Comparative Study of Strategies for Enhancing the Performance of Co3O4/Al2O3 Catalysts for Lean Methane Combustion
Abstract
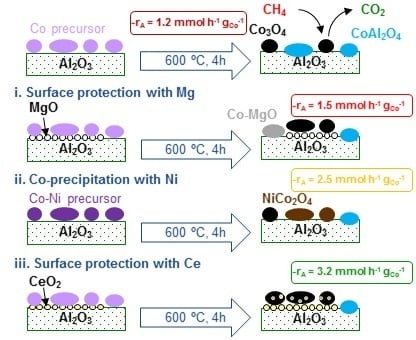
:1. Introduction
- (i)
- Superficial protection of alumina with magnesia prior to the deposition of cobalt (Co/Mg-Al sample). The cobalt content was fixed at 30 wt % and the Mg/Co molar was 0.25.
- (ii)
- (iii)
- Coating the alumina surface with ceria before the addition of cobalt in order to eventually serve as both a surface protector for alumina and redox promoter for cobalt oxide (Co/Ce-Al sample). The cobalt content was fixed at 30 wt % and the Ce/Co molar was 0.20.
2. Results
2.1. Physico-Chemical Characterization
2.2. Catalytic Performance
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Characterisation Techniques
3.3. Catalytic Activity Determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Raj, A. Methane emission control. Johns. Matthey Technol. Rev. 2016, 60, 228–235. [Google Scholar] [CrossRef]
- Khan, M.I.; Yasmin, T.; Shakoor, A. Technical overview of compressed natural gas (CNG) as a transportation fuel. Renew. Sust. Energy Rev. 2015, 51, 785–797. [Google Scholar] [CrossRef]
- Chen, J.; Arandiyan, H.; Gao, X.; Li, J. Recent advances in catalysts for methane combustion. Catal. Surv. Asia 2015, 19, 140–171. [Google Scholar] [CrossRef]
- Choudhary, T.V.; Banerjee, S.; Choudhary, V.R. Catalysts for combustion of methane and lower alkanes. Appl. Catal. A Gen. 2002, 234, 1–23. [Google Scholar] [CrossRef]
- Velin, P.; Ek, M.; Skoglundh, M.; Schaefer, A.; Raj, A.; Thompsett, D.; Smedler, G.; Carlsson, P. Water inhibition in methane oxidation over alumina supported palladium catalysts. J. Phys. Chem. C 2019, 123, 25724–25737. [Google Scholar] [CrossRef]
- Persson, K.; Pfefferle, L.D.; Schwartz, W.; Ersson, A.; Järås, S.G. Stability of palladium-based catalysts during catalytic combustion of methane: The influence of water. Appl. Catal. B Environ. 2007, 74, 242–250. [Google Scholar] [CrossRef]
- Hu, J.; Zhao, W.; Hu, R.; Chang, G.; Li, C.; Wang, L. Catalytic activity of spinel oxides MgCr2O4 and CoCr2O4 for methane combustion. Mater. Res. Bull. 2014, 57, 268–273. [Google Scholar] [CrossRef]
- Ma, Z. Cobalt oxide catalysts for environmental remediation. Curr. Catal. 2014, 3, 15–26. [Google Scholar] [CrossRef]
- Pu, Z.; Zhou, H.; Zheng, Y.; Huang, W.; Li, X. Enhanced methane combustion over Co3O4 catalysts prepared by a facile precipitation method: Effect of aging time. Appl. Surf. Sci. 2017, 410, 14–21. [Google Scholar] [CrossRef]
- Zasada, F.; Piskorz, W.; Janas, J.; Grybos, J.; Indyka, P.; Sojka, Z. Reactive oxygen species on the (100) facet of cobalt spinel nanocatalyst and their relevance in 16O2/18O2 isotopic exchange, deN2O, and deCH4 processes—A theoretical and experimental account. ACS Catal. 2015, 5, 6879–6892. [Google Scholar] [CrossRef]
- Liotta, L.F.; Wu, H.; Pantaleo, G.; Venezia, A.M. Co3O4 nanocrystals and Co3O4-MOx binary oxides for CO, CH4 and VOC oxidation at low temperatures: A review. Catal. Sci. Technol. 2013, 3, 3085–3102. [Google Scholar] [CrossRef]
- Liotta, L.F.; Di Carlo, G.; Pantaleo, G.; Venezia, A.M.; Deganello, G. Co3O4/CeO2 composite oxides for methane emissions abatement: Relationship between Co3O4-CeO2 interaction and catalytic activity. Appl. Catal. B Environ. 2006, 66, 217–227. [Google Scholar] [CrossRef]
- Videla, A.H.M.; Stelmachowski, P.; Ercolino, G.; Specchia, S. Benchmark comparison of Co3O4 spinel-structured oxides with different morphologies for oxygen evolution reaction under alkaline conditions. J. Appl. Electrochem. 2017, 47, 295–304. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, S.; Liu, W.; Gao, X.; Gao, D.; Wang, M.; Wang, S. Morphology-dependent performance of Co3O4 via facile and controllable synthesis for methane combustion. Appl. Catal. A Gen. 2016, 525, 94–102. [Google Scholar] [CrossRef]
- Fei, Z.; He, S.; Li, L.; Ji, W.; Au, C. Morphology-directed synthesis of Co3O4 nanotubes based on modified Kirkendall effect and its application in CH4 combustion. Chem. Commun. 2012, 48, 853–855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, L.; Peng, Q.; Li, Y. Selective synthesis of Co3O4 nanocrystal with different shape and crystal plane effect on catalytic property for methane combustion. J. Am. Chem. Soc. 2008, 130, 16136–16137. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Peng, Y.; Fu, J.; Kyzas, G.Z.; Billah, S.M.R.; An, S. Synthesis, characterization, and catalytic evaluation of Co3O4/γ-Al2O3 as methane combustion catalysts: Significance of Co species and the redox cycle. Appl. Catal. B Environ. 2015, 168-169, 42–50. [Google Scholar] [CrossRef]
- Solsona, B.; Davies, T.E.; Garcia, T.; Vázquez, I.; Dejoz, A.; Taylor, S.H. Total oxidation of propane using nanocrystalline cobalt oxide and supported cobalt oxide catalysts. Appl. Catal. B Environ. 2008, 84, 176–184. [Google Scholar] [CrossRef]
- Liotta, L.F.; Pantaleo, G.; Macaluso, A.; Di Carlo, G.; Deganello, G. CoOx catalysts supported on alumina and alumina-baria: Influence of the support on the cobalt species and their activity in NO reduction by C3H6 in lean conditions. Appl. Catal. A Gen. 2003, 245, 167–177. [Google Scholar] [CrossRef]
- Park, S.; Kwak, G.; Lee, Y.; Jun, K.; Kim, Y.T. Effect of H2O on slurry-phase Fischer–Tropsch synthesis over alumina-supported cobalt catalysts. Bull. Korean Chem. Soc. 2018, 39, 540–547. [Google Scholar] [CrossRef]
- Park, J.; Yeo, S.; Kang, T.; Heo, I.; Lee, K.; Chang, T. Enhanced stability of Co catalysts supported on phosphorus-modified Al2O3 for dry reforming of CH4. Fuel 2018, 212, 77–87. [Google Scholar] [CrossRef]
- Cheng, J.; Yu, J.; Wang, X.; Li, L.; Li, J.; Hao, Z. Novel CH4 combustion catalysts derived from Cu-Co/X-Al (X = Fe, Mn, La, Ce) hydrotalcite-like compounds. Energy Fuels 2008, 22, 2131–2137. [Google Scholar] [CrossRef]
- El-Shobaky, H.G.; Shouman, M.A.; Attia, A.A. Effect of La2O3 and Mn2O3-doping of Co3O4/Al2O3 system on its surface and catalytic properties. Colloids Surf. A Physicochem. Eng. Asp. 2006, 274, 62–70. [Google Scholar] [CrossRef]
- Riad, M. Influence of magnesium and chromium oxides on the physicochemical properties of γ-alumina. Appl. Catal. A Gen. 2007, 327, 13–21. [Google Scholar] [CrossRef]
- Ulla, M.A.; Spretz, R.; Lombardo, E.; Daniell, W.; Knözinger, H. Catalytic combustion of methane on Co/MgO: Characterisation of active cobalt sites. Appl. Catal. B Environ. 2001, 29, 217–229. [Google Scholar] [CrossRef]
- Ji, S.; Ji, S.; Wang, H.; Flahaut, E.; Coleman, K.S.; Green, M.L.H. Catalytic combustion of methane over cobalt-magnesium oxide solid solution catalysts. Catal. Lett. 2001, 75, 65–71. [Google Scholar] [CrossRef]
- Trivedi, S.; Prasad, R. Selection of cobaltite and effect of preparation method of NiCo2O4 for catalytic oxidation of CO–CH4 mixture. Asia-Pac. J. Chem. Eng. 2017, 12, 440–453. [Google Scholar] [CrossRef]
- Lim, T.H.; Cho, S.J.; Yang, H.S.; Engelhard, M.H.; Kim, D.H. Effect of Co/Ni ratios in cobalt nickel mixed oxide catalysts on methane combustion. Appl. Catal. A Gen. 2015, 505, 62–69. [Google Scholar] [CrossRef] [Green Version]
- Dumond, F.; Marceau, E.; Che, M. A study of cobalt speciation in Co/Al2O3 catalysts prepared from solutions of cobalt-ethylenediamine complexes. J. Phys. Chem. C 2007, 111, 4780–4789. [Google Scholar] [CrossRef]
- Pérez-Ramírez, J.; Mul, G.; Kapteijn, F.; Moulijn, J.A. In situ investigation of the thermal decomposition of Co-Al hydrotalcite in different atmospheres. J. Mater. Chem. 2001, 11, 821–830. [Google Scholar] [CrossRef]
- Marco, J.F.; Gancedo, J.R.; Gracia, M.; Gautier, J.L.; Ríos, E.; Berry, F.J. Characterization of the nickel cobaltite, NiCo2O4, prepared by several methods: An XRD, XANES, EXAFS, and XPS study. J. Solid State Chem. 2000, 153, 74–81. [Google Scholar] [CrossRef]
- Lukashuk, L.; Yigit, N.; Rameshan, R.; Kolar, E.; Teschner, D.; Hävecker, M.; Knop-Gericke, A.; Schlögl, R.; Föttinger, K.; Rupprechter, G. Operando insights into CO oxidation on cobalt oxide catalysts by NAP-XPS, FTIR, and XRD. ACS Catal. 2018, 8, 8630–8641. [Google Scholar] [CrossRef] [PubMed]
- Dupin, J.; Gonbeau, D.; Vinatier, P.; Levasseur, A. Systematic XPS studies of metal oxides, hydroxides and peroxides. Phys. Chem. Chem. Phys. 2000, 2, 1319–1324. [Google Scholar] [CrossRef]
- Wu, L.; Jiao, D.; Wang, J.; Chen, L.; Cao, F. The role of MgO in the formation of surface active phases of CoMo/Al2O3-MgO catalysts for hydrodesulfurization of dibenzothiophene. Catal. Commun. 2009, 11, 302–305. [Google Scholar] [CrossRef]
- Cazzanelli, E.; Kuzmin, A.; Mariotto, G.; Mironova-Ulmane, N. Study of vibrational and magnetic excitations in NicMg1-cO solid solutions by Raman spectroscopy. J. Phys. Condens. Matter 2003, 15, 2045–2052. [Google Scholar] [CrossRef] [Green Version]
- Trivedi, S.; Prasad, R.; Gautam, S.K. Design of active NiCo2O4-δ spinel catalyst for abatement of CO-CH4 emissions from CNG fueled vehicles. AIChE J. 2018, 64, 2632–2646. [Google Scholar] [CrossRef]
- Pettiti, I.; Gazzoli, D.; Benito, P.; Fornasari, G.; Vaccari, A. The reducibility of highly stable Ni-containing species in catalysts derived from hydrotalcite-type precursors. RSC Adv. 2015, 5, 82282–82291. [Google Scholar] [CrossRef] [Green Version]
- Zasada, F.; Grybos, J.; Budiyanto, E.; Janas, J.; Sojka, Z. Oxygen species stabilized on the cobalt spinel nano-octahedra at various reaction conditions and their role in catalytic CO and CH4 oxidation, N2O decomposition and oxygen isotopic exchange. J. Catal. 2019, 371, 224–235. [Google Scholar] [CrossRef]
- Zasada, F.; Janas, J.; Piskorz, W.; Gorczynska, M.; Sojka, Z. Total oxidation of lean methane over cobalt spinel nanocubes controlled by the self-adjusted redox state of the catalyst: Experimental and theoretical account for interplay between the Langmuir-Hinshelwood and Mars-Van Krevelen mechanisms. ACS Catal. 2017, 7, 2853–2867. [Google Scholar] [CrossRef]
- Choya, A.; de Rivas, B.; González-Velasco, J.R.; Gutiérrez-Ortiz, J.I.; López-Fonseca, R. Oxidation of residual methane from VNG vehicles over Co3O4-based catalysts: Comparison among bulk, Al2O3-supported and Ce-doped catalysts. Appl. Catal. B Environ. 2018, 237, 844–854. [Google Scholar] [CrossRef]
- Zheng, Y.; Yu, Y.; Zhou, H.; Huang, W.; Pu, Z. Combustion of lean methane over Co3O4 catalysts prepared with different cobalt precursors. RSC Adv. 2020, 10, 4490–4498. [Google Scholar] [CrossRef] [Green Version]
- Liotta, L.F.; Di Carlo, G.; Pantaleo, G.; Deganello, G. Catalytic performance of Co3O4/CeO2 and Co3O4/CeO2–ZrO2 composite oxides for methane combustion: Influence of catalyst pretreatment temperature and oxygen concentration in the reaction mixture. Appl. Catal. B Environ. 2007, 70, 314–322. [Google Scholar] [CrossRef]
- Ji, Y.; Zhao, Z.; Duan, A.; Jiang, G.; Liu, J. Comparative study on the formation and reduction of bulk and Al2O3-supported cobalt oxides by H2-TPR technique. J. Phys. Chem. C 2009, 113, 7186–7199. [Google Scholar] [CrossRef]
- de Rivas, B.; Sampedro, C.; Ramos-Fernández, E.V.; López-Fonseca, R.; Gascon, J.; Makkee, M.; Gutiérrez-Ortiz, J.I. Influence of the synthesis route on the catalytic oxidation of 1,2-dichloroethane over CeO2/H-ZSM5 catalysts. Appl. Catal. A Gen. 2013, 456, 96–104. [Google Scholar] [CrossRef]
- Huang, Y.; Fan, W.; Long, B.; Li, H.; Qiu, W.; Zhao, F.; Tong, Y.; Ji, H. Alkali-modified non-precious metal 3D-NiCo2O4 nanosheets for efficient formaldehyde oxidation at low temperature. J. Mater. Chem. A 2016, 4, 3648–3654. [Google Scholar] [CrossRef]
- Genty, E.; Siffert, S.; Cousin, R. Investigation of reaction mechanism and kinetic modelling for the toluene total oxidation in presence of CoAlCe catalyst. Catal. Today 2019, 333, 28–35. [Google Scholar] [CrossRef]
- Bahlawane, N. Kinetics of methane combustion over CVD-made cobalt oxide catalysts. Appl. Catal. B Environ. 2006, 67, 168–176. [Google Scholar] [CrossRef]
- Stefanov, P.; Todorova, S.; Naydenov, A.; Tzaneva, B.; Kolev, H.; Atanasova, G.; Stoyanova, D.; Karakirova, Y.; Aleksieva, K. On the development of active and stable Pd-Co/γ-Al2O3 catalyst for complete oxidation of methane. Chem. Eng. J. 2015, 266, 329–338. [Google Scholar] [CrossRef]
- Paredes, J.R.; Díaz, E.; Díez, F.V.; Ordónez, S. Combustion of methane in lean mixtures over bulk transition-metal oxides: Evaluation of the activity and self-deactivation. Energy Fuels. 2009, 23, 86–93. [Google Scholar] [CrossRef]
- Choya, A.; de Rivas, B.; Gutiérrez-Ortiz, J.I.; López-Fonseca, R. Effect of residual Na+ on the combustion of methane over Co3O4 bulk catalysts prepared by precipitation. Catalysts 2018, 8, 427. [Google Scholar] [CrossRef] [Green Version]
- Choya, A.; de Rivas, B.; González-Velasco, J.R.; Gutiérrez-Ortiz, J.I.; López-Fonseca, R. Oxidation of lean methane over cobalt catalysts supported on ceria/alumina. Appl. Catal. A Gen. 2020, 591, 117381. [Google Scholar] [CrossRef]
- Choya, A.; de Rivas, B.; González-Velasco, J.R.; Gutiérrez-Ortiz, J.I.; López-Fonseca, R. On the beneficial effect of MgO promoter on the performance of Co3O4/Al2O3 catalysts for combustion of dilute methane. Appl. Catal. A Gen. 2019, 582, 117099. [Google Scholar] [CrossRef]
- EUROKIN Spreadsheet on Requirements for Measurement of Intrinsic Kinetics in the Gas-Solid Fixed-Bed Reactor. Available online: http://eurokin.org/ (accessed on 29 April 2020).
- Aranzabal, A.; González-Marcos, J.A.; Ayastuy, J.L.; González-Velasco, J.R. Kinetics of Pd/alumina catalysed 1,2-dichloroethane gas-phase oxidation. Chem. Eng. Sci. 2006, 61, 3564–3576. [Google Scholar] [CrossRef]











| Sample | Co Loading, wt % | Me Loading, wt % | Me/Co Molar Ratio | BET Surface, m2 g−1 | Pore Volume, cm3 g−1 | Cophase Crystallite Size, nm |
|---|---|---|---|---|---|---|
| Al2O3 | - | - | - | 139 | 0.56 | - |
| Mg-Al | - | 6.7 | - | 145 | 0.50 | - |
| Ce-Al | - | 20.6 | - | 117 | 0.42 | - |
| Co/Al | 27.9 | 0.0 | 0 | 108 | 0.29 | 29 |
| Co/Mg-Al | 28.7 | 3.0 | 0.25 | 99 | 0.27 | 23 |
| Co-Ni/Al | 23.2 | 4.8 | 0.21 | 113 | 0.35 | 21 |
| Co/Ce-Al | 29.5 | 12.4 | 0.18 | 93 | 0.30 | 23 |
| Catalyst | Co, wt % | Me, wt % | Co3+/Co2+ Molar Ratio | Oads/Olatt Molar Ratio |
|---|---|---|---|---|
| Co/Al | 22.6 (27.9) | - | 0.67 | 1.41 |
| Co/Mg-Al | 13.3 (28.7) | 4.4 (3.0) | 0.94 | 1.02 |
| Co-Ni/Al | 25.4 (23.2) | 8.2 (4.8) | 1.21 | 0.94 |
| Co/Ce-Al | 32.9 (29.5) | 3.2 (12.4) | 1.38 | 0.77 |
| H2-TPR | CH4-TPRe | |||
|---|---|---|---|---|
| Catalyst | Low-Temperature H2 Uptake, mmol gCo−1 | High-Temperature H2 Uptake, mmol gCo−1 | Onset Reduction Temperature, °C | Low-Temperature O2 Consumption, mmol gCo−1 |
| Co/Al | 9.8 | 10.2 | 225 | 0.28 |
| Co/Mg-Al | 10.3 | 10.4 | 195 | 0.61 |
| Co-Ni/Al | 12.5 | 12.9 | 175 | 0.83 |
| Co/Ce-Al | 13.6 | 8.5 | 220 | 0.88 |
| Catalyst | T50, °C | Specific Reaction Rate at 400 °C, mmol gCo−1 h−1 | Ea, kJ mol−1 |
|---|---|---|---|
| Co/Al | 550 | 1.2 | 82 ± 2 |
| Co/Mg-Al | 535 | 1.5 | 83 ± 2 |
| Co-Ni/Al | 500 | 2.5 | 84 ± 2 |
| Co/Ce-Al | 480 | 3.2 | 80 ± 2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choya, A.; de Rivas, B.; Gutiérrez-Ortiz, J.I.; López-Fonseca, R. Comparative Study of Strategies for Enhancing the Performance of Co3O4/Al2O3 Catalysts for Lean Methane Combustion. Catalysts 2020, 10, 757. https://doi.org/10.3390/catal10070757
Choya A, de Rivas B, Gutiérrez-Ortiz JI, López-Fonseca R. Comparative Study of Strategies for Enhancing the Performance of Co3O4/Al2O3 Catalysts for Lean Methane Combustion. Catalysts. 2020; 10(7):757. https://doi.org/10.3390/catal10070757
Chicago/Turabian StyleChoya, Andoni, Beatriz de Rivas, Jose Ignacio Gutiérrez-Ortiz, and Rubén López-Fonseca. 2020. "Comparative Study of Strategies for Enhancing the Performance of Co3O4/Al2O3 Catalysts for Lean Methane Combustion" Catalysts 10, no. 7: 757. https://doi.org/10.3390/catal10070757
APA StyleChoya, A., de Rivas, B., Gutiérrez-Ortiz, J. I., & López-Fonseca, R. (2020). Comparative Study of Strategies for Enhancing the Performance of Co3O4/Al2O3 Catalysts for Lean Methane Combustion. Catalysts, 10(7), 757. https://doi.org/10.3390/catal10070757