Black TiO2 Thin Films Production Using Hollow Cathode Hydrogen Plasma Treatment: Synthesis, Material Characteristics and Photocatalytic Activity
Abstract
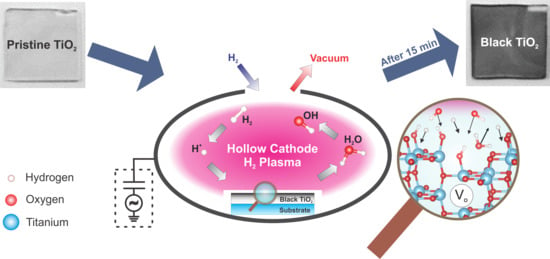
:1. Introduction
2. Results and Discussion
2.1. Morphological Properties
2.2. Structural and Chemical Properties
2.3. Optical and Electrical Properties
2.4. Photocatalytic Activity
3. Materials and Methods
3.1. Substrate Cleaning and Preparation of TiO2 Film
3.2. Preparation of Black TiO2: Hollow Cathode Hydrogen Plasma Process
3.3. Material Characterization
3.4. Photocatalysis Assays
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ikeda, S.; Fujikawa, S.; Harada, T.; Nguyen, T.H.; Nakanishi, S.; Takayama, T.; Iwase, A.; Kudo, A. Photocathode characteristics of a spray-deposited Cu2ZnGeS4 thin film for CO2 reduction in a CO2-saturated aqueous solution. Appl. Energy Mater. 2019, 2, 6911–6918. [Google Scholar] [CrossRef]
- Nogueira, A.E.; Oliveira, J.A.; da Silva, G.T.; Ribeiro, C. Insights into the role of CuO in the CO2 photoreduction process. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, R.; Guzman, M.I. CO2 reduction under periodic illumination of ZnS. J. Phys. Chem. C 2014, 118, 11649–11656. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Sun, C.; Jia, Y.; Yang, X.H.; Yang, H.G.; Yao, X.; Lu, G.Q.; Selloni, A.; Smith, S.C. Hydrogen incorporation and storage in well-defined nanocrystals of anatase titanium dioxide. J. Phys. Chem. C. 2011, 115, 25590–25594. [Google Scholar] [CrossRef]
- Chen, X.; Selloni, A. Introduction: Titanium dioxide (TiO2) nanomaterials. Chem. Rev. 2014, 114, 9281–9282. [Google Scholar] [CrossRef]
- Duarte, D.A.; Massi, M.; Da Silva Sobrinho, A.S. Development of dye-sensitized solar cells with sputtered N-Doped TiO2 thin films: From modeling the growth mechanism of the films to fabrication of the solar cells. Int. J. Photoenergy 2014, 2014, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Duarte, D.A.; Massi, M.; Sagás, J.C.; Da Silva Sobrinho, A.S.; Irala, D.R.; Fontana, L.C. Hysteresis-free deposition of TiOxNy thin films: Effect of the reactive gas mixture and oxidation of the TiN layers on process control. Vacuum 2014, 101, 200–204. [Google Scholar] [CrossRef]
- Pessoa, R.S.; Fraga, M.A.; Santos, L.V.; Massi, M.; Maciel, H.S. Nanostructured thin films based on TiO2 and/or SiC for use in photoelectrochemical cells: A review of the material characteristics, synthesis and recent applications. Mater. Sci. Semicond. Process. 2015, 29, 56–68. [Google Scholar] [CrossRef]
- Zaleska, A. Doped-TiO2: A Review. Recent Patents Eng. 2008, 2, 157–164. [Google Scholar] [CrossRef]
- Li, F.; Dong, B. Construction of novel Z-scheme Cu2O/graphene/α-Fe2O3 nanotube arrays composite for enhanced photocatalytic activity. Ceram. Int. 2017, 43, 16007–16012. [Google Scholar] [CrossRef]
- Aguirre, M.E.; Zhou, R.; Eugene, A.J.; Guzman, M.I.; Grela, M.A. Cu2O/TiO2 heterostructures for CO2 reduction through a direct Z-scheme: Protecting Cu2O from photocorrosion. Appl. Catal. B Environ. 2017, 217, 485–493. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Naldoni, A.; Allieta, M.; Santangelo, S.; Marelli, M.; Fabbri, F.; Cappelli, S.; Bianchi, C.L.; Psaro, R.; Dal Santo, V. Effect of nature and location of defects on bandgap narrowing in black TiO2 nanoparticles. J. Am. Chem. Soc. 2012, 134, 7600–7603. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Wu, R.; Jian, J.; Chen, F.; Sun, Y. Black and yellow anatase titania formed by (H,N)-doping: Strong visible-light absorption and enhanced visible-light photocatalysis. Dalt. Trans. 2015, 44, 1534–1538. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhao, D.; Liu, K.; Wang, C.; Liu, L.; Li, B.; Zhang, Z.; Shen, D. Laser-Modified Black Titanium Oxide Nanospheres and Their Photocatalytic Activities under Visible Light. ACS Appl. Mater. Interfaces 2015, 7, 16070–16077. [Google Scholar] [CrossRef] [PubMed]
- Teng, F.; Li, M.; Gao, C.; Zhang, G.; Zhang, P.; Wang, Y.; Chen, L.; Xie, E. Preparation of black TiO2 by hydrogen plasma assisted chemical vapor deposition and its photocatalytic activity. Appl. Catal. B Environ. 2014, 148, 339–343. [Google Scholar] [CrossRef]
- Zhu, G.; Shan, Y.; Lin, T.; Zhao, W.; Xu, J.; Tian, Z.; Zhang, H.; Zheng, C.; Huang, F. Hydrogenated blue titania with high solar absorption and greatly improved photocatalysis. Nanoscale 2016, 8, 4705–4712. [Google Scholar] [CrossRef]
- Kang, Q.; Cao, J.; Zhang, Y.; Liu, L.; Xu, H.; Ye, J. Reduced TiO2 nanotube arrays for photoelectrochemical water splitting. J. Mater. Chem. A. 2013, 1, 5766–5774. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, C.; Lin, T.; Yin, H.; Chen, P.; Wan, D.; Xu, F.; Huang, F.; Lin, J.; Xie, X.; et al. H-doped black titania with very high solar absorption and excellent photocatalysis enhanced by localized surface plasmon resonance. Adv. Funct. Mater. 2013, 23, 5444–5450. [Google Scholar] [CrossRef]
- Wu, H.; Xu, C.; Xu, J.; Lu, L.; Fan, Z.; Chen, X.; Song, Y.; Li, D. Enhanced supercapacitance in anodic TiO2 nanotube films by hydrogen plasma treatment. Nanotechnology 2013, 24, 455401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, Y.; Hao, B.; Wang, D.; Chen, G.; Markweg, E.; Albrecht, A.; Schaaf, P. Understanding the fast lithium storage performance of hydrogenated TiO2 nanoparticles. J. Mater. Chem. A 2013, 1, 14507–14513. [Google Scholar] [CrossRef]
- Yan, Y.; Han, M.; Kokin, A.; Koppe, T.; Wang, D.; Andreu, T.; Chen, G.; Vetter, U.; Morante, J.R.; Schaaf, P. Slightly hydrogenated TiO2 with enhanced photocatalytic performance. J. Mater. Chem. A 2014, 2, 12708–12716. [Google Scholar] [CrossRef]
- Panomsuwan, G.; Watthanaphanit, A.; Ishizakiae, T.; Saito, N. Water-plasma-assisted synthesis of black titania spheres with efficient visible-light photocatalytic activity. Phys. Chem. Chem. Phys. 2015, 17, 13794–13799. [Google Scholar] [CrossRef]
- Mohammadizadeh, M.R.; Bagheria, M.; Aghabagheria, S.; Abdiba, Y. Photocatalytic activity of TiO2 thin films by hydrogen DC plasma. Appl. Surf. Sci. 2015, 350, 43–49. [Google Scholar] [CrossRef]
- Islam, S.Z.; Reed, A.; Nagpure, S.; Wanninayake, N.; Browning, J.F.; Strzalka, J.; Kim, D.Y.; Rankin, E. Hydrogen incorporation by plasma treatment gives mesoporous black TiO2 thin films with visible photoelectrochemical water oxidation activity. Microporous Mesoporous Mater. 2017, 261, 35–43. [Google Scholar] [CrossRef]
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Ullattil, S.G.; Narendranath, S.B.; Pillai, S.C.; Periyat, P. Black TiO2 Nanomaterials: A Review of Recent Advances. Chem. Eng. J. 2018, 343, 708–736. [Google Scholar] [CrossRef]
- Li, L.; Chen, Y.; Jiao, S.; Fang, Z.; Liu, X.; Xu, Y.; Pang, G.; Feng, S. Synthesis, microstructure, and properties of black anatase and B phase TiO2 nanoparticles. Mater. Des. 2016, 100, 235–240. [Google Scholar] [CrossRef]
- Ge, M.; Cai, J.; Iocozzia, J.; Cao, C.; Huang, J.; Zhang, X.; Shen, J.; Wang, S.; Zhang, S.; Zhang, K.-Q.; et al. A review of TiO2 nanostructured catalysts for sustainable H2 generation. Int. J. Hydrogen Energy 2017, 42, 8418–8449. [Google Scholar] [CrossRef]
- Zhu, G.; Xu, J.; Zhao, W.; Huang, F. Constructing black titania with unique nanocage structure for solar desalination. ACS Appl. Mater. Interfaces 2016, 8, 31716–31721. [Google Scholar] [CrossRef]
- Ullattil, S.G.; Thelappurath, A.V.; Tadka, S.N.; Kavil, J.; Vijayan, B.K.; Periyat, P. A Sol-solvothermal Processed ‘Black TiO2′ as Photoanode Material in Dye Sensitized Solar Cells. Sol. Energy 2017, 155, 490–495. [Google Scholar] [CrossRef]
- Zhi, J.; Yang, C.; Lin, T.; Cui, H.; Wang, Z.; Zhang, H.; Huang, F. Flexible all solid state supercapacitor with high energy density employing black titania nanoparticles as a conductive agent. Nanoscale 2016, 8, 4054–4062. [Google Scholar] [CrossRef] [PubMed]
- Mou, J.; Lin, T.; Huang, F.; Chen, H.; Shi, J. Black titania-based theranostic nanoplatform for single NIR laser induced dual-modal imaging-guided PTT/PDT. Biomaterials 2016, 84, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Muhl, S.; Pérez, A. The use of hollow cathodes in deposition processes: A critical review. Thin Solid Film. 2015, 579, 174–198. [Google Scholar] [CrossRef]
- Pessoa, R.S.; Sagás, J.C.; Rodrigues, B.V.M.; Galvão, N.K.A.M.; Fraga, M.A.; Petraconi, G.; Maciel, H.S. Experimental studies on low-pressure plane-parallel hollow cathode discharges. Braz. J. Phys. 2018, 48, 411–420. [Google Scholar] [CrossRef]
- Pessoa, R.S.; Sismanoglu, B.N.; Amorim, J.; Maciel, H.S.; Petraconi, G. Gas Discharges: Fundamentals and Applications; de Amorim Filho, J., Ed.; Transworld Research Network: Kerala, India, 2017; Volume 1, pp. 175–190. [Google Scholar]
- Wang, F.H.; Chao, J.C.; Liu, H.W.; Liu, F.J. Physical properties of TiO2-doped zinc oxide thin films: Influence of plasma treatment in H2 and/or Ar gas ambient. Vacuum 2017, 140, 155–160. [Google Scholar] [CrossRef]
- Baik, S.J.; Jang, J.H.; Lee, C.H.; Cho, W.Y.; Lim, K.S. Highly textured and conductive undoped ZnO film using hydrogen post-treatment. Appl. Phys. Lett. 1997, 70, 3516–3518. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Xing, Z.; Zhang, Y.; Li, Z.; Wu, X.; Liu, C.; Zhu, Q.; Zhou, W. Ni2+ and Ti3+ co-doped porous black anatase TiO2 with unprecedented-high visible-light-driven photocatalytic degradation performance. RSC Adv. 2015, 5, 107150–107157. [Google Scholar] [CrossRef]
- Tian, F.; Zhang, Y.; Zhang, J.; Pan, C. Raman spectroscopy: A new approach to measure the percentage of anatase TiO2 exposed (001) facets. J. Phys. Chem. C 2012, 116, 7515–7519. [Google Scholar] [CrossRef]
- Testoni, G.E.; Chiappim, W.; Pessoa, R.S.; Fraga, M.A.; Miyakawa, M.; Sakane, K.K.; Galvão, N.K.A.M.; Vieira, L.; Maciel, H.S. Influence of the Al2O3 partial-monolayer number on the crystallization mechanism of TiO2 in ALD TiO2/Al2O3 nanolaminates and its impact on the material properties. J. Phys. D Appl. Phys. 2016, 49, 375301. [Google Scholar] [CrossRef]
- Han, J.X.; Cheng, Y.L.; Tu, W.B.; Zhan, T.Y.; Cheng, Y.L. The black and white coatings on Ti-6Al-4V alloy or pure titanium by plasma electrolytic oxidation in concentrated silicate electrolyte. Appl. Surf. Sci. 2018, 428, 684–697. [Google Scholar] [CrossRef]
- Hannula, M.; Ali-Löytty, H.; Lahtonen, K.; Sarlin, E.; Saari, J.; Valden, M. Improved Stability of Atomic Layer Deposited Amorphous TiO2 Photoelectrode Coatings by Thermally Induced Oxygen Defects. Chem. Mater. 2018, 30, 1199–1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bharti, B.; Kumar, S.; Lee, H.N.; Kumar, R. Formation of oxygen vacancies and Ti3+ state in TiO2 thin film and enhanced optical properties by air plasma treatment. Sci. Rep. 2016, 6, 32355. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, H.; Ling, Y.; Tang, Y.; Yang, X.; Fitzmorris, R.C.; Wang, C.; Zhang, J.Z.; Li, Y. Hydrogen-Treated TiO2 Nanowire Arrays for Photoelectrochemical Water Splitting. Nano Lett. 2011, 11, 3026–3033. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, D.; Meng, M. H2 spillover enhanced hydrogenation capability of TiO2 used for photocatalytic splitting of water: A traditional phenomenon for new applications. Chem. Commun. 2014, 50, 6049–6051. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Yang, Y.; Na, Y.; Fan, R.; Li, L.; Wei, L.; Yang, B.; Cao, W. An insight into the role of oxygen vacancy in hydrogenated TiO2 nanocrystals in the performance of dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2015, 7, 3754–3763. [Google Scholar] [CrossRef]
- Katal, R.; Salehi, M.; Davood, M.H.; Farahani, A.; Masudy-Panah, S.; Ong, S.L.; Hu, J. Preparation of a New Type of Black TiO2 under a Vacuum Atmosphere for Sunlight Photocatalysis. ACS Appl. Mater. Interfaces 2018, 10, 35316–35326. [Google Scholar] [CrossRef]
- Wang, L.; Wu, D.; Guo, Z.; Yan, J.; Hu, Y.; Chang, Z.; Yuan, Q.; Ming, H.; Wang, J. Ultra-thin TiO2 sheets with rich surface disorders for enhanced photocatalytic performance under simulated sunlight. J. Alloys Compd. 2018, 745, 26–32. [Google Scholar] [CrossRef]
- Mattox, D.M. Particle bombardment effects on thin-film deposition: A review. J. Vac. Sci. Technol. A Vac. Surf. Film. 1989, 7, 1105–1114. [Google Scholar] [CrossRef]
- Sanjinés, R.; Tang, H.; Berger, H.; Gozzo, F.; Margaritondo, G.; Lévy, F. Electronic structure of anatase TiO2 oxide. J. Appl. Phys. 1994, 75, 2945–2951. [Google Scholar] [CrossRef]
- Pessoa, R.S.; Tezani, L.L.; Maciel, H.S.; Petraconi, G.; Massi, M. Study of SF6 and SF6/O2 plasmas in a hollow cathode reactive ion etching reactor using Langmuir probe and optical emission spectroscopy techniques. Plasma Sources Sci. Technol. 2010, 19, 025013. [Google Scholar] [CrossRef]
- Fulton, C.C.; Lucovsky, G.; Nemanich, R.J. Electronic states at the interface of Ti–Si oxide on Si(100). J. Vac. Sci. Technol. B Microelectron. Nanom. Struct. 2002, 20, 1726–1732. [Google Scholar] [CrossRef]
- Pereira, A.L.J.; Lisboa Filho, P.N.; Acua, J.; Brandt, I.S.; Pasa, A.A.; Zanatta, A.R.; Vilcarromero, J.; Beltrán, A.; Dias da Silva, J.H. Enhancement of optical absorption by modulation of the oxygen flow of TiO2 films deposited by reactive sputtering. J. Appl. Phys. 2012, 111, 113513. [Google Scholar] [CrossRef] [Green Version]
- Greiner, M.T.; Lu, Z.H. Thin-film metal oxides in organic semiconductor devices: Their electronic structures, work functions and interfaces. NPG Asia Mater. 2013, 5, e55. [Google Scholar] [CrossRef]
- Di Valentin, C.; Pacchioni, G.; Selloni, A. Reduced and n-type doped TiO2: Nature of Ti3+ species. J. Phys. Chem. C 2009, 113, 20543–20552. [Google Scholar] [CrossRef]
- Lepcha, A.; Maccato, C.; Mettenboerger, A.; Andreu, T.; Mayrhofer, L.; Walter, M.; Olthof, S.; Ruoko, T.-P.; Klein, A.; Moseler, M.; et al. Electrospun Black Titania Nanofibres: Influence of Hydrogen Plasma Induced Disorder on the Electronic Structure and Photoelectrochemical Performance. J. Phys. Chem. C 2015, 119, 18835–18842. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, Q.; Liu, L.; Wang, J.; Song, Y. The fabrication of self-floating Ti3+/N co-doped TiO2/diatomite granule catalyst with enhanced photocatalytic performance under visible light irradiation. Appl. Surf. Sci. 2019, 467–468, 514–525. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, K. Fabrication of magnetically recyclable Ce/N co-doped TiO2/NiFe2O4/diatomite ternary hybrid: Improved photocatalytic efficiency under visible light irradiation. J. Alloys Compd. 2017, 697, 161–173. [Google Scholar] [CrossRef]
- Wang, G.; Zheng, J.; Bi, H.; Wang, S.; Wang, J.; Sun, J.; Guo, Y.; Wang, C. Ti3+ self-doping in bulk of rutile TiO2 for enhanced photocatalysis. Scr. Mater. 2019, 162, 28–32. [Google Scholar] [CrossRef]
- Toku, H.; Pessoa, R.S.; Maciel, H.S.; Massi, M.; Mengui, U.A. The effect of oxygen concentration on the low temperature deposition of TiO2 thin films. Surf. Coat. Technol. 2008, 202, 2126–2131. [Google Scholar] [CrossRef]
- Pessoa, R.S.; Maciel, H.S.; Petraconi, G.; Massi, M.; da Silva Sobrinho, A.S. Effect of gas residence time on the morphology of silicon surface etched in SF6 plasmas. Appl. Surf. Sci. 2008, 255, 749–751. [Google Scholar] [CrossRef]
- CasaXPS: Processing Software for XPS, AES. SIMS More. 2019. Version 2.1.0.1. Available online: http://www.casaxps.com/ (accessed on 1 March 2020).
- Chiappim, W.; Testoni, G.E.; Moraes, R.S.; Pessoa, R.S.; Sagás, J.C.; Origo, F.D.; Vieira, L.; Maciel, H.S. Structural, morphological, and optical properties of TiO2 thin films grown by atomic layer deposition on fluorine doped tin oxide conductive glass. Vacuum 2016, 123, 91–102. [Google Scholar] [CrossRef]
- Nogueira, A.C.; Gomes, L.E.; Ferencz, J.A.; Rodrigues, J.E.; Gonçalves, R.V.; Wender, H. Improved Visible Light Photoactivity of CuBi2O4/CuO Heterojunctions for Photodegradation of Methylene Blue and Metronidazole. J. Phys. Chem. C 2019, 123, 25680–25690. [Google Scholar] [CrossRef]
- Gomes, L.E.; da Silva, M.F.; Gonçalves, R.V.; Machado, G.; Alcantara, G.B.; Caires, A.R.; Wender, H. Synthesis and visible-light-driven photocatalytic activity of Ta4+ self-doped gray Ta2O5 nanoparticles. J. Phys. Chem. C 2018, 122, 6014–6025. [Google Scholar] [CrossRef]









| Sample | Thickness (nm) | Surface Area (μm2) | kΩ/sq |
|---|---|---|---|
| Pristine TiO2 | 550 ± 50 | 1.03 | >103 |
| Black TiO2 | 460 ± 50 | 1.28 | 1.93 ± 0.05 |
| Peaks | Pristine TiO2 | Black TiO2 | ||
|---|---|---|---|---|
| BE (eV) | %area | BE (eV) | %area | |
| Ti4+2p3/2 | 459.67 | 100 | 459.01 | 64.44 |
| Ti3+2p3/2 | - | - | 456.98 | 26.89 |
| Ti2+2p3/2 | - | - | 455.39 | 8.67 |
| O-H/TiOx | 532.12 | 47.19 | 531.89 | 54.17 |
| Ti-O | 530.80 | 52.80 | 530.59 | 45.82 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Godoy Junior, A.; Pereira, A.; Gomes, M.; Fraga, M.; Pessoa, R.; Leite, D.; Petraconi, G.; Nogueira, A.; Wender, H.; Miyakawa, W.; et al. Black TiO2 Thin Films Production Using Hollow Cathode Hydrogen Plasma Treatment: Synthesis, Material Characteristics and Photocatalytic Activity. Catalysts 2020, 10, 282. https://doi.org/10.3390/catal10030282
Godoy Junior A, Pereira A, Gomes M, Fraga M, Pessoa R, Leite D, Petraconi G, Nogueira A, Wender H, Miyakawa W, et al. Black TiO2 Thin Films Production Using Hollow Cathode Hydrogen Plasma Treatment: Synthesis, Material Characteristics and Photocatalytic Activity. Catalysts. 2020; 10(3):282. https://doi.org/10.3390/catal10030282
Chicago/Turabian StyleGodoy Junior, Armstrong, André Pereira, Marcilene Gomes, Mariana Fraga, Rodrigo Pessoa, Douglas Leite, Gilberto Petraconi, Adailton Nogueira, Heberton Wender, Walter Miyakawa, and et al. 2020. "Black TiO2 Thin Films Production Using Hollow Cathode Hydrogen Plasma Treatment: Synthesis, Material Characteristics and Photocatalytic Activity" Catalysts 10, no. 3: 282. https://doi.org/10.3390/catal10030282
APA StyleGodoy Junior, A., Pereira, A., Gomes, M., Fraga, M., Pessoa, R., Leite, D., Petraconi, G., Nogueira, A., Wender, H., Miyakawa, W., Massi, M., & da Silva Sobrinho, A. (2020). Black TiO2 Thin Films Production Using Hollow Cathode Hydrogen Plasma Treatment: Synthesis, Material Characteristics and Photocatalytic Activity. Catalysts, 10(3), 282. https://doi.org/10.3390/catal10030282