Homogeneous and Heterogeneous Catalysis Impact on Pyrolyzed Cellulose to Produce Bio-Oil
Abstract
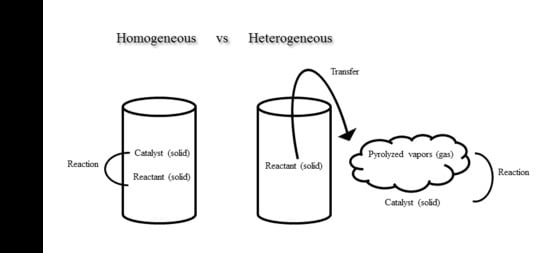
:1. Introduction
2. Results and Discussion
2.1. XRD and SEM Analysis
2.2. Pyrolysis Results
2.2.1. Comparison Among Different Catalysts for Homogeneous Catalysis
2.2.2. Comparison Between Different Catalysts for Heterogeneous Catalysis
3. Experimental Method
3.1. Catalyst Preparation
3.2. Catalyst Preparation
3.3. XRD, BET, and the SEM-EDS Test
3.4. GC–MS Analysis Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dudley, B. BP Statistical Review of World Energy, 68th ed.; B.P.: London, UK, 2019. [Google Scholar]
- Liang, Q.M.; Fan, Y.; Wei, Y.M. The effect of energy end-use efficiency improvement on China’s energy use and CO2 emissions: A CGE model-based analysis. Energy Effic. 2009, 2, 243–262. [Google Scholar] [CrossRef]
- International Energy Outlook 2019 with Projections to 2050. Available online: https://www.eia.gov/ieo (accessed on 18 October 2019).
- Some Snapshots of the Global Energy Situation. Available online: http://conversableeconomist.blogspot.com/2019/06/some-snapshots-of-global-energy.html (accessed on 13 June 2019).
- Okkonen, L.; Lehtonen, O. Local, regional and national level of the socioeconomic impacts of a bio-oil production system—A case in Lieksa, Finland. Renew. Sustain. Energy Rev. 2017, 71, 103–111. [Google Scholar] [CrossRef]
- Biotech Company Euglena Teams with ANA to Fuel Green Commercial Flights, Nikkei Asian Review. Available online: https://asia.nikkei.com/Business/Companies/Jet-biofuel-mass-production-to-begin-in-Japan (accessed on 2 November 2018).
- Lehto, J.; Oasmaa, A.; Solantausta, Y.; Kytö, M.; Chiaramonti, D. Review of fuel oil quality and combustion of fast pyrolysis bio-oils from lignocellulosic biomass. Appl. Energy 2014, 116, 178–190. [Google Scholar] [CrossRef]
- Zhang, B.; Zhong, Z.; Ding, K.; Cao, Y.; Liu, Z. Catalytic Upgrading of Corn Stalk Fast Pyrolysis Vapors with Fresh and Hydrothermally Treated HZSM-5 Catalysts Using Py-GC/MS. Ind. Eng. Chem. Res. 2014, 53, 9979–9984. [Google Scholar] [CrossRef]
- Iliopoulou, E.F.; Stefanidis, S.; Kalogiannis, K.; Psarras, A.C.; Delimitis, A.; Triantafyllidis, K.S.; Lappas, A.A. Pilot-scale validation of Co-ZSM-5 catalyst performance in the catalytic upgrading of biomass pyrolysis vapors. Green Chem. 2014, 16, 662–674. [Google Scholar] [CrossRef]
- Yung, M.M.; Starace, A.K.; Mukarakate, C.; Crow, A.M.; Leshnov, M.A.; Magrini, K.A. Biomass Catalytic Pyrolysis on Ni/ZSM-5: Effects of Nickel Pretreatment and Loading. Energy Fuels 2016, 30, 5259–5268. [Google Scholar] [CrossRef]
- Liu, W.J.; Li, W.W.; Jiang, H.; Yu, H.Q. Fates of Chemical Elements in Biomass during its Pyrolysis. Chem. Rev. 2017, 117, 6367–6398. [Google Scholar] [CrossRef]
- Zhang, H.; Xiao, R.; Huang, H.; Xiao, G. Comparison of non-catalytic and catalytic fast pyrolysis of corncob in a fluidized bed reactor. Bioresour. Technol. 2009, 100, 1428–1434. [Google Scholar] [CrossRef]
- Uzun, B.B.; Sarioglu, N. Rapid and catalytic pyrolysis of corn stalks. Fuel Process. Technol. 2009, 90, 705–716. [Google Scholar] [CrossRef]
- Muneer, B.; Zeeshan, M.; Qaisar, S.; Razzaq, M.; Lftikhar, H. Influence of in-situ and ex-situ HZSM-5 catalyst on co-pyrolysis of corn stalk and polystyrene with a focus on liquid yield and quality. J. Clean. Prod. 2019, 237, 117762. [Google Scholar] [CrossRef]
- Chen, G.Y.; Li, J.; Liu, C.; Yan, B.B.; Cheng, Z.J.; Ma, W.C.; Yao, J.G.; Zhang, H. Low-temperature catalytic cracking of biomass gasification tar over Ni/HZSM-5. Waste Biomass Valorization 2019, 10, 1013–1020. [Google Scholar] [CrossRef]
- Liu, J.C.; Ma, X.L.; Li, Y.; Wang, Y.G.; Xiao, H.; Li, J. Heterogeneous Fe3 single-cluster catalyst for ammonia synthesis via an associative mechanism. Nat. Commun. 2018, 9, 1610. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Chen, J.J.; Mu, Y. Catalytic CO2 reduction to valuable chemicals using NiFe-based nanoclusters: A first-principles theoretical evaluation. Phys. Chem. Chem. Phys. 2017, 19, 28344–28353. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Cross, J.S. Reusability of the Ni2Fe3 metal catalyst for upgrading pyrolyzed bio-oil. In Proceedings of the MATEC Web of Conferences, Sapporo, Japan, 23–27 September 2019. [Google Scholar]
- Nie, L.; de Souza, P.M.; Noronha, F.B.; An, W.; Sooknoi, T.; Resasco, D.E. Selective conversion of m-cresol to toluene over bimetallic Ni–Fe catalysts. J. Mol. Catal. A Chem. 2014, 47–55, 388–389. [Google Scholar] [CrossRef]
- Yao, D.; Wu, C.; Yang, H.; Hu, Q.; Nahil, M.A.; Chen, H.; Williams, P.T. Hydrogen production from catalytic reforming of the aqueous fraction of pyrolysis bio-oil with modified Ni–Al catalysts. Int. J. Hydrogen Energy 2014, 39, 14642–14652. [Google Scholar] [CrossRef] [Green Version]
- Behrens, M.; Cross, J.S.; Akasaka, H.; Ohtake, N. A study of guaiacol, cellulose, and Hinoki wood pyrolysis with silica, ZrO2&TiO2 and ZSM-5 catalysts. J. Anal. Appl. Pyrolysis 2017, 125, 178–184. [Google Scholar]
- Stefanidis, S.D.; Kalogiannis, K.G.; Iliopoulou, E.F.; Michailof, C.M.; Pilavachi, P.A.; Lappas, A.A. A study of lignocellulosic biomass pyrolysis via the pyrolysis of cellulose, hemicellulose and lignin. J. Anal. Appl. Pyrolysis 2014, 105, 143–150. [Google Scholar] [CrossRef]
- Engineering ToolBox. Specific Heat of Some Metals. 2003. Available online: https://www.engineeringtoolbox.com/specific-heat-metals-d_152.html (accessed on 26 January 2020).
- Madelung, O.; White, G.K. Thermal Conductivity of Pure Metals and Alloys; Springer: Berlin, Germany, 1991. [Google Scholar]
- Xia, H.A.; Yan, X.P.; Xu, S.Q.; Yang, L.; Ge, Y.J.; Wang, J.; Zuo, S.L. Effect of Zn/ZSM-5 and FePO4 Catalysts on Cellulose Pyrolysis. J. Chem. 2015, 2015, 749875. [Google Scholar] [CrossRef] [Green Version]
- Fabbri, D.; Torri, C.; Baravelli, V. Effect of zeolites and nanopowder metal oxides on the distribution of chiral anhydrosugars evolved from pyrolysis of cellulose: An analytical study. J. Anal. Appl. Pyrolysis 2007, 80, 24–29. [Google Scholar] [CrossRef]
- Lu, Q.; Wang, Z.; Dong, C.Q.; Zhang, Z.F.; Zhang, Y.; Yang, Y.P.; Zhu, X.F. Selective fast pyrolysis of biomass impregnated with ZnCl2: Furfural production together with acetic acid and activated carbon as by-products. J. Anal. Appl. Pyrolysis 2011, 91, 273–279. [Google Scholar] [CrossRef]
- Li, S.Y.; Yu, D.; Cheng, S.; Cross, J.S. Recycle Metal (Ni, Fe) Cluster Catalyst for Cellulose Pyrolysis to Upgrade Bio-oil. Biomass Bioenergy 2020. under review. [Google Scholar]
- Iliopoulou, E.F.; Stefanidis, S.D.; Kalogiannis, K.G.; Delimitis, A.; Lappas, A.A.; Triantafyllidis, K.S. Catalytic upgrading of biomass pyrolysis vapors using transition metal-modified ZSM-5 zeolite. Appl. Catal. B Environ. 2012, 127, 281–290. [Google Scholar] [CrossRef]
- Cheng, S.; Wei, L.; Julson, J.; Muthukumarappan, K.; Kharel, P.R. Upgrading pyrolysis bio-oil to hydrocarbon enriched biofuel over bifunctional Fe-Ni/HZSM-5 catalyst in supercritical methanol. Fuel Process. Technol. 2017, 167, 117–126. [Google Scholar] [CrossRef]
- De, G.; Tapfer, L.; Catalano, M.; Battaglin, G.; Caccavale, F.; Gonella, F.; Mazzoldi, P.; Haglund, R.F., Jr. Formation of copper and silver nanometer dimension clusters in silica by the sol-gel process. Appl. Phys. Lett. 1996, 68, 3820–3822. [Google Scholar] [CrossRef]
- Jayaprakash, J.; Srinivasan, N.; Chandrasekaran, P.; Girija, E.K. Synthesis and characterization of cluster of grapes like pure and Zinc-doped CuO nanoparticles by sol-gel method. Spectrochim. Acta Part A 2015, 136, 1803–1806. [Google Scholar] [CrossRef] [PubMed]
- Di Serio, M.; Ledda, M.; Cozzolino, M.; Minutillo, G.; Tesser, R.; Santacesaria, E. Transesterification of soybean oil to biodiesel by using heterogeneous basic catalysts. Ind. Eng. Chem. Res. 2006, 45, 3009–3014. [Google Scholar] [CrossRef]
- Delfort, B.; le Pennec, D.; Lendresse, C. Process for Transesterification of Vegetable Oils or Animal Oils by Means of Heterogeneous Catalysts Based on Zinc or Bismuth, Titanium and Aluminum. U.S. Patent 7,151,187 B2, 19 December 2006. [Google Scholar]







| Acid% | Alcohol% | Esters% | Furans% | Ketones% | Phenols% | Sugar% | HC% | Others% | |
|---|---|---|---|---|---|---|---|---|---|
| None | 4.8 ± 0.2 | 2.3 ± 0.1 | 2.0 ± 0.1 | 20.7 ± 0.7 | 17.0 ± 2.6 | 1.6 ± 0.4 | 48.8 ± 1.7 | 0.0 | 2.1 ± 1.2 |
| ZSM-5 | 11.8 ± 0.3 | 0.7 ± 0.1 | 2.1 ± 0.1 | 13.3 ± 0.4 | 25.8 ± 0.5 | 0.0 | 43.3 ± 1.2 | 0.0 | 3.0 ± 0.1 |
| Ni2Fe3/ZSM-5 | 12.6 ± 0.3 | 0.8 ± 0.1 | 2.4 ± 0.1 | 15.7 ± 0.9 | 28.3 ± 1.2 | 0.0 | 38.2 ± 0.1 | 0.0 | 2.0 ± 0.1 |
| Ni2Fe3 | 4.6 ± 0.3 | 2.3 ± 0.1 | 2.4 ± 0.2 | 25.9 ± 0.7 | 27.9 ± 0.8 | 2.2 ± 0.2 | 31.5 ± 0.6 | 0.5 ± 0.4 | 2.6 ± 0.4 |
| Acid% | Alcohol% | Esters% | Furans% | Ketones% | Phenols% | Sugar% | HC% | Others% | |
|---|---|---|---|---|---|---|---|---|---|
| None | 4.8 ± 0.2 | 2.3 ± 0.1 | 2.0 ± 0.1 | 20.7 ± 0.7 | 17.0 ± 2.6 | 1.6 ± 0.4 | 48.8 ± 1.7 | 0.0 | 2.1 ± 1.2 |
| ZSM-5 | 12.3 ± 0.5 | 1.3 ± 0.1 | 4.9 ± 0.2 | 20.7 ± 1.6 | 30.1 ± 0.8 | 2.8 ± 0.3 | 25.6 ± 0.9 | 0.0 | 2.3 ± 0.1 |
| Ni2Fe3/ZSM-5 | 11.2 ± 0.3 | 2.0 ± 0.1 | 3.6 ± 0.4 | 20.3 ± 1.3 | 37.6 ± 2.7 | 1.7 ± 0.3 | 19.1 ± 0.2 | 0.4 ± 0.1 | 4.1 ± 0.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Cheng, S.; Cross, J.S. Homogeneous and Heterogeneous Catalysis Impact on Pyrolyzed Cellulose to Produce Bio-Oil. Catalysts 2020, 10, 178. https://doi.org/10.3390/catal10020178
Li S, Cheng S, Cross JS. Homogeneous and Heterogeneous Catalysis Impact on Pyrolyzed Cellulose to Produce Bio-Oil. Catalysts. 2020; 10(2):178. https://doi.org/10.3390/catal10020178
Chicago/Turabian StyleLi, Siyi, Shuo Cheng, and Jeffrey S. Cross. 2020. "Homogeneous and Heterogeneous Catalysis Impact on Pyrolyzed Cellulose to Produce Bio-Oil" Catalysts 10, no. 2: 178. https://doi.org/10.3390/catal10020178
APA StyleLi, S., Cheng, S., & Cross, J. S. (2020). Homogeneous and Heterogeneous Catalysis Impact on Pyrolyzed Cellulose to Produce Bio-Oil. Catalysts, 10(2), 178. https://doi.org/10.3390/catal10020178