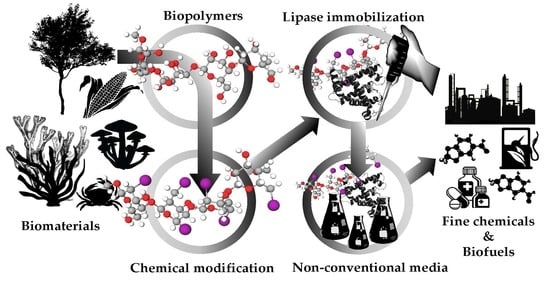
Recent Trends in Biomaterials for Immobilization of Lipases for Application in Non-Conventional Media
Abstract
:1. Introduction
2. Lipases: General Features and Classical Strategy of Immobilization
3. Agarose
4. Chitosan
- (i)
- (ii)
- different agents containing reactive groups can be used to control solubility, anionic properties, and hydrophobicity of chitosan. One example is glutaraldehyde; the bonds formed between the polymer and this agent render the chitosan-matrix quite hydrophobic [62], which may be advantageous for lipase immobilization;
- (iii)
- immobilization may be affected by reactive groups in the chemical agent used to activate the chitosan, such as glutaraldehyde or ethylenediamine, and also by amino, hydroxyl and acetyl amine groups present in chitosan itself. Charged functional groups in the support may cause non-specific binding of the enzyme to the support, which can lead to loss of enzyme activity. In some cases, it may be necessary to block the remaining reactive groups in the support;
- (iv)
- similarly, reactive groups present in chitosan may react with substrates or products of the reaction, depending on the reaction conditions used. For example, the amino groups may react with aldehyde and ketone groups, forming Schiff bases that affect the solubility of the polymer

5. Cellulose
6. Starch
7. Alginate
8. Other Biomaterials
9. Pros and Cons of Using Biopolymers to Immobilize Lipases
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Basso, A.; Serban, S. Industrial applications of immobilized enzymes—A review. Mol. Catal. 2019, 479, 110–607. [Google Scholar] [CrossRef]
- Miranda, A.S.; Miranda, L.S.M.; de Souza, R.O.M.A. Lipases: Valuable catalysts for dynamic kinetic resolutions. Biotechnol. Adv. 2015, 33, 372–393. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, M.; Gupta, M.N. Lipase promiscuity and its biochemical applications. Process. Biochem. 2012, 47, 555–569. [Google Scholar] [CrossRef]
- Zhao, X.; Qi, F.; Yuan, C.; Du, W.; Liu, D. Lipase-catalyzed process for biodiesel production: Enzyme immobilization, process simulation and optimization. Renew. Sustain. Energy Rev. 2015, 44, 182–197. [Google Scholar] [CrossRef]
- Jaeger, K.-E.; Ransac, S.; Dijkstra, B.W.; Colson, C.; van Heuvel, M.; Misset, O. Bacterial lipases. FEMS Microbiol. Rev. 1994, 15, 29–63. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.M.; Alnoch, R.C.; Souza, E.M.; Mitchell, D.A.; Krieger, N. Metagenomics: Is it a powerful tool to obtain lipases for application in biocatalysis? Biochim. Biophys. Acta Proteins Proteom. 2020, 1868, 140320. [Google Scholar] [CrossRef]
- Alnoch, R.C.; Martini, V.P.; Glogauer, A.; Costa, A.C.D.S.; Piovan, L.; Muller-Santos, M.; De Souza, E.M.; Pedrosa, F.D.O.; Mitchell, D.A.; Krieger, N. Immobilization and characterization of a new regioselective and enantioselective lipase obtained from a metagenomic library. PLoS ONE 2015, 10, 1–18. [Google Scholar] [CrossRef]
- Berini, F.; Casciello, C.; Marcone, G.L.; Marinelli, F. Metagenomics: Novel enzymes from non-culturable microbes. FEMS Microbiol. Lett. 2017, 364, 1–19. [Google Scholar] [CrossRef]
- Chow, J.; Kovacic, F.; Dall Antonia, Y.; Krauss, U.; Fersini, F.; Schmeisser, C.; Lauinger, B.; Bongen, P.; Pietruszka, J.; Schmidt, M.; et al. The metagenome-derived enzymes LipS and LipT increase the diversity of known lipases. PLoS ONE 2012, 7, e47665. [Google Scholar] [CrossRef] [Green Version]
- Ferrer, M.; Bargiela, R.; Martínez-Martínez, M.; Mir, J.; Koch, R.; Golyshina, O.V.; Golyshin, P.N. Biodiversity for biocatalysis: A review of the α/β-hydrolase fold superfamily of esterases-lipases discovered in metagenomes. Biocatal. Biotransform. 2016, 2422, 1–15. [Google Scholar] [CrossRef]
- Glogauer, A.; Martini, V.P.; Faoro, H.; Couto, G.H.; Müller-Santos, M.; Monteiro, R.A.; Mitchell, D.A.; de Souza, E.M.; Pedrosa, F.O.; Krieger, N. Identification and characterization of a new true lipase isolated through metagenomic approach. Microb. Cell Fact. 2011, 10, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernal, C.; Rodríguez, K.; Martínez, R. Integrating enzyme immobilization and protein engineering: An alternative path for the development of novel and improved industrial biocatalysts. Biotechnol. Adv. 2018, 36, 1470–1480. [Google Scholar] [CrossRef]
- Gotor-Fernández, V.; Brieva, R.; Gotor, V. Lipases: Useful biocatalysts for the preparation of pharmaceuticals. J. Mol. Catal. B Enzym. 2006, 40, 111–120. [Google Scholar] [CrossRef]
- Verma, M.L.; Puri, M.; Barrow, C.J. Recent trends in nanomaterials immobilised enzymes for biofuel production. Crit. Rev. Biotechnol. 2016, 36, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Ji, P. Enzymes immobilized on carbon nanotubes. Biotechnol. Adv. 2011, 29, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Huang, X.; Chen, P.; Xu, Z. Polymer materials for enzyme immobilization and their application in bioreactors. BMB Rep. 2011, 44, 87–95. [Google Scholar] [CrossRef]
- Zdarta, J.; Meyer, A.S.; Jesionowski, T.; Pinelo, M. A general overview of support materials for enzyme immobilization: Characteristics, properties, practical utility. Catalysts 2018, 8, 92. [Google Scholar] [CrossRef] [Green Version]
- Arpigny, J.L.; Jaeger, K.E. Bacterial lipolytic enzymes: Classification and properties. Biochem. J. 1999, 343, 177. [Google Scholar] [CrossRef] [PubMed]
- Ali, Y.B.; Verger, R.; Abousalham, A. Lipases or esterases: Does it really matter? Toward a new bio-physico-chemical classification. Lipases Phospholipases 2012, 861, 31–51. [Google Scholar]
- Bracco, P.; van Midden, N.; Arango, E.; Torrelo, G.; Ferrario, V.; Gardossi, L.; Hanefeld, U. Bacillus subtilis lipase A—Lipase or esterase? Catalysts 2020, 10, 308. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Lafuente, R.; Armisen, P.; Sabuquillo, P.; Fernández-Lorente, G.; Guisán, J.M. Immobilization of lipases by selective adsorption on hydrophobic supports. Chem. Phys. Lipids 1998, 93, 185–197. [Google Scholar] [CrossRef]
- Palomo, J.M.; Muoz, G.; Fernández-Lorente, G.; Mateo, C.; Fernández-Lafuente, R.; Guisán, J.M. Interfacial adsorption of lipases on very hydrophobic support (octadecyl-Sepabeads): Immobilization, hyperactivation and stabilization of the open form of lipases. J. Mol. Catal. B Enzym. 2002, 19, 279–286. [Google Scholar] [CrossRef]
- Jaeger, K.E.; Dijkstra, B.W.; Reetz, M.T. Bacterial biocatalysts: Molecular biology, three-dimensional structures, and biotechnological applications of lipases. Annu. Rev. Microbiol. 1999, 53, 315–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verger, R. “Interfacial activation” of lipases: Facts and artifacts. Trends Biotechnol. 1997, 15, 32–38. [Google Scholar] [CrossRef]
- Khan, F.I.; Lan, D.; Durrani, R.; Huan, W.; Zhao, Z.; Wang, Y. The lid domain in lipases: Structural and functional determinant of enzymatic properties. Front. Bioeng. Biotechnol. 2017, 5, 16. [Google Scholar] [CrossRef] [Green Version]
- Mateo, C.; Bolivar, J.M.; Godoy, C.A.; Rocha-Martin, J.; Pessela, B.C.; Curiel, J.A.; Munoz, R.; Guisán, J.M.; Fernández-Lorente, G. Improvement of enzyme properties with a two-step immobilizaton process on novel heterofunctional supports. Biomacromolecules 2010, 11, 3112–3117. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, R.C.; Virgen-ortíz, J.J.; José, C.S.; Berenguer-murcia, Á.; Alcantara, A.R.; Barbosa, O.; Ortiz, C.; Fernández-Lafuente, R. Immobilization of lipases on hydrophobic supports: Immobilization mechanism, advantages, problems, and solutions. Biotechnol. Adv. 2019, 37, 746–770. [Google Scholar] [CrossRef] [Green Version]
- Sheldon, R.A. Enzyme Immobilization: The quest for optimum performance. Adv. Synth. Catal. 2007, 349, 1289–1307. [Google Scholar] [CrossRef]
- Xu, M.; Wang, S.; Li, L.; Gao, J.; Zhang, Y. Combined cross-linked enzyme aggregates as biocatalysts. Catalysts 2018, 8, 460. [Google Scholar] [CrossRef] [Green Version]
- Mateo, C.; Palomo, J.M.; Fernández-Lorente, G.; Guisán, J.M.; Fernández-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzym. Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Cabrera, Z.; Palomo, J.M. Enantioselective desymmetrization of prochiral diesters catalyzed by immobilized Rhizopus oryzae lipase. Tetrahedron Asymmetry 2011, 22, 2080–2084. [Google Scholar] [CrossRef]
- Callaghan, C.; Redmond, M.; Alnoch, R.C.; Mateo, C.; Filice, M.; Palomo, J.M. Biocatalytic process optimization for the production of high-added-value 6-O-hydroxy and 3-O-hydroxy glycosyl building blocks. ChemCatChem 2017, 9, 2536–2543. [Google Scholar] [CrossRef]
- Filice, M.; Guisán, J.M.; Terreni, M.; Palomo, J.M. Regioselective monodeprotection of peracetylated carbohydrates. Nat. Protoc. 2012, 7, 1783–1796. [Google Scholar] [CrossRef] [PubMed]
- Palomo, J.M.; Peñas, M.M.; Fernández-Lorente, G.; Mateo, C.; Pisabarro, A.G.; Fernández-Lafuente, R.; Ramírez, L.; Guisán, J.M. Solid-phase handling of hydrophobins: Immobilized hydrophobins as a new tool to study lipases. Biomacromolecules 2003, 4, 204–210. [Google Scholar] [CrossRef]
- Wilson, L.; Palomo, J.M.; Fernández-Lorente, G.; Illanes, A.; Guisán, J.M.; Fernández-Lafuente, R. Effect of lipase-lipase interactions in the activity, stability and specificity of a lipase from Alcaligenes sp. Enzym. Microb. Technol. 2006, 39, 259–264. [Google Scholar] [CrossRef]
- Virgen-Ortíz, J.J.; Tacias-Pascacio, V.G.; Hirata, D.B.; Torrestiana-Sanchez, B.; Rosales-Quintero, A.; Fernández-Lafuente, R. Relevance of substrates and products on the desorption of lipases physically adsorbed on hydrophobic supports. Enzym. Microb. Technol. 2017, 96, 30–35. [Google Scholar] [CrossRef]
- Ortiz, C.; Ferreira, M.L.; Barbosa, O.; Dos Santos, J.C.S.; Rodrigues, R.C.; Berenguer-Murcia, Á.; Briand, L.E.; Fernández-Lafuente, R. Novozym 435: The “perfect” lipase immobilized biocatalyst? Catal. Sci. Technol. 2019, 9, 2380–2420. [Google Scholar] [CrossRef] [Green Version]
- Zucca, P.; Fernández-Lafuente, R.; Sanjust, E. Agarose and its derivatives as supports for enzyme immobilization. Molecules 2016, 21, 1577. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; Fuentes, M.; Betancor, L.; Grazu, V.; Lopez-Gallego, F.; Pessela, B.C.C.; Hidalgo, A.; Fernández-Lorente, G.; Fernández-Lafuente, R.; et al. Glyoxyl agarose: A fully inert and hydrophilic support for immobilization and high stabilization of proteins. Enzym. Microb. Technol. 2006, 39, 274–280. [Google Scholar] [CrossRef]
- Rueda, N.; dos Santos, J.C.S.; Ortiz, C.; Torres, R.; Barbosa, O.; Rodrigues, R.C.; Berenguer-Murcia, Á.; Fernández-Lafuente, R. Chemical modification in the design of immobilized enzyme biocatalysts: Drawbacks and opportunities. Chem. Rec. 2016, 16, 1436–1455. [Google Scholar] [CrossRef]
- Betancor, L.; López-Gallego, F.; Hidalgo, A.; Alonso-Morales, N.; Dellamora-Ortiz, G.; Mateo, C.; Fernández-Lafuente, R.; Guisán, J.M. Different mechanisms of protein immobilization on glutaraldehyde activated supports: Effect of support activation and immobilization conditions. Enzym. Microb. Technol. 2006, 39, 877–882. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Berenguer-Murcia, Á.; Fernández-Lafuente, R. Coupling chemical modification and immobilization to improve the catalytic performance of enzymes. Adv. Synth. Catal. 2011, 353, 2216–2238. [Google Scholar] [CrossRef]
- Rueda, N.; dos Santos, J.C.S.; Torres, R.; Ortiz, C.; Barbosa, O.; Fernández-Lafuente, R. Improved performance of lipases immobilized on heterofunctional octyl-glyoxyl agarose beads. RSC Adv. 2015, 5, 11212–11222. [Google Scholar] [CrossRef] [Green Version]
- Alnoch, R.C.; de Melo, R.R.; Palomo, J.M.; de Souza, E.M.; Krieger, N.; Mateo, C. New tailor-made alkyl-aldehyde bifunctional supports for lipase immobilization. Catalysts 2016, 6, 191. [Google Scholar] [CrossRef] [Green Version]
- Hirata, D.B.; Albuquerque, T.L.; Rueda, N.; Virgen-ortíz, J.J.; Tacias-pascacio, V.G.; Fernández-Lafuente, R. Evaluation of different immobilized lipases in transesterification reactions using tributyrin: Advantages of the heterofunctional octyl agarose beads. J. Mol. Catal. B Enzym. 2016, 133, 117–123. [Google Scholar] [CrossRef]
- Fernández-Lopez, L.; Rueda, N.; Bartolome-Cabrero, R.; Rodriguez, M.D.; Albuquerque, T.L.; Dos Santos, J.C.S.; Barbosa, O.; Fernández-Lafuente, R. Improved immobilization and stabilization of lipase from Rhizomucor miehei on octyl-glyoxyl agarose beads by using CaCl2. Process Biochem. 2016, 51, 48–52. [Google Scholar] [CrossRef]
- Suescun, A.; Rueda, N.; Dos Santos, J.C.S.; Castillo, J.J.; Ortiz, C.; Torres, R.; Barbosa, O.; Fernández-Lafuente, R. Immobilization of lipases on glyoxyl-octyl supports: Improved stability and reactivation strategies. Process Biochem. 2015, 50, 1211–1217. [Google Scholar] [CrossRef]
- Rueda, N.; Dos Santos, C.S.; Rodriguez, M.D.; Albuquerque, T.L.; Barbosa, O.; Torres, R.; Ortiz, C.; Fernández-Lafuente, R. Reversible immobilization of lipases on octyl-glutamic agarose beads: A mixed adsorption that reinforces enzyme immobilization. J. Mol. Catal. B Enzym. 2016, 128, 10–18. [Google Scholar] [CrossRef]
- Arana-Peña, S.; Mendez-Sanchez, C.; Rios, N.S.; Ortiz, C.; Gonçalves, L.R.B.; Fernández-Lafuente, R. New applications of glyoxyl-octyl agarose in lipases co-immobilization: Strategies to reuse the most stable lipase. Int. J. Biol. Macromol. 2019, 131, 989–997. [Google Scholar] [CrossRef]
- Rios, N.S.; Arana-Peña, S.; Mendez-Sanchez, C.; Ortiz, C.; Gonçalves, L.R.B.; Fernández-Lafuente, R. Reuse of lipase from Pseudomonas fluorescens via its step-by-step coimmobilization on glyoxyl-octyl agarose beads with least stable lipases. Catalysts 2019, 9, 487. [Google Scholar] [CrossRef] [Green Version]
- Rivero, C.; Palomo, J. Covalent immobilization of Candida rugosa lipase at alkaline pH and their application in the regioselective deprotection of per-O-acetylated thymidine. Catalysts 2016, 6, 115. [Google Scholar] [CrossRef] [Green Version]
- Rueda, N.; Dos Santos, J.C.S.; Ortiz, C.; Barbosa, O.; Fernández-Lafuente, R.; Torres, R. Chemical amination of lipases improves their immobilization on octyl-glyoxyl agarose beads. Catal. Today 2016, 259, 107–118. [Google Scholar] [CrossRef]
- De Melo, R.R.; Alnoch, R.C.; Vilela, A.F.L.; De Souza, E.M.; Krieger, N.; Ruller, R.; Sato, H.H.; Mateo, C. New heterofunctional supports based on glutaraldehyde-activation: A tool for enzyme immobilization at neutral pH. Molecules 2017, 22, 1088. [Google Scholar] [CrossRef] [Green Version]
- Godoy, A.C. New Strategy for the immobilization of lipases on glyoxyl–agarose supports: Production of robust biocatalysts for natural oil transformation. Int. J. Mol. Sci. 2017, 18, 2130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krajewska, B. Application of chitin- and chitosan-based materials for enzyme immobilizations: A review. Enzym. Microb. Technol. 2004, 35, 126–139. [Google Scholar] [CrossRef]
- Shukla, S.K.; Mishra, A.K.; Arotiba, O.A.; Mamba, B.B. Chitosan-based nanomaterials: A state-of-the-art review. Int. J. Biol. Macromol. 2013, 59, 46–58. [Google Scholar] [CrossRef]
- Lusiana, R.A.; Siswanta, D.; Mudasir, M.; Hayashita, T. The influence of PVA.pc.citric acid/chitosan membrane hydrophicility on the transport of creatinine and urea. Indones. J. Chem. 2013, 13, 262–270. [Google Scholar] [CrossRef]
- Pellá, M.C.G.; Lima-Tenório, M.K.; Tenório-Neto, E.T.; Guilherme, M.R.; Muniz, E.C.; Rubira, A.F. Chitosan-based hydrogels: From preparation to biomedical applications. Carbohydr. Polym. 2018, 196, 233–245. [Google Scholar] [CrossRef]
- Sung, H.W.; Huang, R.N.; Huang, L.H.; Tsai, C.C. In vitro evaluation of cytotoxicity of a naturally occurring cross-linking reagent for biological tissue fixation. J. Biomater. Sci. Polym. Ed. 1999, 10, 63–78. [Google Scholar] [CrossRef]
- Chatterjee, S.; Lee, M.W.; Woo, S.H. Enhanced mechanical strength of chitosan hydrogel beads by impregnation with carbon nanotubes. Carbon N. Y. 2009, 47, 2933–2936. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M.N. Naturally-derived biopolymers: Potential platforms for enzyme immobilization. Int. J. Biol. Macromol. 2019, 130, 462–482. [Google Scholar] [CrossRef] [PubMed]
- Manzo, R.M.; de Sousa, M.; Fenoglio, C.L.; Gonçalves, L.R.B.; Mammarella, E.J. Chemical improvement of chitosan-modified beads for the immobilization of Enterococcus faecium DBFIQ E36 l-arabinose isomerase through multipoint covalent attachment approach. J. Ind. Microbiol. Biotechnol. 2015, 42, 1325–1340. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.F.; Ng, Y.F.; Pudney, P.D.A. Mechanism and kinetics of the crosslinking reaction between biopolymers containing primary amine groups and genipin. J. Polym. Sci. Part. A Polym. Chem. 2003, 41, 3941–3953. [Google Scholar] [CrossRef]
- Santos, J.C.S.D.; Bonazza, H.L.; de Matos, L.J.B.L.; Carneiro, E.A.; Barbosa, O.; Fernández-Lafuente, R.; Gonçalves, L.R.B.; Ana, H.B.D.S.; Santiago-Aguiar, R.S. Immobilization of CALB on activated chitosan: Application to enzymatic synthesis in supercritical and near-critical carbon dioxide. Biotechnol. Rep. 2017, 14, 16–26. [Google Scholar] [CrossRef]
- Costa-Silva, T.A.; Carvalho, A.K.F.; Souza, C.R.F.; De Castro, H.F.; Said, S.; Oliveira, W.P. Enzymatic transesterification of coconut oil using chitosan-immobilized lipase produced by fluidized-bed system. Energy Fuels 2017, 31, 12209–12216. [Google Scholar] [CrossRef]
- Zhao, K.; Chen, B.; Li, C.; Li, X.; Li, K.; Shen, Y. Immobilization of Candida rugosa lipase on glutaraldehyde-activated Fe3O4@chitosan as a magnetically separable catalyst for hydrolysis of castor oil. Eur. J. Lipid. Sci. Technol. 2018, 120, 1700373. [Google Scholar] [CrossRef]
- Mehdi, S.; Min, S.; Sayed, M.; Younesi, H. Lipase-immobilized chitosan-crosslinked magnetic nanoparticle as a biocatalyst for ring opening esterification of itaconic anhydride. Biochem. Eng. J. 2019, 143, 141–150. [Google Scholar]
- Ziegler-Borowska, M.; Chelminiak-Dudkiewicz, D.; Siódmiak, T.; Sikora, A.; Wegrzynowska-Drzymalska, K.; Skopinska-Wisniewska, J.; Kaczmarek, H.; Marszałł, M.P. Chitosan–collagen coated magnetic nanoparticles for lipase immobilization—New type of “enzyme friendly” polymer shell crosslinking with squaric acid. Catalysts 2017, 7, 26. [Google Scholar] [CrossRef] [Green Version]
- Suo, H.; Xu, L.; Xu, C.; Chen, H.; Yu, D.; Gao, Z.; Huang, H.; Hu, Y. Enhancement of catalytic performance of porcine pancreatic lipase immobilized on functional ionic liquid modified Fe3O4-chitosan nanocomposites. Int. J. Biol. Macromol. 2018, 119, 624–632. [Google Scholar] [CrossRef]
- Xiang, X.; Suo, H.; Xu, C.; Hu, Y. Covalent immobilization of lipase onto chitosan-mesoporous silica hybrid nanomaterials by carboxyl functionalized ionic liquids as the coupling agent. Colloids Surf. B Biointerfaces 2018, 165, 262–269. [Google Scholar] [CrossRef]
- Xiang, X.; Ding, S.; Suo, H.; Xu, C.; Gao, Z.; Hu, Y. Fabrication of chitosan-mesoporous silica SBA-15 nanocomposites via functional ionic liquid as the bridging agent for PPL immobilization. Carbohydr. Polym. 2018, 182, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Myra, F.; Manan, A.; Attan, N.; Zakaria, Z.; Mahat, N.A.; Wahab, R.A. Insight into the Rhizomucor miehei lipase supported on chitosan-chitin nanowhiskers assisted esterification of eugenol to eugenyl benzoate. J. Biotechnol. 2018, 280, 19–30. [Google Scholar]
- Melo, A.D.Q.; Silva, F.F.M.; Dos Santos, J.C.S.; Fernández-Lafuente, R.; Lemos, T.L.G.; Dias Filho, F.A. Synthesis of benzyl acetate catalyzed by lipase immobilized in nontoxic chitosan-polyphosphate beads. Molecules 2017, 22, 2165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinheiro, B.B.; Rios, N.S.; Rodríguez Aguado, E.; Fernández-Lafuente, R.; Freire, T.M.; Fechine, P.B.A.; dos Santos, J.C.S.; Gonçalves, L.R.B. Chitosan activated with divinyl sulfone: A new heterofunctional support for enzyme immobilization. Application in the immobilization of lipase B from Candida antarctica. Int. J. Biol. Macromol. 2019, 130, 798–809. [Google Scholar] [CrossRef]
- Dos Santos, J.C.S.; Rueda, N.; Barbosa, O.; Fernández-Sánchez, J.F.; Medina-Castillo, A.L.; Ramón-Márquez, T.; Arias-Martos, M.C.; Millán-Linares, M.C.; Pedroche, J.; Yust, M.; et al. Characterization of supports activated with divinyl sulfone as a tool to immobilize and stabilize enzymes via multipoint covalent attachment. Application to chymotrypsin. RSC Adv. 2015, 5, 20639–20649. [Google Scholar] [CrossRef] [Green Version]
- Palla, C.A.; Pacheco, C.; Carrín, M.E. Preparation and modification of chitosan particles for Rhizomucor miehei lipase immobilization. Biochem. Eng. J. 2011, 55, 199–207. [Google Scholar] [CrossRef]
- Urrutia, P.; Arrieta, R.; Alvarez, L.; Cardenas, C.; Mesa, M.; Wilson, L. Immobilization of lipases in hydrophobic chitosan for selective hydrolysis of fish oil: The impact of support functionalization on lipase activity, selectivity and stability. Int. J. Biol. Macromol. 2018, 108, 674–686. [Google Scholar] [CrossRef]
- Argüelles-Monal, W.; Lizardi-Mendoza, J.; Fernández-Quiroz, D.; Recillas-Mota, M.; Montiel-Herrera, M. Chitosan derivatives: Introducing new functionalities with a controlled molecular architecture for innovative materials. Polymers (Basel). 2018, 10, 342. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.J.; Park, S.; Kim, S.H.; Kim, J.H.; Yu, H.; Kim, H.J.; Yang, Y.-H.; Kan, E.; Kim, Y.H.; Lee, S.H. Biocompatible cellulose nanocrystals as supports to immobilize lipase. J. Mol. Catal. B Enzym. 2015, 122, 170–178. [Google Scholar] [CrossRef]
- Cao, S.-L.; Huang, Y.-M.; Li, X.-H.; Xu, P.; Wu, H.; Li, N.; Lou, W.-Y.; Zong, M.-H. Preparation and characterization of immobilized lipase from Pseudomonas Cepacia onto magnetic cellulose nanocrystals. Sci. Rep. 2016, 6, 20420. [Google Scholar] [CrossRef] [Green Version]
- Karra-Châabouni, M.; Bouaziz, I.; Boufi, S.; Botelho do Rego, A.M.; Gargouri, Y. Physical immobilization of Rhizopus oryzae lipase onto cellulose substrate: Activity and stability studies. Colloids Surf. B Biointerfaces 2008, 66, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Koga, H.; Kitaoka, T.; Isogai, A. Paper-immobilized enzyme as a green microstructured catalyst. J. Mater. Chem. 2012, 22, 11591–11597. [Google Scholar] [CrossRef]
- Souza, S.P.; Junior, I.I.; Silva, G.M.A.; Miranda, L.S.M.; Santiago, M.F.; Leung-Yuk Lam, F.; Dawood, A.; Bornscheuer, U.T.; de Souza, R.O.M.A. Cellulose as an efficient matrix for lipase and transaminase immobilization. RSC Adv. 2016, 6, 6665–6671. [Google Scholar] [CrossRef]
- Singh, A.K.; Mukhopadhyay, M. Immobilization of Candida antarctica lipase onto cellulose acetate-coated Fe2O3 nanoparticles for glycerolysis of olive oil. Korean J. Chem. Eng. 2014, 31, 1225–1232. [Google Scholar] [CrossRef]
- Elias, N.; Chandren, S.; Razak, F.I.A.; Jamalis, J.; Widodo, N.; Wahab, R.A. Characterization, optimization and stability studies on Candida rugosa lipase supported on nanocellulose reinforced chitosan prepared from oil palm biomass. Int. J. Biol. Macromol. 2018, 114, 306–316. [Google Scholar] [CrossRef]
- Cai, Q.; Hu, C.; Yang, N.; Wang, Q.; Wang, J.; Pan, H.; Hu, Y.; Ruan, C. Enhanced activity and stability of industrial lipases immobilized onto spherelike bacterial cellulose. Int. J. Biol. Macromol. 2018, 109, 1174–1181. [Google Scholar] [CrossRef]
- Wu, S.-C.; Wu, S.-M.; Su, F.-M. Novel process for immobilizing an enzyme on a bacterial cellulose membrane through repeated absorption. J. Chem. Technol. Biotechnol. 2017, 92, 109–114. [Google Scholar] [CrossRef]
- Badgujar, K.C.; Bhanage, B.M. Lipase immobilization on hyroxypropyl methyl cellulose support and its applications for chemo-selective synthesis of α-amino ester compounds. Process. Biochem. 2016, 51, 1420–1433. [Google Scholar] [CrossRef]
- Zeeman, S.C.; Kossmann, J.; Smith, A.M. Starch: Its metabolism, evolution, and biotechnological modification in plants. Annu. Rev. Plant. Biol. 2010, 61, 209–234. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, I.; Silva, V.D.; Nascimento, M.D.G. Enantioselective resolution of (R,S)-1-phenylethanol catalyzed by lipases immobilized in starch films. J. Braz. Chem. Soc. 2011, 22, 1559–1567. [Google Scholar] [CrossRef]
- Nascimento, M.D.G.; Silva, J.M.R.D.; Silva, J.C.D.; Alves, M.M. The use of organic solvents/ionic liquids mixtures in reactions catalyzed by lipase from Burkholderia cepacia immobilized in different supports. J. Mol. Catal. B Enzym. 2015, 112, 1–8. [Google Scholar] [CrossRef]
- Silva, J.C.D.; Nascimento, M.D.G. The influence of organic medium and supports in the resolution of (R,S)-Menthol catalyzed by lipases. J. Braz. Chem. Soc. 2016, 27, 2226–2233. [Google Scholar] [CrossRef]
- Masina, N.; Choonara, Y.E.; Kumar, P.; du Toit, L.C.; Govender, M.; Indermun, S.; Pillay, V. A review of the chemical modification techniques of starch. Carbohydr. Polym. 2017, 157, 1226–1236. [Google Scholar] [CrossRef] [PubMed]
- Vanier, N.L.; El Halal, S.L.M.; Dias, A.R.G.; da Rosa Zavareze, E. Molecular structure, functionality and applications of oxidized starches: A review. Food Chem. 2017, 221, 1546–1559. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Y.; Yao, S.; Qian, J.; Guo, H.; Cai, X. Preparation of immobilized lipase on magnetic nanoparticles dialdehyde starch. Carbohydr. Polym. 2019, 218, 324–332. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- Day, D.F. Enzymatically modified alginates as useful biopolymers. Ann. N. Y. Acad. Sci. 1996, 799, 584–587. [Google Scholar] [CrossRef]
- Tudorache, M.; Gheorghe, A.; Negoi, A.; Enache, M.; Maria, G.; Parvulescu, V.I. Bifunctional carbohydrate biopolymers entrapped lipase as catalyst for the two consecutive conversions of -pinene to oxy-derivatives. Carbohydr. Polym. 2016, 152, 726–733. [Google Scholar] [CrossRef]
- Tanaka, H.; Matsumura, M.; Veliky, I.A. Diffusion characteristics of substrates in Ca-alginate gel beads. Biotechnol. Bioeng. 1984, 26, 53–58. [Google Scholar] [CrossRef]
- Pereira, A.D.S.; Fraga, L.J.; Diniz, M.M.; Fontes-Sant’Ana, C.G.; Amaral, F.F.P. High catalytic activity of lipase from Yarrowia lipolytica immobilized by microencapsulation. Int. J. Mol. Sci. 2018, 19, 3393. [Google Scholar] [CrossRef] [Green Version]
- Bhushan, I.; Parshad, R.; Qazi, G.; Gupta, V. Immobilization of lipase by entrapment in Ca-alginate beads. J. Bioact. Compat. Polym. 2008, 23, 552–562. [Google Scholar] [CrossRef]
- Bonine, B.M.; Polizelli, P.P.; Bonilla-Rodriguez, G.O. Immobilization of a plant lipase from Pachira aquatica in Alginate and Alginate/PVA beads. Enzym. Res. 2014, 2014, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Abdulla, R.; Ravindra, P. Characterization of cross linked Burkholderia cepacia lipase in alginate and κ-carrageenan hybrid matrix. J. Taiwan Inst. Chem. Eng. 2013, 44, 545–551. [Google Scholar] [CrossRef]
- Huang, J.; Yan, R.; He, J.; Wang, P. Purification and immobilization of a novel enantioselective lipase from Tsukamurella tyrosinosolvents for efficient resolution of ethyl 2-(2-oxopyrrolidin-1-yl) butyrate. Appl. Biochem. Biotechnol. 2016, 180, 609–622. [Google Scholar] [CrossRef] [PubMed]
- Sipponen, M.; Muhammad, F.; Koivisto, J.; Pellis, A.; Seitsonen, J.; Österberg, M. Spatially confined lignin nanospheres for biocatalytic ester synthesis in aqueous media. Nat. Commun. 2018, 9, 2300. [Google Scholar] [CrossRef]
- Pawar, S.N.; Edgar, K.J. Alginate derivatization: A review of chemistry, properties and applications. Biomaterials 2012, 33, 3279–3305. [Google Scholar] [CrossRef]
- Hou, C.; Qi, Z.; Zhu, H. Preparation of core-shell magnetic polydopamine/alginate biocomposite for Candida rugosa lipase immobilization. Colloids Surf. B Biointerfaces 2015, 128, 544–551. [Google Scholar] [CrossRef]
- Mendes, A.A.; Oliveira, P.C.; Vélez, A.M.; Giordano, R.C.; Giordano, R.D.L.C.; de Castro, H.F. Evaluation of immobilized lipases on poly-hydroxybutyrate beads to catalyze biodiesel synthesis. Int. J. Biol. Macromol. 2012, 50, 503–511. [Google Scholar] [CrossRef]
- Miranda, J.S.; Silva, N.C.A.; Bassi, J.J.; Corradini, M.C.C.; Lage, F.A.P.; Hirata, D.B.; Mendes, A.A. Immobilization of Thermomyces lanuginosus lipase on mesoporous poly-hydroxybutyrate particles and application in alkyl esters synthesis: Isotherm, thermodynamic and mass transfer studies. Chem. Eng. J. 2014, 251, 392–403. [Google Scholar] [CrossRef]
- Silva, N.C.A.; Miranda, J.S.; Bolina, I.C.A.; Silva, W.C.; Hirata, D.B.; Castro, H.F.D.; Mendes, A.A. Immobilization of porcine pancreatic lipase on poly-hydroxybutyrate particles for the production of ethyl esters from macaw palm oils and pineapple flavor. Biochem. Eng. J. 2014, 82, 139–149. [Google Scholar] [CrossRef]
- Ramos, E.Z.; Júnior, R.H.M.; Castro, P.F.D.; Tardioli, P.W.; Mendes, A.A.; Fernández-Lafuente, R.; Hirata, D.B. Production and immobilization of Geotrichum candidum lipase via physical adsorption on eco-friendly support: Characterization of the catalytic properties in hydrolysis and esterification reactions. J. Mol. Catal. B Enzym. 2015, 118, 43–51. [Google Scholar] [CrossRef]
- Sidebotham, R.L. Dextrans. Adv. Carbohydr. Chem. Biochem. 1974, 30, 371–444. [Google Scholar] [PubMed]
- Tacias-Pascacio, V.G.; Ortiz, C.; Rueda, N.; Berenguer-Murcia, Á.; Acosta, N.; Aranaz, I.; Civera, C.; Fernández-Lafuente, R.; Alcántara, A.R. Dextran aldehyde in biocatalysis: More than a mere immobilization system. Catalysts 2019, 9, 622. [Google Scholar] [CrossRef] [Green Version]
- Tahir, M.N.; Adnan, A.; Mischnick, P. Lipase immobilization on O-propargyl and O-pentynyl dextrans and its application for the synthesis of click beetle pheromones. Process Biochem. 2009, 44, 1276–1283. [Google Scholar] [CrossRef]
- Orrego, A.H.; Ghobadi, R.; Moreno-Perez, S.; Mendoza, A.J.; Fernández-Lorente, G.; Guisán, J.M.; Rocha-Martin, J. Stabilization of immobilized lipases by intense intramolecular cross-linking of their surfaces by using aldehyde-dextran polymers. Int. J. Mol. Sci. 2018, 19, 553. [Google Scholar] [CrossRef] [Green Version]
- Cejudo-Sanches, J.; Orrego, A.H.; Jaime-Mendoza, A.; Ghobadi, R.; Moreno-Perez, S.; Fernández-Lorente, G.; Rocha-Martin, J.; Guisán, J.M. High stabilization of immobilized Rhizomucor miehei lipase by additional coating with hydrophilic crosslinked polymers: Poly-allylamine/aldehyde–dextran. Process Biochem. 2020, 92, 156–163. [Google Scholar] [CrossRef]
- Bi, Y.; Yu, M.; Zhou, H.; Zhou, H.; Wei, P. Biosynthesis of oleyl oleate in solvent-free system by Candida rugosa lipase (CRL) immobilized in macroporous resin with cross-linking of aldehyde-dextran. J. Mol. Catal. B Enzym. 2016, 133, 1–5. [Google Scholar] [CrossRef]
- Tahir, M.N.; Adnan, A.; Strömberg, E.; Mischnick, P. Stability of lipase immobilized on O-pentynyl dextran. Bioprocess Biosyst. Eng. 2012, 35, 535–544. [Google Scholar] [CrossRef]
- Singh, R.S.; Kaur, N.; Kennedy, J.F. Pullulan and pullulan derivatives as promising biomolecules for drug and gene targeting. Carbohydr. Polym. 2015, 123, 190–207. [Google Scholar] [CrossRef]
- Xu, L.; Cui, G.; Ke, C.; Fan, Y.; Yan, Y. Immobilized Burkholderia cepacia lipase on pH-responsive pullulan derivatives with improved enantioselectivity in chiral resolution. Catalysts 2018, 8, 13. [Google Scholar] [CrossRef] [Green Version]
- Krieger, N.; Dias, G.S.; Alnoch, R.C.; Mitchell, D.A. Fermented solids and their application in the production of organic compounds of biotechnological interest. Adv. Biochem. Eng. Biotechnol. 2019, 169, 125–146. [Google Scholar] [PubMed]
- Ittrat, P.; Chacho, T.; Pholprayoon, J.; Suttiwarayanon, N.; Charoenpanich, J. Application of agriculture waste as a support for lipase immobilization. Biocatal. Agric. Biotechnol. 2014, 3, 77–82. [Google Scholar] [CrossRef]
- Cespugli, M.; Lotteria, S.; Navarini, L.; Lonzarich, V.; Del Terra, L.; Vita, F.; Zweyer, M.; Baldini, G.; Ferrario, V.; Ebert, C.; et al. Rice husk as an inexpensive renewable immobilization carrier for biocatalysts employed in the food, cosmetic and polymer sectors. Catalysts. 2018, 8, 471. [Google Scholar] [CrossRef] [Green Version]
- Tan, T.; Lu, J.; Nie, K.; Deng, L.; Wang, F. Biodiesel production with immobilized lipase: A review. Biotechnol. Adv. 2010, 28, 628–634. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, S.; Kim, H.; Kim, H.J.; Yang, Y.-H.; Kim, Y.H.; Jung, S.-K.; Kan, E.; Lee, S.H. Alginate/bacterial cellulose nanocomposite beads prepared using Gluconacetobacter xylinus and their application in lipase immobilization. Carbohydr. Polym. 2017, 157, 137–145. [Google Scholar] [CrossRef]
- Doğaç, Y.İ.; Deveci, İ.; Mercimek, B.; Teke, M. A comparative study for lipase immobilization onto alginate based composite electrospun nanofibers with effective and enhanced stability. Int. J. Biol. Macromol. 2017, 96, 302–311. [Google Scholar] [CrossRef]
- Hussin, F.N.N.M.; Attan, N.; Wahab, R.A. Taguchi design-assisted immobilization of Candida rugosa lipase onto a ternary alginate/nanocellulose/montmorillonite composite: Physicochemical characterization, thermal stability and reusability studies. Enzym. Microb. Technol. 2020, 136, 109506. [Google Scholar] [CrossRef]
- Cantone, S.; Ferrario, V.; Corici, L.; Ebert, C.; Fattor, D.; Spizzo, P.; Gardossi, L. Efficient immobilisation of industrial biocatalysts: Criteria and constraints for the selection of organic polymeric carriers and immobilisation methods. Chem. Soc. Rev. 2013, 42, 6262–6276. [Google Scholar] [CrossRef] [Green Version]
- Hanefeld, U.; Gardossi, L.; Magner, E. Understanding enzyme immobilisation. Chem. Soc. Rev. 2009, 38, 453–458. [Google Scholar] [CrossRef]
- Reslow, M.; Adlercreutz, P.; Mattiasson, B. On the importance of the support material for bioorganic synthesis. Eur. J. Biochem. 1988, 172, 573–578. [Google Scholar] [CrossRef]
- Soares, D.; Daci, J.D.; Corazza, M.L.; Mitchell, D.A.; Gonçalves, A.G.; Krieger, N. Analysis of multiphasic behavior during the ethyl esterification of fatty acids catalyzed by a fermented solid with lipolytic activity in a packed-bed bioreactor in a closed-loop batch system. Fuel 2015, 159, 364–372. [Google Scholar] [CrossRef]
- Dias, G.S.; Luz, L.F.D.L.; Mitchell, D.A.; Krieger, N. Scale-up of biodiesel synthesis in a closed-loop packed-bed bioreactor system using the fermented solid produced by Burkholderia lata LTEB11. Chem. Eng. J. 2017, 316, 341–349. [Google Scholar] [CrossRef]
- Bolivar, J.M.; Nidetzky, B. The microenvironment in immobilized enzymes: Methods of characterization and its role in determining enzyme performance. Molecules 2019, 24, 3460. [Google Scholar] [CrossRef] [PubMed] [Green Version]




















© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alnoch, R.C.; Alves dos Santos, L.; Marques de Almeida, J.; Krieger, N.; Mateo, C. Recent Trends in Biomaterials for Immobilization of Lipases for Application in Non-Conventional Media. Catalysts 2020, 10, 697. https://doi.org/10.3390/catal10060697
Alnoch RC, Alves dos Santos L, Marques de Almeida J, Krieger N, Mateo C. Recent Trends in Biomaterials for Immobilization of Lipases for Application in Non-Conventional Media. Catalysts. 2020; 10(6):697. https://doi.org/10.3390/catal10060697
Chicago/Turabian StyleAlnoch, Robson Carlos, Leandro Alves dos Santos, Janaina Marques de Almeida, Nadia Krieger, and Cesar Mateo. 2020. "Recent Trends in Biomaterials for Immobilization of Lipases for Application in Non-Conventional Media" Catalysts 10, no. 6: 697. https://doi.org/10.3390/catal10060697
APA StyleAlnoch, R. C., Alves dos Santos, L., Marques de Almeida, J., Krieger, N., & Mateo, C. (2020). Recent Trends in Biomaterials for Immobilization of Lipases for Application in Non-Conventional Media. Catalysts, 10(6), 697. https://doi.org/10.3390/catal10060697