Enhanced Visible and Ultraviolet Light-Induced Gas-Phase Photocatalytic Activity of TiO2 Thin Films Modified by Increased Amount of Acetylacetone in Precursor Solution for Spray Pyrolysis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Material Characterization
2.2. Gas-Phase Photocatalytic Activity of the TiO2 Thin Films
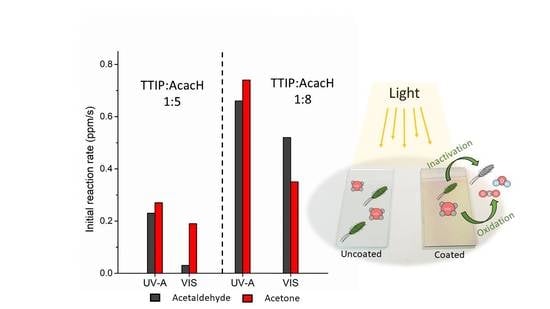
2.2.1. Photocatalytic Oxidation of Acetone and Acetaldehyde under Ultraviolet (UV-A) Light
2.2.2. Effect of Pollutant’s Initial Concentration on Its Photocatalytic Oxidation
2.2.3. Effect of Air Flow Rate and Relative Humidity
2.2.4. Photocatalytic Oxidation of Acetone and Acetaldehyde under Visible Light
2.2.5. Reusability of the Film
2.3. Antibacterial Activity
3. Materials and Methods
3.1. Preparation and Characterization of TiO2 Thin Films
3.2. Evaluation of Gas-Phase Photocatalytic Activity of the TiO2 Thin Films
3.3. Study of Antibacterial Activity of the TiO2 Thin Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jafari, M.J.; Khajevandi, A.A.; Najarkola, S.A.M.; Yekaninejad, M.S.; Pourhoseingholi, M.A.; Omidi, L.; Kalantary, S. Association of sick building syndrome with indoor air parameters. Tanaffos 2015, 14, 55–62. [Google Scholar]
- Hay, S.O.; Obee, T.; Luo, Z.; Jiang, T.; Meng, Y.; He, J.; Murphy, S.C.; Suib, S. The Viability of Photocatalysis for Air Purification. Molecules 2015, 20, 1319–1356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nath, R.K.; Zain, M.F.M.; Jamil, M. An environment-friendly solution for indoor air purification by using renewable photocatalysts in concrete: A. review. Renew. Sust. Energ. Rev. 2016, 62, 1184–1194. [Google Scholar] [CrossRef]
- Franklin, P.J. Indoor air quality and respiratory health of children. Paediatr. Respir. Rev. 2007, 8, 281–286. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Air pollution, Household Air Pollution: Pollutants. Available online: https://www.who.int/airpollution/household/pollutants/en/ (accessed on 10 December 2019).
- Dundar, I.; Krichevskaya, M.; Katerski, A.; Krunks, M.; Oja Acik, I. Photocatalytic degradation of different vocs in the gas-phase over tio2 thin films prepared by ultrasonic spray pyrolysis. Catalyst 2019, 9, 915. [Google Scholar] [CrossRef] [Green Version]
- Luga-Vega, C.S.; Moreira, J.; Serrano-Roseles, B.; de Lasa, H. Kinetics of the pollutant photocatalytic conversion in a Photo-CREC-Air Reactor. Chem. Eng. J. 2017, 317, 1069–1082. [Google Scholar] [CrossRef]
- Bianchi, C.L.; Gatto, S.; Pirola, C.; Naldoni, A.; Di Michele, A.; Cerrato, G.; Crocellà, V.; Capucci, V. Photocatalytic degradation of acetone, acetaldehyde and toluene in gas-phase: Comparison between nano and micro-sized TiO2. Appl. Catal. B-Environ. 2014, 146, 123–130. [Google Scholar] [CrossRef]
- Tomida, T.; Okada, N.; Katoh, M. Adsorption and photocatalytic decomposition of volatile organic compounds on photocatalyst of TiO2—Silica beads. Adsorption 2005, 11, 865–869. [Google Scholar] [CrossRef]
- Bianchi, C.L.; Pirola, C.; Stucchi, M.; Sacchi, B.; Cerrato, G.; Morandi, S.; Di Michele, A.; Carletti, A.; Capucci, V. A New Frontier of Photocatalysis Employing Micro-Sized Tio2: Air/Water Pollution Abatement and Self-Cleaning/Antibacterial Applications. In Semiconductor Photocatalysis–Materials, Mechanisms and Applications; Cao, W., Ed.; IntechOpen: London, UK, 2015. [Google Scholar]
- European Chemical Agency (ECHA), Substance Infocard, Acetaldehyde. Available online: https://echa.europa.eu (accessed on 20 April 2020).
- Exploratory Survey of Occupational Exposure Limits for Carcinogens, Mutagens and Reprotoxic Substances at EU Member States Level. In European Agency for Safety and Health at Work; European Risk Observatory Report. 2007. Available online: https://osha.europa.eu/en/publications (accessed on 2 September 2020).
- Augugliaro, V.; Loddo, V.; Pagliaro, M.; Giovanni, P.; Palmisano, L. Clean by Light Irradiation: Practical Applications of Supported TiO2. In Royal Society of Chemistry; RSC publishing: Cambridge, UK, 2010. [Google Scholar]
- Airscience Technology Co., Ltd., Air Steril. Available online: http://www.airpurifier.com.hk/product-en.htm (accessed on 13 March 2020).
- Son Le, T.; Hien Dao, T.; Cuong Nguyen, D.; Chau Nguyen, H.; Balikhin, I.L. Air purification equipment combining a filter coated by silver nanoparticles with a nano-TiO2 photocatalyst for use in hospitals. Adv. Nat. Sci. Nanosci. Nanotechnol. 2015, 6, 8. [Google Scholar] [CrossRef] [Green Version]
- Pant, B.; Park, M.; Park, S.J. Recent advances in tio2 films prepared by sol-gel methods for photocatalytic degradation of organic pollutants and antibacterial activities. Coatings 2019, 9, 613. [Google Scholar] [CrossRef] [Green Version]
- Dundar, I.; Krichevskaya, M.; Katerski, A.; Oja Acik, I. TiO2 thin films by ultrasonic spray pyrolysis as photocatalytic material for air purification. R. Soc. Open Sci. 2019, 6, 181578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saad, P.S.M.; Sutan, H.B.; Shariffudin, S.S.; Hashim, H.; Noor, U.M. TiO2 thin film via sol-gel method: Investigation on molarity effect. IOP Conf. Ser. Mater. Sci. Eng. 2015, 99, 012006. [Google Scholar] [CrossRef]
- Stefanov, B.I.; Niklasson, G.A.; Granqvist, G.G.; Österlund, L. Gas-phase photocatalytic activity of sputter-deposited anatase TiO2 films: Effect of <001> preferential orientation, surface temperature and humidity. J. Catal. 2016, 335, 187–196. [Google Scholar] [CrossRef] [Green Version]
- Al-Hajji, L.A.; Ismail, A.A. Photooxidation of acetaldehyde over TiO2 film: Impact of the substrate glassy structure on the photonic efficiency. Superlatt. Microst. 2019, 129, 259–267. [Google Scholar] [CrossRef]
- Antonello, A.; Soliveri, G.; Meroni, D.; Cappelletti, G.; Ardizzone, S. Photocatalytic remediation of indoor pollution by transparent TiO2 films. Catal. Today 2014, 230, 35–40. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, S.B.; Kim, G.S.; Jang, H.T.; Hong, S.C. Kinetic study on degradation of gaseous acetone over thin-film TiO2 photocatalyst in a continuous flow system. React. Kinet. Catal. Lett. 2007, 90, 85–91. [Google Scholar] [CrossRef]
- Liang, W.; Li, J.; He, H. Photo-Catalytic Degradation of Volatile Organic Compounds (VOCs) over Titanium Dioxide Thin Film, Advanced Aspects of Spectroscopy; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef] [Green Version]
- Simonsen, M.E.; Sorensen, M.B.; Sogaard, E.G. Comparison of methods for evaluation of activity of photocatalytic films. Environ. Sci. Pollut. Res. 2012, 19, 3772–3781. [Google Scholar] [CrossRef]
- Adachi, T.; Latthe, S.S.; Gosavi, S.W.; Roy, N.; Suzuki, N.; Ikari, H.; Kato, K.; Katsumata, L.; Nakata, K.; Furudate, M.; et al. Photocatalytic, superhydrophilic, self-cleaning TiO2 coating on cheap, light-weight, flexible polycarbonate substrates. Appl. Surf. Sci. 2018, 458, 917–923. [Google Scholar] [CrossRef]
- Spiridonova, J.; Katerski, A.; Danilson, M.; Krichevskaya, M.; Krunks, M.; Oja Acik, I. Effect of the titanium isopropoxide:acetylacetone molar ratio on the photocatalytic activity of TiO2 thin films. Molecules 2019, 24, 4326. [Google Scholar] [CrossRef] [Green Version]
- Yao, N.; Yeung, K.L. Investigation of the performance of TiO2 photocatalytic coatings. Chem. Eng. J. 2011, 167, 13–21. [Google Scholar] [CrossRef]
- Abidi, M.; Assadi, A.A.; Bouzaza, A.; Hajjaji, A.; Bessais, B.; Rtimi, S. Photocatalytic indoor/outdoor air treatment and bacterial inactivation on CuxO/TiO2 prepared by HiPIMS on polyester cloth under low intensity visible light. Appl. Catal. B-Environ. 2019, 259, 118074. [Google Scholar] [CrossRef]
- Nakano, R.; Hara, M.; Ishiguri, H.; Yao, Y.; Ochiai, T.; Nakata, K.; Murakami, T.; Kajioka, J.; Sunada, K.; Hashimoto, K.; et al. Broad spectrum microbicidal activity of photocatalysis by TiO2. Catalyst 2013, 3, 310–323. [Google Scholar] [CrossRef] [Green Version]
- De Falco, G.; Porta, A.; Petrone, A.M.; Del Gaudio, P.; El Hassanin, A.; Commodo, M.; Minutolo, P.; Squillaceband, A.; D’Anna, A. Antimicrobial activity of flame-synthesized nano-TiO2 coatings. Environ. Sci. Nano 2017, 4, 1095. [Google Scholar] [CrossRef]
- Peerakiatkhajorn, P.; Chawengkijwanich, C.; Onreabroy, W.; Chiarakorn, S. Novel Photocatalytic Ag/TiO2 Thin Film on Polyvinyl Chloride for gaseous BTEX Treatment. Mater. Sci. Forum 2012, 712, 133–145. [Google Scholar] [CrossRef]
- Garlisi, C.; Scandura, G.; Alabi, A.; Aderemi, A.; Palmosano, G. Self-Cleaning Coatings Activated by Solar and Visible Radiation. J. Adv. Chem. Eng. 2015, 5, 3. [Google Scholar] [CrossRef] [Green Version]
- Raza, N.; Kim, K.-H.; Agbe, H.; Kailase, S.K.; Szulejko, J.E.; Brown, R.J.C. Recent advances in titania-based composites for photocatalytic degradation of indoor volatile organic compounds. Asian J. Atmos. Environ. 2017, 11, 217–234. [Google Scholar] [CrossRef]
- Di Valentin, C.; Pacchioni, G.; Selloni, A. Theory of carbon doping of titanium dioxide. Chem. Mater. 2015, 17, 6656–6665. [Google Scholar] [CrossRef]
- Klauson, D.; Portjanskaya, E.; Budarnaja, O.; Krichevskaya, M.; Preis, S. The synthesis of sulphur and boron-containing titania photocatalysts and the evaluation of their photocatalytic activity. Catal. Commun. 2010, 11, 715–720. [Google Scholar] [CrossRef]
- Ratova, M.; Klaysri, R.; Praserthdam, P.; Kelly, P.J. Visible light active photocatalytic C-doped titanium dioxide films deposited via reactive pulsed DC magnetron co-sputtering: Properties and photocatalytic activity. Vacuum 2018, 149, 214–224. [Google Scholar] [CrossRef]
- Rasoulnezhad, H.; Kavei, G.; Ahmadi, K.; Rahimipour, M.R. Visible light photocatalytic degradation of paraoxon and parathion pesticides on carbon-doped TiO2 nanorod thin films. J. Mater. Sci. Mater. Electron. 2017, 28, 18337–18347. [Google Scholar] [CrossRef]
- Park, Y.; Kim, W.; Park, H.; Tachikawa, T.; Majima, T.; Choi, W. Carbon-doped TiO2 photocatalyst synthesized without using an external carbon precursor and the visible light activity. Appl. Catal. B Environ. 2009, 91, 355–361. [Google Scholar] [CrossRef]
- Oja, I.; Mere, A.; Krunks, M.; Solterbeck, C.-H.; Es-Souni, M. Properties of TiO2 films prepared by the spray pyrolysis method. Solid State Phenom. 2004, 99–100, 259–262. [Google Scholar] [CrossRef]
- Krunks, M.; Dedova, T.; Oja Acik, I. Spray pyrolysis deposition of zinc oxide nanostructured layers. Thin Solid Film. 2006, 515, 1157–1160. [Google Scholar] [CrossRef]
- Juma, A.O.; Acik, O.I.; Mikli, V.; Mere, A.; Krunks, M. Effect of solution composition on anatase to rutile transformation of sprayed TiO2 thin films. Thin Solid Film. 2015, 594, 287–292. [Google Scholar] [CrossRef]
- Kaltsum, U.; Kurniawan, A.F.; Nurhasanah, I.; Priyono, P. The role of concentration ratio of ttip:acac on the photocatalytic activity of Tio2 thin film in reducing degradation products of used frying oil. Bull. Chem. React. Eng. Catal. 2017, 12, 430–436. [Google Scholar] [CrossRef] [Green Version]
- Duminica, F.D.; Maury, F.; Abisset, S. Pyrosol deposition of anatase TiO2 thin films starting from Ti(OiPr)4/acetylacetone solutions. Thin Solid Film. 2007, 515, 7732–7739. [Google Scholar] [CrossRef] [Green Version]
- Krunks, M.; Oja, I.; Tonsuaadu, K.; Es-Souni, M.; Gruselle, M.; Niinisto, L. Thermoanalytical study of acetylacetonate-modified titanium(IV) isopropoxide as a precursor for TiO2 films. J. Therm. Anal. Calorim. 2005, 80, 483–488. [Google Scholar] [CrossRef]
- Oja Acik, I.; Madarasz, J.; Krunks, M.; Tõnsuaadu, K.; Janke, D.; Pokol, G.; Niinistö, L. Thermoanalytical studies of titanium(IV) acetylacetonate xerogels with emphasis on evolved gas analysis. J. Therm. Anal. Calorim. 2007, 88, 557–563. [Google Scholar] [CrossRef]
- Ansari, S.A.; Cho, M.H. Highly visible light responsive, narrow band gap TiO2 nanoparticles modified by elemental red phosphorus for photocatalysis and photoelectrochemical applications. Sci. Rep. 2016, 6, 25405. [Google Scholar] [CrossRef]
- Yaghoubi, H.; Li, Z.; Chen, Y.; Ngo, H.T.; Bhethanabotla, V.R.; Joseph, B.; Ma, S.; Schlaf, R.; Takshi, A. Toward a visible light-driven photocatalyst: The effect of midgap-states-induced energy gap of undoped TiO2 nanoparticles. Am. Chem. Soc. 2015, 5, 327–335. [Google Scholar] [CrossRef] [Green Version]
- Demeestere, K.; Dewulf, J.; Van Langenhove, H. Heterogeneous photocatalysis as an advanced oxidation process for the abatement of chlorinated, monocyclic aromatic and sulfurous volatile organic compounds in air: State of the art. Crit. Rev. Environ. Sci. Tec. 2007, 37, 489–538. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, X.; Terashima, C.; Fujishima, A.; Nakata, K. Thermodynamic and kinetic analysis of heterogeneous photocatalysis for semiconductor systems. Phys. Chem. Chem. Phys. 2014, 16, 8751–8760. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.A.; Huie, R.E.; Koppenol, W.H.; Lymar, S.V.; Merenyi, G.; Neta, P.; Ruscic, B.; Stanbury, D.M.; Steenken, S.; Wardman, P. Standard electrode potentials involving radicals in aqueous solution: Inorganic radicals. IUPAC Tech. Rep. 2016, 87, 1365–3075. [Google Scholar] [CrossRef] [Green Version]
- Yao, M.; Ji, Y.; Wang, H.; Ao, Z.; Li, G.; An, T. Adsorption mechanisms of typical carbonyl-containing volatile organic compounds on anatase TiO2 (001) surface: A DFT investigation. J. Phys. Chem. C 2017, 121, 13717–13722. [Google Scholar] [CrossRef]
- Li, L.; Sun, Z.; Li, H.; Keener, T.C. Effects of activated carbon surface properties on the adsorption of volatile organic compounds. J. Air Waste Manag. 2012, 62, 1196–1202. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.P.; Chen, J.N.; Lu, M.C. Heterogeneous photocatalytic oxidation of acetone for air purification by near uv-irradiated titanium dioxide. J. Environ. Sci. Health 2003, 38, 1131–1143. [Google Scholar] [CrossRef]
- Aisien, F.A.; Amenaghawon, N.A.; Urhobotie, O.I. Potential application of a locally sourced photocatalyst for the photocatalytic decolourisation of methyl orange in aqueous solution. J. Eng. Sci. Technol. 2015, 10, 1641–1653. [Google Scholar]
- Maudhuit, A.; Raillard, C.; Hequet, V.; Le Coq, L.; Sablayrolles, J.; Molins, L. Adsorption phenomena in photocatalytic reactions: The case of toluene, acetone and heptane. Chem. Eng. J. 2011, 170, 464–470. [Google Scholar] [CrossRef]
- Han, Y.; Kanno, H.; Ahn, Y.J.; Shikazono, N. Measurement of liquid film thickness in micro tube annular flow. Int. J. Multiph. Flow 2015, 72, 264–274. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.B.; Hwang, H.T.; Hong, S.C. Photocatalytic degradation of volatile organic compounds at the gas–solid interface of a TiO2 photocatalyst. Chemosphere 2002, 48, 437–444. [Google Scholar] [CrossRef]
- Seo, H.O.; Park, E.J.; Kim, I.H.; Han, S.W.; Cha, B.J.; Wo, T.G.; Kim, Y.D. Influence of humidity on the photo-catalytic degradation ofacetaldehyde over TiO2 surface under UV light irradiation. Catal. Today 2017, 295, 102–109. [Google Scholar] [CrossRef]
- Visnapuu, M.; Rosenberg, M.; Truska, E.; Nõmmiste, E.; Šutka, A.; Kahru, A.; Rähn, M.; Vija, H.; Orupõld, K.; Kisand, V.; et al. UVA-induced antimicrobial activity of ZnO/Ag nanocomposite covered surfaces. Colloids Surf. B 2018, 169, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Maness, P.C.; Blake, D.M.; Wolfrum, E.J.; Smolinski, S.L.; Jacoby, W.A. Bactericidal mode of titanium dioxide photocatalysis. J. Photochem. Photobiol. A 2000, 130, 16–170. [Google Scholar] [CrossRef]
- Jacoby, W.A.; Maness, P.C.; Wolfrum, E.J.; Blake, D.M.; Fennell, J.A. Mineralization of bacterial cell mass on a photocatalytic surface in air. Environ. Sci. Technol. 1998, 32, 2650–2653. [Google Scholar] [CrossRef]
- Kim, D.S.; Kwak, S.J. Photocatalytic inactivation of E. coli with a mesoporous TiO2 coated film using the film adhesion method. Environ. Sci. Technol. 2009, 43, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Kask, M.; Bolobajev, J.; Krichevskaya, M. Gas-phase photocatalytic degradation of acetone and toluene, and their mixture in the presence of ozone in continuous multi-section reactor as possible air post-treatment for exhaust from pulsed corona discharge. Chem. Eng. J. 2020, 399, 125815. [Google Scholar] [CrossRef]
- Hu, Z.; Tan, K.; Lustig, W.P.; Wang, H.; Zhao, Y.; Zheng, C.; Banerjee, D.J.; Emge, T.J.; Chabal, V.; Li, J. Effective sensing of RDX via instant and selective detection of ketone vapors. Chem. Sci. 2014, 5, 4873. [Google Scholar] [CrossRef]
- Tsuji, M.; Miyano, M.; Kamo, N.; Kawahara, T.; Uto, K.; Hayashi, J.; Tsuji, T. Photochemical removal of acetaldehyde using 172 nm vacuum ultraviolet excimer lamp in N2 or air at atmospheric pressure. Environ. Sci. Pollut. Res. 2019, 26, 11324–11325. [Google Scholar] [CrossRef]











| TTIP:AcacH in Precursor Solution | kr ppm/s | K 1/ppm |
|---|---|---|
| 1:5 | 0.728 | 0.0452 |
| 1:8 | 2.395 | 0.0225 |
| Residence Time, s | Catalyst Surface Area, cm2 | Air Flow, L/min | Conversion, % | |||
|---|---|---|---|---|---|---|
| Acetone | Acetaldehyde | |||||
| 40 ppm | 10 ppm | 40 ppm | 10 ppm | |||
| 15.6 | 120 | 0.5 | 46 | 67 | 75 | 68 |
| 240 | 1 | 27 | 71 | 79 | 100 | |
| 31.2 | 240 | 0.5 | 87 | 100 | 100 | 100 |
| 420 | 1 | 77 | 100 | 100 | 100 | |
| Air Flow Rate, L/min | Concentration, ppm | Relative Humidity (RH), % | Acetone | Acetaldehyde | ||
|---|---|---|---|---|---|---|
| Initial Reaction Rate | Initial Reaction Rate | |||||
| ppm/s | ppm/(s cm2) | ppm/s | ppm/(s cm2) | |||
| 0.5 | 40 | 6 | 1.70 | 0.0142 | 2.01 | 0.0167 |
| 1 | 40 | 6 | 1.59 | 0.0132 | 2.38 | 0.0199 |
| 0.5 | 10 | 6 | 0.74 | 0.0061 | 0.66 | 0.0055 |
| 1 | 10 | 6 | 0.86 | 0.0074 | 1.17 | 0.0084 |
| 0.5 | 10 | 40 | 0.20 | 0.0016 | 0.60 | 0.0050 |
| 1 | 10 | 40 | 0.37 | 0.0031 | 0.72 | 0.0060 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spiridonova, J.; Mere, A.; Krunks, M.; Rosenberg, M.; Kahru, A.; Danilson, M.; Krichevskaya, M.; Oja Acik, I. Enhanced Visible and Ultraviolet Light-Induced Gas-Phase Photocatalytic Activity of TiO2 Thin Films Modified by Increased Amount of Acetylacetone in Precursor Solution for Spray Pyrolysis. Catalysts 2020, 10, 1011. https://doi.org/10.3390/catal10091011
Spiridonova J, Mere A, Krunks M, Rosenberg M, Kahru A, Danilson M, Krichevskaya M, Oja Acik I. Enhanced Visible and Ultraviolet Light-Induced Gas-Phase Photocatalytic Activity of TiO2 Thin Films Modified by Increased Amount of Acetylacetone in Precursor Solution for Spray Pyrolysis. Catalysts. 2020; 10(9):1011. https://doi.org/10.3390/catal10091011
Chicago/Turabian StyleSpiridonova, Jekaterina, Arvo Mere, Malle Krunks, Merilin Rosenberg, Anne Kahru, Mati Danilson, Marina Krichevskaya, and Ilona Oja Acik. 2020. "Enhanced Visible and Ultraviolet Light-Induced Gas-Phase Photocatalytic Activity of TiO2 Thin Films Modified by Increased Amount of Acetylacetone in Precursor Solution for Spray Pyrolysis" Catalysts 10, no. 9: 1011. https://doi.org/10.3390/catal10091011
APA StyleSpiridonova, J., Mere, A., Krunks, M., Rosenberg, M., Kahru, A., Danilson, M., Krichevskaya, M., & Oja Acik, I. (2020). Enhanced Visible and Ultraviolet Light-Induced Gas-Phase Photocatalytic Activity of TiO2 Thin Films Modified by Increased Amount of Acetylacetone in Precursor Solution for Spray Pyrolysis. Catalysts, 10(9), 1011. https://doi.org/10.3390/catal10091011