Efficient Multifunctional Catalytic and Sensing Properties of Synthesized Ruthenium Oxide Nanoparticles
Abstract
:1. Introduction
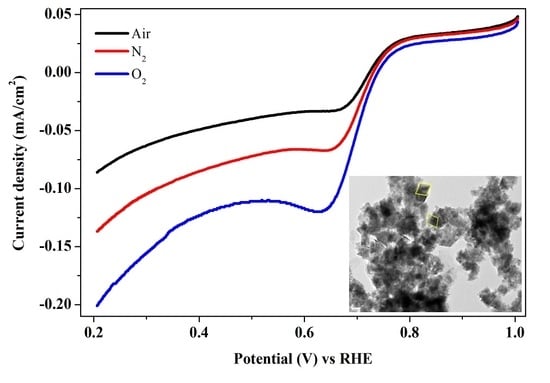
2. Results and Discussion
3. Experimental
3.1. Physical Characterization
3.2. Electrochemical Measurements
3.3. Catalytic Activity of RuO2 Nanoparticles and HRP
3.4. Detection of H2O2
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Solomonn, S.; Plattner, G.-K.; Knutti, R.; Friedlingstein, P. Irreversible climate change due to carbon dioxide emissions. Proc. Natl. Acad. Sci. USA 2009, 106, 1704–1709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chhow, J.; Kopp, R.J.; Portney, P.R. Energy resources and global development. Science 2003, 302, 1528–1531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, T.; Lone, I.H.; Ansari, S.G.; Ahmed, J.; Ahamad, T.; Alshehri, S.M. Multifunctional properties and applications of yttrium ferrite nanoparticles prepared by citrate precursor route. Mater. Des. 2017, 126, 331–338. [Google Scholar] [CrossRef]
- Vondrák, J.; Klápště, B.; Velická, J.; Sedlaříková, M.; Ćerný, R. Hydrogen-oxygen fuel cells. J. Solid State Electrochem. 2003, 8, 44–47. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.-G.; Xie, Z.; Zhou, Z. Recent progress in rechargeable alkali metal-air batteries. Green Energy Environ. 2016, 1, 4–17. [Google Scholar] [CrossRef] [Green Version]
- Gutsche, C.; Moeller, C.J.; Knipper, M.; Borchert, H.; Parisi, J.; Plaggenborg, T. Synthesis, structure, and electrochemical stability of Ir-decorated RuO2 nanoparticles and Ptnanorods as oxygen catalysts. J. Phys. Chem. C. 2016, 120, 1137–1146. [Google Scholar] [CrossRef]
- Tseng, H.-W.; Zong, R.; Muckerman, J.T.; Thummel, R. Mononuclear ruthenium (II) complexes that catalyze water oxidation. Inorg. Chem. 2008, 47, 11763–11773. [Google Scholar] [CrossRef]
- Reier, T.; Oezaslan, M.; Strasser, P. Electrocatalytic oxygen evolution reaction (OER) on Ru, Ir, and Ptcatalysts: A comparative study of nanoparticles and bulk materials. ACS Catal. 2012, 2, 1765–1772. [Google Scholar] [CrossRef]
- Karlsson, E.A.; Lee, B.-L.; Åkermark, T.; Johnston, E.V.; Kärkäs, M.D.; Sun, J.; Hansson, Ö.; Bäckvall, J.-E.; Åkermark, B. Photosensitized water oxidation by use of a bioinspired manganese catalyst. Angew. Chem. Int. Ed. 2011, 50, 11715–11718. [Google Scholar] [CrossRef]
- Ellis, W.C.; McDaniel, N.D.; Bernhard, S.; Collins, T.J. Fast water oxidation using iron. J. Am. Chem. Soc. 2010, 132, 10990–10991. [Google Scholar] [CrossRef]
- Alshehri, S.M.; Ahmed, J.; Alhabarah, A.N.; Ahamad, T.; Ahmad, T. Nitrogen doped cobalt ferrite/carbon nanocomposites for supercapacitor application. ChemElectroChem 2017, 4, 2952–2958. [Google Scholar] [CrossRef]
- Coggins, M.; Zhang, K.M.T.; Chen, Z.; Song, N.; Meyer, T.J. Single-site copper (II) water oxidation electrocatalysis: Rate enhancements with HPO42- as a proton acceptor at pH-8. Angew. Chem. Int. Ed. Engl. 2014, 53, 12226–12230. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, S.M.; Ahmed, J.; Ahamad, T.; Alhokbany, N.; Arunachalam, P.; Al-Mayouf, A.M.; Ahmad, T. Synthesis, characterization, multifunctional electrochemical (OGR/ORR/SCs) and photodegradable activities of ZnWO4 nanobricks. J. Sol-Gel Sci. Technol. 2018, 87, 137–146. [Google Scholar] [CrossRef]
- Fang, Y.-H.; Liu, Z.-P. Mechanism and tafel lines of electro-oxidation of water to oxygen on RuO2 (110). J. Am. Chem. Soc. 2010, 132, 18214–18222. [Google Scholar] [CrossRef]
- Michas, A.; Andolfatto, F.; Lyons, M.E.G.; Durand, R. Gas evolution reactions at conductive metallic oxide electrodes for solid polymer electrolyte water electrolysis. Key Eng. Mater. 1992, 535, 72–74. [Google Scholar] [CrossRef]
- Cui, X.; Zhou, J.; Ye, Z.; Chen, H.; Li, L.; Ruan, M.; Shi, J. Selective catalytic oxidation of ammonia to nitrogen over mesoporous CuO/RuO2 synthesized by co-nanocasting-replication method. J. Catal. 2010, 270, 310–317. [Google Scholar] [CrossRef]
- Seki, K. Development of RuO2/Rutile-TiO2 catalyst for industrial HCl oxidation process. Catal. Surv. Asia 2010, 14, 168–175. [Google Scholar] [CrossRef]
- Liu, H.; Iglesia, E. Selective oxidation of methanol and ethanol on supported ruthenium oxide clusters at low temperatures. J. Phys. Chem. B 2005, 109, 2155–2163. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.; Liu, C.; Liao, J.; Su, Y.; Xue, X.; Xing, W. Study of ruthenium oxide catalyst for electrocatalytic performance in oxygen evolution. J. Mol. Catal. A-Chem. 2006, 247, 7–13. [Google Scholar] [CrossRef]
- Kiele, N.M.; Herrero, C.; Ranjbari, A.; Aukauloo, A.; Grigoriev, S.A.; Villagra, A.; Millet, P. Ruthenium-based molecular compounds for oxygen evolution in acidic media. Int. J. Hydrogen Energy 2013, 38, 8590–8596. [Google Scholar] [CrossRef]
- Jeon, H.S.; Permana, A.D.C.; Kim, J.; Min, B.K. Water splitting for hydrogen production using a high surface area RuO2 electrocatalyst synthesized in supercritical water. Int. J. Hydrogen Energy 2013, 38, 6092–6096. [Google Scholar] [CrossRef]
- Tilley, S.D.; Schreier, M.; Azevedo, J.; Stefik, M.; Grätzel, M. Ruthenium oxide hydrogen evolution catalysis on composite cuprous oxide water-splitting photocathodes. Adv. Funct. Mater. 2014, 24, 303–311. [Google Scholar] [CrossRef]
- Ryden, W.D.; Lawson, A.W.; Sartain, C.C. Temperature dependence of the resistivity of RuO2 and IrO2. Phys. Lett. 1968, 26, 209–210. [Google Scholar] [CrossRef]
- Hu, J.-M.; Zhang, J.-Q.; Cao, C.-N. Oxygen evolution reaction on IrO2-based DSA® type electrodes: Kinetics analysis of tafel lines and EIS. Int. J. Hydrogen Energy 2004, 29, 791–797. [Google Scholar] [CrossRef]
- Lee, Y.; Suntivich, J.; May, K.J.; Perry, E.E.; Shao-Horn, Y. Synthesis and activities of rutile IrO2 and RuO2 nanoparticles for oxygen evolution in acid and alkaline solutions. J. Phys. Chem. Lett. 2012, 3, 399–404. [Google Scholar] [CrossRef]
- Ahmed, J.; Mao, Y. Ultrafine iridium oxide nanorods synthesized by molten salt method toward electrocatalytic oxygen and hydrogen evolution reactions. Electrochim. Acta 2016, 212, 686–693. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.M.; Korotcov, A.; Hsu, H.P.; Huang, Y.S.; Tsai, D.S. Raman scattering characterization of well-aligned RuO2 nanocrystals grown on sapphire substrates. New J. Phys. 2007, 9, 1–11. [Google Scholar] [CrossRef]
- Shen, W.; Shi, J.; Chen, H.; Gu, J.; Zhu, Y.; Dong, X. Synthesis and CO oxidation catalytic character of high surface area ruthenium dioxide replicated by cubic mesoporous silica. Chem. Lett. 2005, 34, 390–391. [Google Scholar] [CrossRef]
- Ryan, J.V.; Berry, A.D.; Anderson, M.L.; Long, J.W.; Stroud, R.M.; Cepak, V.M.; Browning, V.M.; Rolison, D.R.; Merzbacher, C.I. Electronic connection to the interior of a mesoporous insulator with nanowires of crystalline RuO2. Nature 2000, 406, 169–172. [Google Scholar] [CrossRef]
- Viswanathamurthi, P.; Bhattarai, N.; Kim, C.K.; Kim, H.Y.; Lee, D.R. Ruthenium doped TiO2 fibers by electrospinning. Inorg. Chem. Commun. 2004, 7, 679–682. [Google Scholar] [CrossRef]
- Browne, M.P.; Nolan, H.; Duesberg, G.S.; Colavita, P.E.; Lyons, M.E.G. Low-overpotential high-activity mixed manganese and ruthenium oxide electrocatalysts for oxygen evolution reaction in alkaline media. ACS Catal. 2016, 6, 2408–2415. [Google Scholar] [CrossRef]
- Gustafson, K.P.J.; Shatskiy, A.; Verho, O.; Kärkäs, M.D.; Schluschass, B.; Tai, C.-W.; Åkermark, B.; Bäckvall, J.-E.; Johnston, E.V. Water oxidation mediated by ruthenium oxide nanoparticles supported on siliceous mesocellular foam. Catal. Sci. Technol. 2017, 7, 293–299. [Google Scholar]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, X.; Hu, J. Pt-DNA complexes as peroxidase mimetics and their applications in colorimetric detection of H2O2 and glucose. Anal. Methods 2012, 4, 2183–2187. [Google Scholar] [CrossRef]
- Chen, T.; Tian, L.; Chen, Y.; Liu, B.; Zhang, J. A facile one-pot synthesis of Au/Cu2O nanocomposites for nonenzymatic detection of hydrogen peroxide. Nanoscale Res. Lett. 2015, 10, 935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, J.; Peng, J.; Jin, X. Synthesis of copper sulfide nanorods as peroxidase mimics for the colorimetric detection of hydrogen peroxide. Anal. Methods 2015, 7, 5454–5461. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, Y.; Liu, Z.; Guo, M. A Novel Profluorescent Probe for detecting oxidative stress induced by metal and H2O2 in living cells. Chem. Commun. 2010, 46, 4472–4474. [Google Scholar] [CrossRef]
- Dickinson, B.C.; Chang, C.J. A targetable fluorescent probe for imaging hydrogen peroxide in the mitochondria of living cells. J. Am. Chem. Soc. 2008, 130, 9638–9639. [Google Scholar] [CrossRef] [Green Version]
- Finkel, T.; Serrano, M.; Blasco, M.A. The common biology of cancer and ageing. Nature 2007, 448, 767–774. [Google Scholar] [CrossRef] [Green Version]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2001. [Google Scholar]
- Stoerzinger, K.A.; Qiao, L.; Biegalski, M.D.; Shao-Horn, Y. Orientation-dependent oxygen evolution activities of rutile IrO2 and RuO2. J. Phys. Chem. Lett. 2014, 5, 1636–1641. [Google Scholar] [CrossRef]
- AlShehri, S.M.; Ahmed, J.; Ahamad, T.; Arunachalam, P.; Ahmad, T.; Khan, A. Bifunctional electro-catalytic performances of CoWO4 nanocubes for water redox reactions (OER/ORR). RSC Adv. 2017, 7, 45615–45623. [Google Scholar] [CrossRef] [Green Version]
- Jiang, R.; Tran, D.T.; Li, J.; Chu, D. Ru@RuO2 core-shell nanorods: A highly active and stable bifunctional catalyst for oxygen evolution and hydrogen evolution reactions. Energy Environ. Mater. 2019, 2, 201–208. [Google Scholar] [CrossRef] [Green Version]
- Bhowmik, T.; Kundu, M.K.; Barman, S. Growth of one-dimensional RuO2 nanowires on g-carbon nitride: An active and stable bifunctional electrocatalyst for hydrogen and oxygen evolution reactions at all pH values. ACS Appl. Mater. Inter. 2016, 8, 28678–28688. [Google Scholar] [CrossRef] [PubMed]
- Cherevko, S.; Geiger, S.; Kasian, O.; Kulyk, N.; Grote, J.-P.; Savan, A.; Shrestha, B.R.; Merzlikin, S.; Breitbach, B.; Ludwig, A.; et al. Oxygen and hydrogen evolution reactions on Ru, RuO2, Ir, and IrO2 thin film electrodes in acidic and alkaline electrolytes: A comparative study on activity and stability. Catal. Today 2016, 262, 170–180. [Google Scholar] [CrossRef]
- Farooq, U.; Phul, P.; Alshehri, S.M.; Ahmed, J.; Ahmad, T. Electrocatalytic and enhanced photocatalytic applications of sodium niobate nanoparticles developed by citrate precursor route. Sci. Rep. 2019, 9, 4488. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, J.; Ubaidullah, M.; Ahmad, T.; Alhokbany, N.; Alshehri, S.M. Synthesis of graphite oxide/cobalt molybdenum oxide hybrid nanosheets for enhanced electrochemical performances in supercapacitors and OER. ChemElectroChem 2019, 6, 2524–2530. [Google Scholar] [CrossRef]
- Bos, E.S.; van der Doelen, A.A.; van Rooy, N.; Schuurs, A.H.W.M. 3,3′,5,5′-etramethylbenzidine as an ames test negative chromogen for horse-radish peroxidase in enzyme immunoassay. J. Immunoass. 1981, 2, 187–204. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, J.; Guo, Z.; Tan, H.; Zhu, X. A simple colorimetric method for determination of hydrogen peroxide in plant tissues. Plant Growth Regul. 2006, 49, 113–118. [Google Scholar] [CrossRef]






| Catalyst | Electrolyte | Scan Rate (mVs−1) | Onset Potential (V/RHE) | TafelSlope (mVdec−1) | Ref. | |
|---|---|---|---|---|---|---|
| OER | ORR | |||||
| r-RuO2 | 0.1M KOH | 10 | 1.4 | - | - | [25] |
| Ru@RuO2 | 0.1M KOH | 10 | 1.3 | 86 | - | [43] |
| 1D-RuO2-CNx | 0.5M KOH | 10 | 1.42 | 56 | - | [44] |
| RuO2 | 0.05M NaOH | 10 | 1.27 | - | - | [45] |
| Mn25Ru75@450 | 1M NaOH | 10 | 1.4 | 66 | - | [31] |
| RuO2 nanoparticles | 0.5M KOH | 25 | 1.5 | 47 | 49 | Present work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phul, R.; Perwez, M.; Ahmed, J.; Sardar, M.; M. Alshehri, S.; Alhokbany, N.; Khan, M.A.M.; Ahmad, T. Efficient Multifunctional Catalytic and Sensing Properties of Synthesized Ruthenium Oxide Nanoparticles. Catalysts 2020, 10, 780. https://doi.org/10.3390/catal10070780
Phul R, Perwez M, Ahmed J, Sardar M, M. Alshehri S, Alhokbany N, Khan MAM, Ahmad T. Efficient Multifunctional Catalytic and Sensing Properties of Synthesized Ruthenium Oxide Nanoparticles. Catalysts. 2020; 10(7):780. https://doi.org/10.3390/catal10070780
Chicago/Turabian StylePhul, Ruby, Mohammad Perwez, Jahangeer Ahmed, Meryam Sardar, Saad M. Alshehri, Norah Alhokbany, Mohd A. Majeed Khan, and Tokeer Ahmad. 2020. "Efficient Multifunctional Catalytic and Sensing Properties of Synthesized Ruthenium Oxide Nanoparticles" Catalysts 10, no. 7: 780. https://doi.org/10.3390/catal10070780
APA StylePhul, R., Perwez, M., Ahmed, J., Sardar, M., M. Alshehri, S., Alhokbany, N., Khan, M. A. M., & Ahmad, T. (2020). Efficient Multifunctional Catalytic and Sensing Properties of Synthesized Ruthenium Oxide Nanoparticles. Catalysts, 10(7), 780. https://doi.org/10.3390/catal10070780