Hydrodeoxygenation of Benzofuran over Bimetallic Ni-Cu/γ-Al2O3 Catalysts
Abstract
:1. Introduction
2. Results and Discussion
2.1. X-ray Diffractometry (XRD)
2.2. Brunauer–Emmett–Teller Analysis
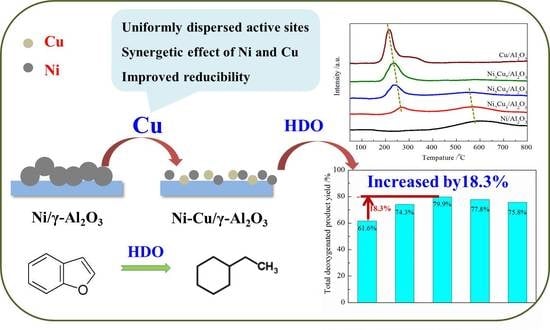
2.3. H2-Temperature Programmed Reduction (H2-TPR)
2.4. X-ray Photoelectron Spectroscopy (XPS)
2.5. Transmission Electron Microscopy (TEM)
2.6. Hydrodeoxygenation (HDO) Performance of Bimetallic NixCu(10-x)/γ-Al2O3 Catalysts
3. Experimental
3.1. Materials and Preparation of Catalysts
3.2. Characterization
3.3. HDO Performance test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gollakota, A.R.K.; Reddy, M.; Subramanyam, M.D.; Kishore, N. A review on the upgradation techniques of pyrolysis oil. Renew. Sust. Energy Rev. 2016, 58, 1543–1568. [Google Scholar] [CrossRef]
- Wang, H.; Male, J.; Wang, Y. Recent advances in hydrotreating of pyrolysis bio-oil and its oxygen-containing model compounds. Acs Catal. 2013, 3, 1047–1070. [Google Scholar] [CrossRef]
- Huber, G.W.; O’Connor, P.; Corma, A. Processing biomass in conventional oil refineries: Production of high quality diesel by hydrotreating vegetable oils in heavy vacuum oil mixtures. Appl. Catal. A Gen. 2007, 329, 120–129. [Google Scholar] [CrossRef]
- Yildiz, G.; Pronk, M.; Djokic, M.; van Geem, K.M.; Ronsse, F.; van Duren, R.; Prins, W. Validation of a new set-up for continuous catalytic fast pyrolysis of biomass coupled with vapour phase upgrading. J. Anal. Appl. Pyrol. 2013, 103, 343–351. [Google Scholar] [CrossRef]
- Prajitno, H.; Insyani, R.; Park, J.; Ryu, C.; Kim, J. Non-catalytic upgrading of fast pyrolysis bio-oil in supercritical ethanol and combustion behavior of the upgraded oil. Appl. Energy 2016, 172, 12–22. [Google Scholar] [CrossRef]
- Romero, Y.; Richard, F.; Brunet, S. Hydrodeoxygenation of 2-ethylphenol as a model compound of bio-crude over sulfided Mo-based catalysts: Promoting effect and reaction mechanism. Appl. Catal. B Environ. 2010, 98, 213–223. [Google Scholar] [CrossRef]
- Brillouet, S.; Baltag, E.; Brunet, S.; Richard, F. Deoxygenation of decanoic acid and its main intermediates over unpromoted and promoted sulfided catalysts. Appl. Catal. B Environ. 2014, 148, 201–211. [Google Scholar] [CrossRef]
- Gonçalves, V.O.O.; Brunet, S.; Richard, F. Hydrodeoxygenation of cresols over Mo/Al2O3 and CoMo/Al2O3 sulfided catalysts. Catal. Lett. 2016, 146, 1562–1573. [Google Scholar] [CrossRef]
- Şenol, O.İ.; Viljava, T.R.; Krause, A.O.I. Effect of sulphiding agents on the hydrodeoxygenation of aliphatic esters on sulphided catalysts. Appl. Catal. A Gen. 2007, 326, 236–244. [Google Scholar] [CrossRef]
- Nie, L.; Resasco, D.E. Kinetics and mechanism of m-cresol hydrodeoxygenation on a Pt/SiO2 catalyst. J. Catal. 2014, 317, 22–29. [Google Scholar] [CrossRef]
- De Souza, P.M.; Rabelo-Neto, R.C.; Borges, L.E.P.; Jacobs, G.; Davis, B.H.; Sooknoi, T.; Resasco, D.E.; Noronha, F.B. Role of keto intermediates in the hydrodeoxygenation of phenol over Pd on oxophilic supports. Acs Catal. 2015, 5, 1318–1329. [Google Scholar] [CrossRef]
- Newman, C.; Zhou, X.; Goundie, B.; Ghampson, I.T.; Pollock, R.A.; Ross, Z.; Wheeler, M.C.; Meulenberg, R.W.; Austin, R.N.; Frederick, B.G. Effects of support identity and metal dispersion in supported ruthenium hydrodeoxygenation catalysts. Appl. Catal. A Gen. 2014, 477, 64–74. [Google Scholar] [CrossRef]
- Zhou, M.; Ye, J.; Liu, P.; Xu, J.; Jiang, J. Water-assisted selective hydrodeoxygenation of guaiacol to cyclohexanol over supported Ni and Co bimetallic catalysts. ACS Sustain. Chem. Eng. 2017, 5, 8824–8835. [Google Scholar] [CrossRef]
- Leng, S.; Wang, X.; He, X.; Liu, L.; Liu, Y.; Zhong, X.; Zhuang, G.; Wang, J. NiFe/γ-Al2O3: A universal catalyst for the hydrodeoxygenation of bio-oil and its model compounds. Catal. Commun. 2013, 41, 34–37. [Google Scholar] [CrossRef]
- Cheng, S.; Wei, L.; Julson, J.; Rabnawaz, M. Upgrading pyrolysis bio-oil through hydrodeoxygenation (HDO) using non-sulfided Fe-Co/SiO2 catalyst. Energy Convers. Manag. 2017, 150, 331–342. [Google Scholar] [CrossRef]
- Carrero, A.; Calles, J.A.; García-Moreno, L.; Vizcaino, A.J. Production of renewable hydrogen from glycerol steam reforming over bimetallic Ni-(Cu, Co, Cr) catalysts supported on SBA-15 Silica. Catalysts 2017, 7, 55. [Google Scholar] [CrossRef] [Green Version]
- Tuan, L.A.; Luong, N.T.; Ishihara, K.N. Low-temperature catalytic performance of Ni-Cu/Al2O3 catalysts for gasoline reforming to produce hydrogen applied in spark ignition engines. Catalysts 2016, 6, 45. [Google Scholar] [CrossRef] [Green Version]
- Berenguer, A.; Sankaranarayanan, T.M.; Gómez, G.; Moreno, I.; Coronado, J.M.; Pizarro, P.; Serrano, D.P. Evaluation of transition metal phosphides supported on ordered mesoporous materials as catalysts for phenol hydrodeoxygenation. Green Chem. 2016, 18, 1938–1951. [Google Scholar] [CrossRef]
- Sutton, A.D.; Waldie, F.D.; Wu, R.; Schlaf, M.; Silks, L.A.; Gordon, J.C. The hydrodeoxygenation of bioderived furans into alkanes. Nat. Chem. 2013, 5, 428–432. [Google Scholar] [CrossRef]
- Liu, R.; Pang, M.; Chen, X.; Li, C.; Xu, C.; Liang, C. W2C nanorods with various amounts of vacancy defects: Determination of catalytic active sites in the hydrodeoxygenation of benzofuran. Catal. Sci. Technol. 2017, 7, 1333–1341. [Google Scholar] [CrossRef]
- Bunch, A.Y.; Ozkan, U.S. Investigation of the reaction network of benzofuran hydrodeoxygenation over sulfided and reduced Ni–Mo/Al2O3 catalysts. J. Catal. 2002, 206, 177–187. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, G.; Xie, H.; Jiao, Z.; Zhang, X. Hydrodeoxygenation of methyl laurate over the sulfur-free Ni/γ-Al2O3 catalysts. Appl. Catal. A Gen. 2019, 569, 35–44. [Google Scholar] [CrossRef]
- Ham, H.; Baek, S.W.; Shin, C.H.; Bae, J.W. Roles of Structural Promoters for Direct CO2 Hydrogenation to Dimethyl Ether over Ordered Mesoporous Bifunctional Cu/M–Al2O3 (M = Ga or Zn). ACS Catal. 2018, 9, 679–690. [Google Scholar] [CrossRef]
- Cai, F.; Pan, D.; Ibrahim, J.J.; Zhang, J.; Xiao, G. Hydrogenolysis of glycerol over supported bimetallic Ni/Cu catalysts with and without external hydrogen addition in a fixed-bed flow reactor. Appl. Catal. A Gen. 2018, 564, 172–182. [Google Scholar] [CrossRef]
- Hou, Z.; Yokota, O.; Tanaka, T.; Yashima, T. Characterization of Ca-promoted Ni/α-Al2O3 catalyst for CH4 reforming with CO2. Appl. Catal. A Gen. 2003, 253, 381–387. [Google Scholar] [CrossRef]
- Nie, R.; Yang, H.; Zhang, H.; Yu, X.; Lu, X.; Zhou, D.; Xia, Q. Mild-temperature hydrodeoxygenation of vanillin over porous nitrogen-doped carbon black supported nickel nanoparticles. Green Chem. 2017, 19, 3126–3134. [Google Scholar] [CrossRef]
- Liu, L.; Lou, H.; Chen, M. Selective hydrogenation of furfural to tetrahydrofurfuryl alcohol over Ni/CNTs and bimetallic Cu-Ni/CNTs catalysts. Int. J. Hydrogen Energy 2016, 41, 1721–14731. [Google Scholar] [CrossRef]
- Xin, J.; Cui, H.; Cheng, Z.; Zhou, Z. Bimetallic Ni-Co/SBA-15 catalysts prepared by urea co-precipitation for dry reforming of methane. Appl. Catal. A Gen. 2018, 554, 95–104. [Google Scholar] [CrossRef]
- Ambursa, M.M.; Ali, T.H.; Lee, H.V.; Sudarsanam, P.; Bhargava, S.K.; Hamid, S.B.A. Hydrodeoxygenation of dibenzofuran to bicyclic hydrocarbons using bimetallic Cu–Ni catalysts supported on metal oxides. Fuel 2016, 180, 767–776. [Google Scholar] [CrossRef]
- Yang, F.; Liu, D.; Zhao, Y.; Wang, H.; Han, J.; Ge, Q.; Zhu, X. Size dependence of vapor phase hydrodeoxygenation of m-cresol on Ni/SiO2 catalysts. ACS Catal. 2018, 8, 1672–1682. [Google Scholar] [CrossRef]
- Resasco, D.E. What should we demand from the catalysts responsible for upgrading biomass pyrolysis oil? J. Phys. Chem. Lett. 2011, 2, 2294–2295. [Google Scholar] [CrossRef]
- Song, H.; Gong, J.; Song, H.; Li, F. A novel surface modification approach for synthesizing supported nickel phosphide catalysts with high activity for hydrodeoxygenation of benzofuran. Appl. Catal. A Gen. 2015, 505, 267–275. [Google Scholar] [CrossRef]
- Zhu, T.; Song, H.; Dai, X.; Song, H. Preparation of Ni2P/Al-SBA-15 catalyst and its performance for benzofuran hydrodeoxygenation. Chin. J. Chem. Eng. 2017, 25, 1784–1790. [Google Scholar] [CrossRef]
- Iino, A.; Cho, A.; Takagaki, A.; Kikuchi, R.; Oyama, S.T. Kinetic studies of hydrodeoxygenation of 2-methyltetrahydrofuran on a Ni2P/SiO2 catalyst at medium pressure. J. Catal. 2014, 311, 17–27. [Google Scholar] [CrossRef]
- Jiang, B.; Zhu, T.; Song, H.; Li, F. Hydrodeoxygenation and hydrodesulfurization over Fe promoted Ni2P/SBA-15 catalyst. J. Alloys Compd. 2019, 806, 254–262. [Google Scholar] [CrossRef]










| Samples | Actual Loading a (wt.%) | BET Surface Area (m2·g−1) | Pore Volume (cm3·g−1) | Pore Diameter (nm) | |
|---|---|---|---|---|---|
| Ni | Cu | ||||
| γ-Al2O3 | - | - | 257 | 0.70 | 10.9 |
| Ni/γ-Al2O3 | 8.3 | - | 130 | 0.32 | 8.0 |
| Ni7Cu3/γ-Al2O3 | 5.2 | 2.9 | 136 | 0.33 | 8.5 |
| Ni5Cu5/γ-Al2O3 | 3.3 | 4.8 | 153 | 0.35 | 8.2 |
| Ni3Cu7/γ-Al2O3 | 2.4 | 5.9 | 151 | 0.36 | 8.3 |
| Cu/γ-Al2O3 | - | 8.7 | 139 | 0.35 | 9.0 |
| Samples | Temperature (K) | Pressure (MPa) | Conversion (%) | Ref. no. |
|---|---|---|---|---|
| Sulfided NiMo/Al2O3a | 553 | 2.0 | 74.6 | 43 |
| Sulfided NiMo/Al2O3a | 553 | 5.0 | 82.5 | 43 |
| NiMoP/Al2O3a | 613 | 7.0 | 80.7 | 44 |
| Pt/SiO2-Al2O3 | 553 | 3.0 | 80 | 45 |
| Pd/SiO2-Al2O3 | 553 | 3.0 | 97 | 45 |
| W2C(Ar-2-1023 K-1 h) | 613 | 4.0 | 41 | 46 |
| Ni2P-N/MCM-41 | 493 | 3.0 | 31 | 40 |
| Ni2P-O/MCM-41 | 493 | 3.0 | 57 | 40 |
| Ni2P/Al2O3 | 573 | 3.0 | 78 | 47 |
| Ni2P/TiO2 | 573 | 3.0 | 85 | 47 |
| Ni2P/Al2O3@TiO2 | 573 | 3.0 | 95 | 47 |
| Ni5Cu5/γ-Al2O3 | 573 | 3.0 | 95 | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, T.; Song, H.; Li, F.; Chen, Y. Hydrodeoxygenation of Benzofuran over Bimetallic Ni-Cu/γ-Al2O3 Catalysts. Catalysts 2020, 10, 274. https://doi.org/10.3390/catal10030274
Zhu T, Song H, Li F, Chen Y. Hydrodeoxygenation of Benzofuran over Bimetallic Ni-Cu/γ-Al2O3 Catalysts. Catalysts. 2020; 10(3):274. https://doi.org/10.3390/catal10030274
Chicago/Turabian StyleZhu, Tianhan, Hua Song, Feng Li, and Yanguang Chen. 2020. "Hydrodeoxygenation of Benzofuran over Bimetallic Ni-Cu/γ-Al2O3 Catalysts" Catalysts 10, no. 3: 274. https://doi.org/10.3390/catal10030274
APA StyleZhu, T., Song, H., Li, F., & Chen, Y. (2020). Hydrodeoxygenation of Benzofuran over Bimetallic Ni-Cu/γ-Al2O3 Catalysts. Catalysts, 10(3), 274. https://doi.org/10.3390/catal10030274