The Influence of the Gold Particle Size on the Catalytic Oxidation of 5-(Hydroxymethyl)furfural
Abstract
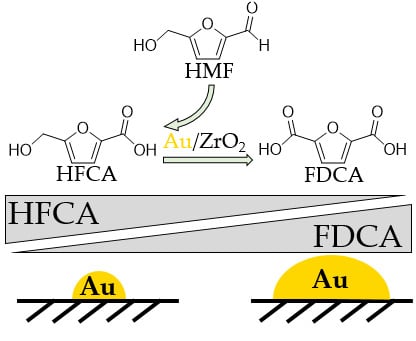
:1. Introduction
2. Results
2.1. Catalyst Characterization
2.2. Catalytic Activity
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Catalyst Preparation
4.3. Catalyst Characterization
4.4. Selective Oxidation of HMF
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nikolau, B.J.; Perera, M.A.D.N.; Brachova, L.; Shanks, B. Platform biochemicals for a biorenewable chemical industry. Plant J. 2008, 54, 536–545. [Google Scholar] [CrossRef]
- Henrich, E.; Dahmen, N.; Dinjus, E.; Sauer, J. The role of biomass in a future world without fossil fuels. Chem. Ing. Tech. 2015, 87, 1667–1685. [Google Scholar] [CrossRef]
- Esposito, D.; Antonietti, M. Redefining biorefinery: The search for unconventional building blocks for materials. Chem. Soc. Rev. 2015, 44, 5821–5835. [Google Scholar] [CrossRef] [Green Version]
- van Putten, R.-J.; van der Waal, J.C.; de Jong, E.; Rasrendra, C.B.; Heeres, H.J.; de Vries, J.G. Hydroxymethylfurfural, A Versatile Platform Chemical Made from Renewable Resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef]
- Rosatella, A.A.; Simeonov, S.P.; Frade, R.F.M.; Afonso, C.A.M. 5-Hydroxymethylfurfural (HMF) as a building block platform: Biological properties, synthesis and synthetic applications. Green Chem. 2011, 13, 754–793. [Google Scholar] [CrossRef]
- Kuster, B.F.M. 5-Hydroxymethylfurfural (HMF). A Review Focussing on its Manufacture. Starch-Stärke 1990, 42, 314–321. [Google Scholar] [CrossRef] [Green Version]
- Steinbach, D.; Kruse, A.; Sauer, J. Pretreatment technologies of lignocellulosic biomass in water in view of furfural and 5-hydroxymethylfurfural production- A review. Biomass Convers. Biorefin. 2017, 7, 247–274. [Google Scholar] [CrossRef]
- Chatterjee, M.; Ishizaka, T.; Kawanami, H. Selective hydrogenation of 5-hydroxymethylfurfural to 2,5-bis-(hydroxymethyl)furan using Pt/MCM-41 in an aqueous medium: A simple approach. Green Chem. 2014, 16, 4734–4739. [Google Scholar] [CrossRef]
- Chatterjee, M.; Ishizaka, T.; Chatterjee, A.; Kawanami, H. Dehydrogenation of 5-hydroxymethylfurfural to diformylfuran in compressed carbon dioxide: An oxidant free approach. Green Chem. 2017, 19, 1315–1326. [Google Scholar] [CrossRef]
- Hengst, K.; Schubert, M.; Kleist, W.; Grunwaldt, J.-D. Hydrodeoxygenation of Lignocellulose-Derived Platform Molecules. In Catalytic Hydrogenation for Biomass Valorization; Rinaldi, R., Ed.; The Royal Society of Chemistry: Cambridge, UK, 2014; pp. 125–150. [Google Scholar]
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass; Natural Renewable Energy Laboratory: Golden, CO, USA, 2004. [Google Scholar]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates-the US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Sousa, A.F.; Vilela, C.; Fonseca, A.C.; Matos, M.; Freire, C.S.R.; Gruter, G.-J.M.; Coelho, J.F.J.; Silvestre, A.J.D. Biobased polyesters and other polymers from 2,5-furandicarboxylic acid: A tribute to furan excellency. Polym. Chem. 2015, 6, 5961–5983. [Google Scholar] [CrossRef]
- Zhang, D.; Dumont, M.-J. Advances in polymer precursors and bio-based polymers synthesized from 5-hydroxymethylfurfural. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 1478–1492. [Google Scholar] [CrossRef] [Green Version]
- Miura, T.; Kakinuma, H.; Kawano, T.; Matsuhisa, H. Method for Producing Furan-2,5-dicarboxylic Acid. U.S. Patent 7,411,078, 12 August 2008. [Google Scholar]
- Krystof, M.; Pérez-Sánchez, M.; Domínguez de María, P. Lipase-Mediated Selective Oxidation of Furfural and 5-Hydroxymethylfurfural. ChemSusChem 2013, 6, 826–830. [Google Scholar] [CrossRef] [PubMed]
- Dijkman, W.P.; Groothuis, D.E.; Fraaije, M.W. Enzyme-Catalyzed Oxidation of 5-Hydroxymethylfurfural to Furan-2,5-dicarboxylic Acid. Angew. Chem. Int. Ed. 2014, 53, 6515–6518. [Google Scholar] [CrossRef] [PubMed]
- Vuyyuru, K.R.; Strasser, P. Oxidation of biomass derived 5-hydroxymethylfurfural using heterogeneous and electrochemical catalysis. Catal. Today 2012, 195, 144–154. [Google Scholar] [CrossRef]
- Barwe, S.; Weidner, J.; Cychy, S.; Morales, D.M.; Dieckhöfer, S.; Hiltrop, D.; Masa, J.; Muhler, M.; Schuhmann, W. Electrocatalytic Oxidation of 5-(Hydroxymethyl)furfural Using High-Surface-Area Nickel Boride. Angew. Chem. Int. Ed. 2018, 57, 11460–11464. [Google Scholar] [CrossRef]
- Taitt, B.J.; Nam, D.-H.; Choi, K.-S. A Comparative Study of Nickel, Cobalt, and Iron Oxyhydroxide Anodes for the Electrochemical Oxidation of 5-Hydroxymethylfurfural to 2,5-Furandicarboxylic Acid. ACS Catal. 2018, 9, 660–670. [Google Scholar] [CrossRef]
- Partenheimer, W.; Grushin, V.V. Synthesis of 2,5-Diformylfuran and Furan-2,5-Dicarboxylic Acid by Catalytic Air-Oxidation of 5-Hydroxymethylfurfural. Unexpectedly Selective Aerobic Oxidation of Benzyl Alcohol to Benzaldehyde with Metal=Bromide Catalysts. Adv. Synth. Catal. 2001, 343, 102–111. [Google Scholar] [CrossRef]
- Ait Rass, H.; Essayem, N.; Besson, M. Selective aqueous phase oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid over Pt/C catalysts: Influence of the base and effect of bismuth promotion. Green Chem. 2013, 15, 2240–2251. [Google Scholar] [CrossRef]
- Davis, S.E.; Houk, L.R.; Tamargo, E.C.; Datye, A.K.; Davis, R.J. Oxidation of 5-hydroxymethylfurfural over supported Pt, Pd and Au catalysts. Catal. Today 2011, 160, 55–60. [Google Scholar] [CrossRef]
- Yu, H.; Kim, K.-A.; Kang, M.J.; Hwang, S.Y.; Cha, H.G. Carbon Support with Tunable Porosity Prepared by Carbonizing Chitosan for Catalytic Oxidation of 5-Hydroxylmethylfurfural. ACS Sus. Chem. Eng. 2018, 7, 3742–3748. [Google Scholar] [CrossRef]
- Siyo, B.; Schneider, M.; Radnik, J.; Pohl, M.-M.; Langer, P.; Steinfeldt, N. Influence of support on the aerobic oxidation of HMF into FDCA over preformed Pd nanoparticle based materials. Appl. Catal. A 2014, 478, 107–116. [Google Scholar] [CrossRef]
- Chen, C.; Li, X.; Wang, L.; Liang, T.; Wang, L.; Zhang, Y.; Zhang, J. Highly Porous Nitrogen- and Phosphorus-Codoped Graphene: An Outstanding Support for Pd Catalysts to Oxidize 5-Hydroxymethylfurfural into 2,5-Furandicarboxylic Acid. ACS Sus. Chem. Eng. 2017, 5, 1300–11306. [Google Scholar] [CrossRef]
- Lei, D.; Yu, K.; Li, M.-R.; Wang, Y.; Wang, Q.; Liu, T.; Liu, P.; Lou, L.-L.; Wang, G.; Liu, S. Facet Effect of Single-Crystalline Pd Nanocrystals for Aerobic Oxidation of 5-Hydroxymethyl-2-furfural. ACS Catal. 2017, 7, 421–432. [Google Scholar] [CrossRef]
- Casanova, O.; Iborra, S.; Corma, A. Biomass into Chemicals: Aerobic Oxidation of 5-Hydroxymethyl-2-furfural into 2,5-Furandicarboxylic Acid with Gold Nanoparticle Catalysts. ChemSusChem 2009, 2, 1138–1144. [Google Scholar] [CrossRef] [PubMed]
- Gorbanev, Y.Y.; Klitgaard, S.K.; Woodley, J.M.; Christensen, C.H.; Riisager, A. Gold-Catalyzed Aerobic Oxidation of 5-Hydroxymethylfurfural in Water at Ambient Temperature. ChemSusChem 2009, 2, 672–675. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Ma, H.; Zhang, J.; Song, Q.; Du, Z.; Huang, Y.; Xu, J. Gold Nanoclusters Confined in a Supercage of Y Zeolite for Aerobic Oxidation of HMF under Mild Conditions. Chem. Eur. J. 2013, 19, 14215–14223. [Google Scholar] [CrossRef]
- Miao, Z.; Zhang, Y.; Pan, X.; Wu, T.; Zhang, B.; Li, J.; Yi, T.; Zhang, Z.; Yang, X. Superior catalytic performance of Ce1-xBixO2-δ solid solution and Au/Ce1-xBixO2-δ for 5-hydroxymethylfurfural conversion in alkaline aqueous solution. Catal. Sci. Technol. 2015, 5, 1314–1322. [Google Scholar] [CrossRef]
- Gupta, N.K.; Nishimura, S.; Takagaki, A.; Ebitani, K. Hydrotalcite-supported gold-nanoparticle-catalyzed highly efficient base-free aqueous oxidation of 5-hydroxymethylfurfural into 2,5-furandicarboxylic acid under atmospheric oxygen pressure. Green Chem. 2011, 13, 824–827. [Google Scholar] [CrossRef]
- Gao, T.; Gao, T.; Fang, W.; Cao, Q. Base-free aerobic oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid in water by hydrotalcite-activated carbon composite supported gold catalyst. Mol. Catal. 2017, 439, 171–179. [Google Scholar] [CrossRef]
- Masoud, N.; Donoeva, B.; de Jongh, P.E. Stability of gold nanocatalysts supported on mesoporous silica for the oxidation of 5-hydroxymethyl furfural to furan-2,5-dicarboxylic acid. Appl. Catal. A 2018, 561, 150–157. [Google Scholar] [CrossRef]
- Schade, O.R.; Dannecker, P.-K.; Kalz, K.F.; Steinbach, D.; Meier, M.A.; Grunwaldt, J.-D. Direct Catalytic Route to Biomass-Derived 2,5-Furandicarboxylic Acid and Its Use as Monomer in a Multicomponent Polymerization. ACS Omega 2019, 4, 16972–16979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haruta, M.; Kobayashi, T.; Sano, H.; Yamada, N. Novel Gold Catalysts for the Oxidation of Carbon Monoxide at a Temperature far Below 0 °C. Chem. Lett. 1987, 16, 405–408. [Google Scholar] [CrossRef]
- Hashmi, A.S.K.; Hutchings, G.J. Gold Catalysis. Angew. Chem. Int. Ed. 2006, 45, 7896–7936. [Google Scholar] [CrossRef]
- Grunwaldt, J.-D.; Kiener, C.; Wögerbauer, C.; Baiker, A. Preparation of Supported Gold Catalysts for Low-Temperature CO Oxidation via “Size-Controlled” Gold Colloids. J. Catal. 1999, 181, 223–232. [Google Scholar] [CrossRef]
- Haruta, M.; Uphade, B.; Tsubota, S.; Miyamoto, A. Selective oxidation of propylene over gold deposited on titanium-based oxides. Res. Chem. Intermed. 1998, 24, 329–336. [Google Scholar] [CrossRef]
- Haruta, M. When gold is not noble: Catalysis by nanoparticles. Chem. Rec. 2003, 3, 75–87. [Google Scholar] [CrossRef]
- Herzing, A.A.; Kiely, C.J.; Carley, A.F.; Landon, P.; Hutchings, G.J. Identification of active gold nanoclusters on iron oxide supports for CO oxidation. Science 2008, 321, 1331–1335. [Google Scholar] [CrossRef]
- Bogdanchikova, N.; Pestryakov, A.; Farias, M.; Diaz, J.A.; Avalos, M.; Navarrete, J. Formation of TEM-and XRD-undetectable gold clusters accompanying big gold particles on TiO2–SiO2 supports. Solid State Sci. 2008, 10, 908–914. [Google Scholar] [CrossRef]
- Haider, P.; Kimmerle, B.; Krumeich, F.; Kleist, W.; Grunwaldt, J.-D.; Baiker, A. Gold-catalyzed aerobic oxidation of benzyl alcohol: Effect of gold particle size on activity and selectivity in different solvents. Catal. Lett. 2008, 125, 169–176. [Google Scholar] [CrossRef] [Green Version]
- Adnan, R.H.; Andersson, G.G.; Polson, M.I.; Metha, G.F.; Golovko, V.B. Factors influencing the catalytic oxidation of benzyl alcohol using supported phosphine-capped gold nanoparticles. Catal. Sci. Technol. 2015, 5, 1323–1333. [Google Scholar] [CrossRef] [Green Version]
- Tsunoyama, H.; Ichikuni, N.; Tsukuda, T. Microfluidic synthesis and catalytic application of PVP-stabilized, ∼ 1 nm gold clusters. Langmuir 2008, 24, 11327–11330. [Google Scholar] [CrossRef] [PubMed]
- Tofighi, G.; Lichtenberg, H.; Pesek, J.; Sheppard, T.L.; Wang, W.; Schöttner, L.; Rinke, G.; Dittmeyer, R.; Grunwaldt, J.-D. Continuous microfluidic synthesis of colloidal ultrasmall gold nanoparticles: In Situ study of the early reaction stages and application for catalysis. React. Chem. Eng. 2017, 2, 876–884. [Google Scholar] [CrossRef]
- Sasirekha, N.; Sangeetha, P.; Chen, Y.-W. Bimetallic Au–Ag/CeO2 catalysts for preferential oxidation of CO in hydrogen-rich stream: Effect of calcination temperature. J. Phys. Chem. C 2014, 118, 15226–15233. [Google Scholar] [CrossRef]
- Li, Q.; Wang, H.; Tian, Z.; Weng, Y.; Wang, C.; Ma, J.; Zhu, C.; Li, W.; Liu, Q.; Ma, L. Selective oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid over Au/CeO2 catalysts: The morphology effect of CeO2. Catal. Sci. Technol. 2019, 9, 1570–1580. [Google Scholar] [CrossRef]
- Albonetti, S.; Lolli, A.; Morandi, V.; Migliori, A.; Lucarelli, C.; Cavani, F. Conversion of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid over Au-based catalysts: Optimization of active phase and metal–support interaction. Appl. Catal. B 2015, 163, 520–530. [Google Scholar] [CrossRef]
- Schade, O.R.; Kalz, K.F.; Neukum, D.; Kleist, W.; Grunwaldt, J.-D. Supported gold- and silver-based catalysts for the selective aerobic oxidation of 5-(hydroxymethyl)furfural to 2,5-furandicarboxylic acid and 5-hydroxymethyl-2-furancarboxylic acid. Green Chem. 2018, 20, 3530–3541. [Google Scholar] [CrossRef] [Green Version]
- Davis, S.E.; Zope, B.N.; Davis, R.J. On the mechanism of selective oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid over supported Pt and Au catalysts. Green Chem. 2012, 14, 143–147. [Google Scholar] [CrossRef]
- Megías-Sayago, C.; Lolli, A.; Bonincontro, D.; Penkova, A.; Albonetti, S.; Cavani, F.; Odriozola, J.A.; Ivanova, S. Effect of gold particles size over Au/C catalyst selectivity in HMF oxidation reaction. ChemCatChem 2020, 12, 1177–1183. [Google Scholar] [CrossRef]
- Ishida, T.; Kinoshita, N.; Okatsu, H.; Akita, T.; Takei, T.; Haruta, M. Influence of the support and the size of gold clusters on catalytic activity for glucose oxidation. Angew. Chem. Int. Ed. 2008, 47, 9265–9268. [Google Scholar] [CrossRef]
- Sahu, R.; Dhepe, P.L. Synthesis of 2,5-furandicarboxylic acid by the aerobic oxidation of 5-hydroxymethyl furfural over supported metal catalysts. React. Kinet. Mech. Catal. 2014, 112, 173–187. [Google Scholar] [CrossRef]
- Megías-Sayago, C.; Chakarova, K.; Penkova, A.; Lolli, A.; Ivanova, S.; Albonetti, S.; Cavani, F.; Odriozola, J.A. Understanding the role of the acid sites in HMF oxidation to FDCA reaction over gold catalysts: Surface investigation on CexZr1-xO2 compounds. ACS Catal. 2018, 8, 11154–11164. [Google Scholar] [CrossRef]
- Porta, F.; Prati, L.; Rossi, M.; Coluccia, S.; Martra, G. Metal sols as a useful tool for heterogeneous gold catalyst preparation: Reinvestigation of a liquid phase oxidation. Catal. Today 2000, 61, 165–172. [Google Scholar] [CrossRef]
- Sun, K.-Q.; Luo, S.-W.; Xu, N.; Xu, B.-Q. Gold nano-size effect in Au/SiO2 for selective ethanol oxidation in aqueous solution. Catal. Lett. 2008, 124, 238–242. [Google Scholar] [CrossRef]
- Guan, Y.; Hensen, E.J. Ethanol dehydrogenation by gold catalysts: The effect of the gold particle size and the presence of oxygen. Appl. Catal. A 2009, 361, 49–56. [Google Scholar] [CrossRef]
- Zope, B.N.; Hibbitts, D.D.; Neurock, M.; Davis, R.J. Reactivity of the Gold/Water Interface during Selective Oxidation Catalysis. Science 2010, 330, 74–78. [Google Scholar] [CrossRef] [Green Version]
- Mondelli, C.; Ferri, D.; Grunwaldt, J.-D.; Krumeich, F.; Mangold, S.; Psaro, R.; Baiker, A. Combined liquid-phase ATR-IR and XAS study of the Bi-promotion in the aerobic oxidation of benzyl alcohol over Pd/Al2O3. J. Catal. 2007, 252, 77–87. [Google Scholar] [CrossRef]
- Kartusch, C.; van Bokhoven, J.A. Hydrogenation over gold catalysts: The interaction of gold with hydrogen. Gold Bull. 2009, 42, 343–348. [Google Scholar] [CrossRef] [Green Version]
- Bergeret, G.; Gallezot, P. Particle size and dispersion measurements. In Handbook of Heterogeneous Catalysis; Ertl, G., Knözinger, H., Schüth, F., Weitkamp, J., Eds.; Wiley-VCH: Weinheim, Germany, 2008; pp. 738–765. [Google Scholar]






| Entry | Support/Catalyst | Au Loading/wt.% | Specific Surface Area/m2 g−1 |
|---|---|---|---|
| 1 | ZrO2 | - | 99 |
| 2 | AuZ2.1 | 1.4 | 97 |
| 3 | AuZ2.4 | 1.8 | 98 |
| 4 | AuZ2.7 | 1.8 | 100 |
| 5 | AuZ2.9 | 1.8 | 102 |
| Entry | Catalyst | C/at% | O/at% | P/at% | Au/at% | Zr/at% | Cl/at% |
|---|---|---|---|---|---|---|---|
| 1 | AuZ2.1 | 49.4 | 33.1 | 1.4 | 1.0 | 6.9 | 8.2 |
| 2 | AuZ2.4 | 42.9 | 38.9 | 0.9 | 1.0 | 8.4 | 7.9 |
| 3 | AuZ2.7 | 43.0 | 37.6 | 1.8 | 1.6 | 7.4 | 8.6 |
| 4 | AuZ2.9 | 35.5 | 38.3 | 1.6 | 0.4 | 12.0 | 11.0 |
| Entry | Catalyst | HMF Conversion/% | Yield/% | C-Balance/% | Productivity a/molFDCA h−1 molAu−1 | |
|---|---|---|---|---|---|---|
| HFCA | FDCA | |||||
| 1 [50] | Au/ZrO2 | 100 | 0 | 75 | 75 | 19 (72) |
| 2 | AuZ2.1 | 100 | 9 | 16 | 25 | 5 (10) |
| 3 | AuZ2.4 | 100 | 0 | 35 | 35 | 8 (18) |
| 4 | AuZ2.7 | 100 | 0 | 43 | 43 | 10 (25) |
| 5 | AuZ2.9 | 100 | 1 | 30 | 31 | 7 (18) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schade, O.; Dolcet, P.; Nefedov, A.; Huang, X.; Saraçi, E.; Wöll, C.; Grunwaldt, J.-D. The Influence of the Gold Particle Size on the Catalytic Oxidation of 5-(Hydroxymethyl)furfural. Catalysts 2020, 10, 342. https://doi.org/10.3390/catal10030342
Schade O, Dolcet P, Nefedov A, Huang X, Saraçi E, Wöll C, Grunwaldt J-D. The Influence of the Gold Particle Size on the Catalytic Oxidation of 5-(Hydroxymethyl)furfural. Catalysts. 2020; 10(3):342. https://doi.org/10.3390/catal10030342
Chicago/Turabian StyleSchade, Oliver, Paolo Dolcet, Alexei Nefedov, Xiaohui Huang, Erisa Saraçi, Christof Wöll, and Jan-Dierk Grunwaldt. 2020. "The Influence of the Gold Particle Size on the Catalytic Oxidation of 5-(Hydroxymethyl)furfural" Catalysts 10, no. 3: 342. https://doi.org/10.3390/catal10030342
APA StyleSchade, O., Dolcet, P., Nefedov, A., Huang, X., Saraçi, E., Wöll, C., & Grunwaldt, J. -D. (2020). The Influence of the Gold Particle Size on the Catalytic Oxidation of 5-(Hydroxymethyl)furfural. Catalysts, 10(3), 342. https://doi.org/10.3390/catal10030342