeneous Enantioselective Hydrogenation of Aromatic Ketones Catalyzed by Cinchona-and Phosphine-Modified Iridium Catalysts. Angew Chem. Int. Ed. 2008, 47, 9240–9244. [Google Scholar] [CrossRef]
- Liu, P.N.; Gu, P.M.; Deng, J.G.; Tu, Y.Q.; Ma, Y.P. Synthesis of dendritic catalysts and application in asymmetric transfer hydrogenation. Eur.J. Org. Chem. 2005, 2005, 3221–3227. [Google Scholar] [CrossRef]
- Liu, P.N.; Gu, P.M.; Wang, F.; Tu, Y.Q. Efficient heterogeneous asymmetric transfer hydrogenation of ketones using highly recyclable and accessible silica-immobilized Ru-TsDPEN catalysts. Org. Lett. 2004, 6, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.N.; Deng, J.G.; Tu, Y.Q.; Wang, S.H. Highly efficient and recyclable heterogeneous asymmetric transfer hydrogenation of ketones in water. Chem. Commun. 2004, 18, 2070–2071. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Jing, Q.; Li, X.; Shi, L.; Ding, K. Programmed assembly of two different ligands with metallic ions: Generation of self-supported Noyori-type catalysts for heterogeneous asymmetric hydrogenation of ketones. J. Am. Chem. Soc. 2005, 127, 7694–7695. [Google Scholar] [CrossRef] [PubMed]












| Serial No. | Catalyst, Amount (mg) | Base | Temperature (°C) | Time (h) | Yield (%) b | ee(%) c |
|---|---|---|---|---|---|---|
| 1 | 0 | KOH | 82 | 24 | 12 | - |
| 2 | G-CLRu(II), 5 | KOH | 82 | 24 | 43 | 95 |
| 3 | G-CLRu(II), 10 | KOH | 82 | 24 | 95 | 97 |
| 4 | G-CLRu(II), 15 | KOH | 82 | 24 | 95 | 96 |
| 5 d | G-CLRu(II), 10 | NaOH | 82 | 24 | 99 | 83 |
| 6 d | G-CLRu(II), 10 | (CH3)3COK | 82 | 24 | 99 | 89 |
| 7 | G-CLRu(II), 10 | KOH | 27 | 24 | 9 | - |
| 8 | G-CLRu(II), 10 | KOH | 50 | 24 | 23 | 93 |
| 9 | G-CLRu(II), 10 | KOH | 70 | 24 | 47 | 95 |
| 10 | G-CLRu(II), 10 | KOH | 82 | 2 | 9 | - |
| 11 | G-CLRu(II), 10 | KOH | 82 | 4 | 18 | 92 |
| 12 | G-CLRu(II), 10 | KOH | 82 | 8 | 36 | 94 |
| 13 | G-CLRu(II), 10 | KOH | 82 | 12 | 45 | 97 |
| 14 | G-CLRu(II), 10 | KOH | 82 | 16 | 64 | 95 |
| 15 | G-CLRu(II), 10 | KOH | 82 | 20 | 88 | 95 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mayakrishnan, G.; Ick Soo, K.; Ill Min, C. Stepwise Construction of Ru(II)Center Containing Chiral Thiourea Ligand on Graphene Oxide: First Efficient, Reusable, and Stable Catalyst for Asymmetric Transfer Hydrogenation of Ketones. Catalysts 2020, 10, 175. https://doi.org/10.3390/catal10020175
Mayakrishnan G, Ick Soo K, Ill Min C. Stepwise Construction of Ru(II)Center Containing Chiral Thiourea Ligand on Graphene Oxide: First Efficient, Reusable, and Stable Catalyst for Asymmetric Transfer Hydrogenation of Ketones. Catalysts. 2020; 10(2):175. https://doi.org/10.3390/catal10020175
Chicago/Turabian StyleMayakrishnan, Gopiraman, Kim Ick Soo, and Chung Ill Min. 2020. "Stepwise Construction of Ru(II)Center Containing Chiral Thiourea Ligand on Graphene Oxide: First Efficient, Reusable, and Stable Catalyst for Asymmetric Transfer Hydrogenation of Ketones" Catalysts 10, no. 2: 175. https://doi.org/10.3390/catal10020175
APA StyleMayakrishnan, G., Ick Soo, K., & Ill Min, C. (2020). Stepwise Construction of Ru(II)Center Containing Chiral Thiourea Ligand on Graphene Oxide: First Efficient, Reusable, and Stable Catalyst for Asymmetric Transfer Hydrogenation of Ketones. Catalysts, 10(2), 175. https://doi.org/10.3390/catal10020175