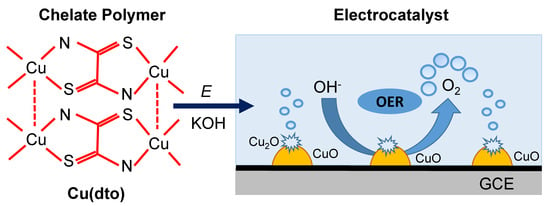
An Efficient Electrocatalyst for Oxygen Evolution Reaction in Alkaline Solutions Derived from a Copper Chelate Polymer via In Situ Electrochemical Transformation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Cu(dto) Compound
2.2. Electrochemical Characterization
2.3. Electrocatalytic Characterization
2.4. Surface Morphology and Composition after Electrochemical Cycling
2.5. Proposed Catalytic Cycle
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Copper Dithiooxamide Cu(dto) Compound
3.3. Materials Characterization
3.4. Catalyst Slurry and Electrode Preparation
3.5. Electrochemical Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tahir, M.; Pan, L.; Idrees, F.; Zhang, X.; Wang, L.; Zou, J.-J.; Wang, Z.L. Electrocatalytic Oxygen Evolution Reaction for Energy Conversion and Storage: A Comprehensive Review. Nano Energy 2017, 37, 136–157. [Google Scholar] [CrossRef]
- Park, S.; Shao, Y.; Liu, J.; Wang, Y. Oxygen Electrocatalysts for Water Electrolyzers and Reversible Fuel Cells: Status and Perspective. Energy Environ. Sci. 2012, 5, 9331–9344. [Google Scholar] [CrossRef]
- Wang, Z.-L.; Xu, D.; Xu, J.-J.; Zhang, X.-B. Oxygen Electrocatalysts in Metal-air Batteries: From Aqueous to Nonaqueous Electrolytes. Chem. Soc. Rev. 2014, 43, 7746–7786. [Google Scholar] [CrossRef] [PubMed]
- Kondo, M.; Masaoka, S. Water Oxidation Catalysts Constructed by Biorelevant First-row Metal Complexes. Chem. Lett. 2016, 45, 1220–1231. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Handoko, A.D.; Du, Y.; Xi, S.; Yeo, B.S. In Situ Raman Spectroscopy of Copper and Copper Oxide Surfaces during Electrochemical Oxygen Evolution Reaction: Identification of CuIII Oxides as Catalytically Active Species. ACS Catal. 2016, 6, 2473–2481. [Google Scholar] [CrossRef]
- Lee, Y.; Suntivich, J.; May, K.J.; Perry, E.E.; Shao-Horn, Y. Synthesis and Activities of Rutile IrO2 and RuO2 Nanoparticles for Oxygen Evolution in Acid and Alkaline Solutions. J. Phys. Chem. Lett. 2012, 3, 399–404. [Google Scholar] [CrossRef]
- Reier, T.; Oezaslan, M.; Strasser, P. Electrocatalytic Oxygen Evolution Reaction (OER) on Ru, Ir, and Pt Catalysts: A Comparative Study of Nanoparticles and Bulk Materials. ACS Catal. 2012, 2, 1765–1772. [Google Scholar] [CrossRef]
- Song, F.; Bai, L.; Moysiadou, A.; Lee, S.; Hu, C.; Liardet, L.; Hu, X. Transition Metal Oxides as Electrocatalysts for the Oxygen Evolution Reaction in Alkaline Solutions: An Application-Inspired Renaissance. J. Am. Chem. Soc. 2018, 140, 7748–7759. [Google Scholar] [CrossRef]
- McCrory, C.C.L.; Jung, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking Heterogeneous Electrocatalysts for the Oxygen Evolution Reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. [Google Scholar] [CrossRef]
- Eftekhari, A. Tuning the Electrocatalysts for Oxygen Evolution Reaction. Mater. Today Energy 2017, 5, 37–57. [Google Scholar] [CrossRef]
- Liu, X.; Cui, S.; Qian, M.; Sun, Z.; Du, P. In Situ Generated Highly Active Copper Oxide Catalysts for the Oxygen Evolution Reaction at Low Overpotential in Akaline Solutions. Chem. Commun. 2016, 52, 5546–5549. [Google Scholar] [CrossRef]
- Najafpour, M.M.; Mehrabani, S.; Mousazade, Y.; Hołyńska, M. Water Oxidation by A Copper(II) Complex: New Findings, Questions, Challenges and A New Hypothesis. Dalton Trans. 2018, 47, 9021–9029. [Google Scholar] [CrossRef]
- Liu, X.; Cui, S.; Sun, Z.; Du, P. Copper Oxide Nanomaterials Synthesized from simple Copper Salts as active Catalysts for Electrocatalytic Water Oxidation. Electrochim. Acta 2015, 160, 202–208. [Google Scholar] [CrossRef]
- Silva, N.; Ramírez, S.; Díaz, I.; Garcia, A.; Hassan, N. Easy, Quick, and Reproducible Sonochemical Synthesis of CuO Nanoparticles. Materials 2019, 12, 804. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Cui, S.; Sun, Z.; Ren, Y.; Zhang, X.; Du, P. Self-Supported Copper Oxide Electrocatalyst for Water Oxidation at Low Overpotential and Confirmation of Its Robustness by Cu K-Edge X-ray Absorption Spectroscopy. J. Phys. Chem. C 2016, 120, 831–840. [Google Scholar] [CrossRef]
- Abboudi, M.; Mosset, A.; Galy, J. Metal Complexes of Rubeanic Acid. Large-angle X-ray Scattering Studies of Amorphous Copper(II) and Nickel(II) Complexes. Inorg. Chem. 1985, 24, 2091–2094. [Google Scholar] [CrossRef]
- Kanda, S.; Ito, K.; Nogaito, T. Magnetic and Electrical Properties of Coordination Polymers Formed with Copper and Rubeanic Acid. J. Polym. Sci. Polym. Symp. 1967, 17, 151–162. [Google Scholar] [CrossRef]
- Kanda, S.; Suzuki, A.; Ohkawa, K. Syntheses of Nonstereospecific and Stereospecific Lamellar Coordination Polymers. N,N -Disubstituted Dithiooxamides Copper Coordination Polymers. Ind. Eng. Chem. Prod. Res. Develop. 1973, 12, 88–96. [Google Scholar] [CrossRef]
- Ambrose, J.; Barradas, R.G.; Shoesmith, D.W. Investigations of Copper in Aqueous Alkaline Solutions by Cyclic Voltammetry. J. Electroanal. Chem. Interfacial Electrochem. 1973, 47, 47–64. [Google Scholar] [CrossRef]
- El Din, A.M.S.; El Wahab, F.M.A. The Behaviour of the Copper Electrode in Alkaline Solutions upon Alternate Anodic and Cathodic Polarization. Electrochim. Acta 1964, 9, 113–121. [Google Scholar] [CrossRef]
- Abd el Haleem, S.M.; Ateya, B.G. Cyclic Voltammetry of Copper in Sodium Hydroxide Solutions. J. Electroanal. Chem. Interfacial Electrochem. 1981, 117, 309–319. [Google Scholar] [CrossRef]
- Zhu, Y.P.; Jing, Y.; Vasileff, A.; Heine, T.; Qiao, S.-Z. 3D Synergistically Active Carbon Nanofibers for Improved Oxygen Evolution. Adv. Energy Mater. 2017, 7, 1602928. [Google Scholar] [CrossRef]
- Hara, M.; Waraksa, C.C.; Lean, J.T.; Lewis, B.A.; Mallouk, T.E. Photocatalytic Water Oxidation in a Buffered Tris(2,2′-bipyridyl)ruthenium Complex-Colloidal IrO2 System. J. Phys. Chem. A 2000, 104, 5275–5280. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.-X.; He, C.-T.; Li, G.-R.; Zhang, J.-P. An Inorganic-MOF-inorganic Approach to Ultrathin CuO Decorated Cu–C Hybrid Nanorod Arrays for an Efficient Oxygen Evolution Reaction. J. Mater. Chem. A 2018, 6, 19176–19181. [Google Scholar] [CrossRef]
- Xu, H.; Feng, J.-X.; Tong, Y.-X.; Li, G.-R. Cu2O–Cu Hybrid Foams as High-Performance Electrocatalysts for Oxygen Evolution Reaction in Alkaline Media. ACS Catal. 2017, 7, 986–991. [Google Scholar] [CrossRef]
- Serov, A.; Andersen, N.I.; Roy, A.J.; Matanovic, I.; Artyushkova, K.; Atanassov, P. CuCo2O4 ORR/OER Bi-functional Catalyst: Influence of Synthetic Approach on Performance. J. Electrochem. Soc. 2015, 162, F449–F454. [Google Scholar] [CrossRef]
- Hinogami, R.; Toyoda, K.; Aizawa, M.; Kawasaki, T.; Gyoten, H. Copper Delafossite Anode for Water Electrolysis. ECS Trans. 2013, 58, 27–31. [Google Scholar] [CrossRef]
- Chen, P.; Xu, K.; Tong, Y.; Li, X.; Tao, S.; Fang, Z.; Chu, W.; Wu, X.; Wu, C. Cobalt Nitrides as A Class of Metallic Electrocatalysts for the Oxygen Evolution Reaction. Inorg. Chem. Front. 2016, 3, 236–242. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Yamada, S.; Nishida, T.; Sato, E. Oxygen Evolution on La1 − xSrxFe1 − yCoyO3 Series Oxides. J. Electrochem. Soc. 1980, 127, 2360–2364. [Google Scholar] [CrossRef]
- Wu, J.; Subramaniam, J.; Liu, Y.; Geng, D.; Meng, X. Facile Assembly of Ni(OH)2 Nanosheets on Nitrogen-doped Carbon Nanotubes Network as High-performance Electrocatalyst for Oxygen Evolution Reaction. J. Alloys Compd. 2018, 731, 766–773. [Google Scholar] [CrossRef]
- Zhu, W.; Yue, X.; Zhang, W.; Yu, S.; Zhang, Y.; Wang, J.; Wang, J. Nickel Sulfide Microsphere Film on Ni foam as An Efficient Bifunctional Electrocatalyst for Overall Water Splitting. Chem. Commun. 2016, 52, 1486–1489. [Google Scholar] [CrossRef] [PubMed]
- Meghana, S.; Kabra, P.; Chakraborty, S.; Padmavathy, N. Understanding the Pathway of Antibacterial Activity of Copper Oxide Nanoparticles. RSC Adv. 2015, 5, 12293–12299. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, D.; Wu, Q.; Diao, P. Cu2O/CuO Bilayered Composite as a High-Efficiency Photocathode for Photoelectrochemical Hydrogen Evolution Reaction. Sci. Rep. 2016, 6, 35158. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Song, F.; Hu, X. A Nickel Iron Diselenide-derived Efficient Oxygen-evolution Catalyst. Nat. Commun. 2016, 7, 12324. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.G.; Jones, C.W.; Linic, S.; Stamenkovic, V.R. Best Practices in Pursuit of Topics in Heterogeneous Electrocatalysis. ACS Catal. 2017, 7, 6392–6393. [Google Scholar] [CrossRef] [Green Version]







| Catalysts | η (mV) at J = 1 mA cm−2 | EOER (V)a at J = 1 mA cm−2 | η (mV) at J = 10 mA cm−2 | EOER (V)a at J = 10 mA cm−2 | Tafel Slope (mV dec−1) |
|---|---|---|---|---|---|
| Cu(dto)/C | 279 | 1.509 | 400 | 1.630 | 81 |
| CuO/C | 312 | 1.542 | 496 | 1.726 | 160 |
| IrO2/C | 379 | 1.609 | - | - | 189 |
| Cu | 525 | 1.755 | - | - | 271 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Putra, R.P.; Horino, H.; Rzeznicka, I.I. An Efficient Electrocatalyst for Oxygen Evolution Reaction in Alkaline Solutions Derived from a Copper Chelate Polymer via In Situ Electrochemical Transformation. Catalysts 2020, 10, 233. https://doi.org/10.3390/catal10020233
Putra RP, Horino H, Rzeznicka II. An Efficient Electrocatalyst for Oxygen Evolution Reaction in Alkaline Solutions Derived from a Copper Chelate Polymer via In Situ Electrochemical Transformation. Catalysts. 2020; 10(2):233. https://doi.org/10.3390/catal10020233
Chicago/Turabian StylePutra, Ridwan P., Hideyuki Horino, and Izabela I. Rzeznicka. 2020. "An Efficient Electrocatalyst for Oxygen Evolution Reaction in Alkaline Solutions Derived from a Copper Chelate Polymer via In Situ Electrochemical Transformation" Catalysts 10, no. 2: 233. https://doi.org/10.3390/catal10020233
APA StylePutra, R. P., Horino, H., & Rzeznicka, I. I. (2020). An Efficient Electrocatalyst for Oxygen Evolution Reaction in Alkaline Solutions Derived from a Copper Chelate Polymer via In Situ Electrochemical Transformation. Catalysts, 10(2), 233. https://doi.org/10.3390/catal10020233