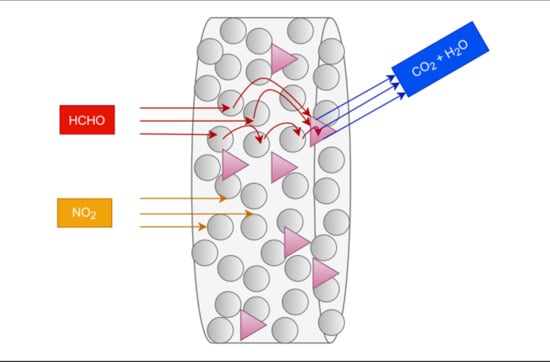
Novel Materials for Combined Nitrogen Dioxide and Formaldehyde Pollution Control under Ambient Conditions
Abstract
:1. Introduction
1.1. Formaldehyde Emissions and Health Effects
1.2. Nitrogen Dioxide Emissions and Health Effects
1.3. Problems with the Current State-of-the-Art Pollution Control Methods
1.4. Scope of This Article
2. Results and Discussion
2.1. HCHO Removal
2.1.1. Adsorbents
2.1.2. Catalysts
2.1.3. Composites
2.2. Repeat HCHO Removal Tests
2.3. NO2 Removal
2.3.1. Adsorbents
2.3.2. Composites
2.3.3. Catalysts
2.4. Combined NO2 and HCHO Removal
3. Materials and Methods.
3.1. Materials
3.2. Characterization
3.3. Experimental Setup and Procedure
3.3.1. Single-Pass
3.3.2. Chamber
3.4. Material Effectiveness
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Household Air Pollution and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/household-air-pollution-and-health (accessed on 11 August 2019).
- Lelieveld, J.; Pozzer, A.; Pöschl, U.; Fnais, M.; Haines, A.; Münzel, T. Loss of life expectancy from air pollution compared to other risk factors: A worldwide perspective. Cardiovasc. Res. 2020. [Google Scholar] [CrossRef]
- Hodgson, A.; Beal, D.; McIlvaine, J.E.R. Sources of formaldehyde, other aldehydes and terpenes in a new manufactured house. Indoor Air 2002, 12, 235–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonald, B.C.; De Gouw, J.; Gilman, J.B.; Jathar, S.H.; Akherati, A.; Cappa, C.D.; Jimenez, J.L.; Lee-Taylor, J.; Hayes, P.L.; McKeen, S.A.; et al. Volatile chemical products emerging as largest petrochemical source of urban organic emissions. Science 2018, 359, 760–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salthammer, T.; Mentese, S.; Marutzky, R. Formaldehyde in the indoor environment. Chem. Rev. 2010, 110, 2536–2572. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhao, Y.; Zhao, B. Emission rates of multiple air pollutants generated from Chinese residential cooking. Environ. Sci. Technol. 2018, 52, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Spiru, P.; Simona, P.L. A review on interactions between energy performance of the buildings, outdoor air pollution and the indoor air quality. Energy Procedia 2017, 128, 179–186. [Google Scholar] [CrossRef]
- Yoshida, T.; Matsunaga, I. A case study on identification of airborne organic compounds and time courses of their concentrations in the cabin of a new car for private use. Environ. Int. 2006, 32, 58–79. [Google Scholar] [CrossRef]
- Yoshida, T.; Matsunaga, I.; Tomioka, K.; Kumagai, S. Interior air pollution in automotive cabins by volatile organic compounds diffusing from interior materials: II. Influence of manufacturer, specifications and usage status on air pollution, and estimation of air pollution levels in initial phases of delivery as a new car. Indoor Built Environ. 2006, 15, 445–462. [Google Scholar] [CrossRef]
- Schupp, T.; Bolt, H.; Hengstler, J.G. Maximum exposure levels for xylene, formaldehyde and acetaldehyde in cars. Toxicology 2005, 206, 461–470. [Google Scholar] [CrossRef]
- Zhang, G.-S.; Li, T.; Luo, M.; Liu, J.-F.; Liu, Z.-R.; Bai, Y.-H. Air pollution in the microenvironment of parked new cars. Build. Environ. 2008, 43, 315–319. [Google Scholar] [CrossRef]
- Huang, S.; Wei, W.; Weschler, L.B.; Salthammer, T.; Kan, H.; Bu, Z.; Zhang, Y. Indoor formaldehyde concentrations in urban China: Preliminary study of some important influencing factors. Sci. Total. Environ. 2017, 590, 394–405. [Google Scholar] [CrossRef]
- Salthammer, T. Formaldehyde in the ambient atmosphere: From an indoor pollutant to an outdoor pollutant? Angew. Chem. Int. Ed. 2013, 52, 3320–3327. [Google Scholar] [CrossRef]
- Nielsen, G.D.; Larsen, S.T.; Wolkoff5P. Re-evaluation of the WHO (2010) formaldehyde indoor air quality guideline for cancer risk assessment. Arch. Toxicol. 2016, 91, 35–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwilk, E.; Zhang, L.; Smith, M.T.; Smith, A.H.; Steinmaus, C. Formaldehyde and leukemia: An updated meta-analysis and evaluation of bias. J. Occup. Environ. Med. 2010, 52, 878–886. [Google Scholar] [CrossRef] [PubMed]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans Formaldehyde, 2-butoxyethanol and 1-tert-butoxypropan-2-ol. IARC Monogr Eval Carcinog Risks Hum 2006, 88, 1–478.
- Zhang, X.; Zhao, Y.; Song, J.; Yang, X.; Zhang, J.; Zhang, Y.; Li, R. differential health effects of constant versus intermittent exposure to formaldehyde in mice: Implications for building ventilation strategies. Environ. Sci. Technol. 2018, 52, 1551–1560. [Google Scholar] [CrossRef]
- IPCC in Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Ed.; Cambridge University Press: New York, NY, USA, 2013. [Google Scholar]
- Cofala, J.; Amann, M.; Klimont, Z.; Kupiainen, K.; Höglund-Isaksson, L. Scenarios of global anthropogenic emissions of air pollutants and methane until 2030. Atmos. Environ. 2007, 41, 8486–8499. [Google Scholar] [CrossRef]
- Dennekamp, M.; Howarth, S.; Dick, C.A.J.; Cherrie, J.; Donaldson, K.; Seaton, A. Ultrafine particles and nitrogen oxides generated by gas and electric cooking. Occup. Environ. Med. 2001, 58, 511–516. [Google Scholar] [CrossRef] [Green Version]
- Logue, J.M.; Klepeis, N.E.; Lobscheid, A.B.; Singer, B.C. Pollutant exposures from natural gas cooking burners: A simulation-based assessment for Southern California. Environ. Heal. Perspect. 2014, 122, 43–50. [Google Scholar] [CrossRef]
- Żak, M.; Melaniuk-Wolny, E.; Widziewicz-Rzońca, K. The exposure of pedestrians, drivers and road transport passengers to nitrogen dioxide. Atmos. Pollut. Res. 2017, 8, 781–790. [Google Scholar] [CrossRef]
- Son, B.; Yang, W.; Breysse, P.; Chung, T.; Lee, Y. Estimation of occupational and nonoccupational nitrogen dioxide exposure for Korean taxi drivers using a microenvironmental model. Environ. Res. 2004, 94, 291–296. [Google Scholar] [CrossRef]
- Lewné, M.; Nise, G.; Lind, M.-L.; Gustavsson, P. Exposure to particles and nitrogen dioxide among taxi, bus and lorry drivers. Int. Arch. Occup. Environ. Heal. 2005, 79, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Samoli, E.; Aga, E.; Touloumi, G.; Nisiotis, K.; Forsberg, B.; Lefranc, A.; Pekkanen, J.; Wojtyniak, B.; Schindler, C.; Niciu, E.; et al. Short-term effects of nitrogen dioxide on mortality: An analysis within the APHEA project. Eur. Respir. J. 2006, 27, 1129–1138. [Google Scholar] [CrossRef] [PubMed]
- WHO Air Quality Guidelines: Global Update 2005: Particulate Matter, Ozone, Nitrogen Dioxide, and Sulfur Dioxide; World Health Organization: Copenhagen, Denmark, 2006; ISBN 92-890-2192-6.
- Bernabe, D.; Herrera, R.A.S.; Doma, B.; Fu, M.-L.; Dong, Y.; Wang, Y.-F. Adsorption of low concentration formaldehyde in air using ethylene-diamine-modified diatomaceous earth. Aerosol Air Qual. Res. 2015, 15, 1652–1661. [Google Scholar] [CrossRef] [Green Version]
- Pei, J.; Zhang, J.S. Critical review of catalytic oxidization and chemisorption methods for indoor formaldehyde removal. Hvac&R Res. 2011, 17, 476–503. [Google Scholar]
- Peng, J.; Wang, S. Performance and characterization of supported metal catalysts for complete oxidation of formaldehyde at low temperatures. Appl. Catal. B Environ. 2007, 73, 282–291. [Google Scholar] [CrossRef]
- Na, C.-J.; Yoo, M.-J.; Tsang, D.C.; Kim, H.W.; Kim, K.-H. High-performance materials for effective sorptive removal of formaldehyde in air. J. Hazard. Mater. 2019, 366, 452–465. [Google Scholar] [CrossRef]
- Pei, J.; Zhang, J.S. On the performance and mechanisms of formaldehyde removal by chemi-sorbents. Chem. Eng. J. 2011, 167, 59–66. [Google Scholar] [CrossRef]
- Wang, Q.; Zhong, Z. (Eds.) Functional catalysts for catalytic removal of formaldehyde from air. In Environmental Functional Nanomaterials; De Gruyter: Berlin, Germany, 2019; pp. 89–126. ISBN 978-3-11-054418-3. [Google Scholar]
- Gandolfo, A.; Marque, S.; Temime-Roussel, B.; Gemayel, R.; Wortham, H.; Truffier-Boutry, D.; Bartolomei, V.; Gligorovski, S. Unexpectedly high levels of organic compounds released by indoor photocatalytic paints. Environ. Sci. Technol. 2018, 52, 11328–11337. [Google Scholar] [CrossRef]
- Sidheswaran, M.; Chen, W.; Chang, A.; Miller, R.; Cohn, S.; Sullivan, D.; Fisk, W.J.; Kumagai, K.; Destaillats, H. Formaldehyde emissions from ventilation filters under different relative humidity conditions. Environ. Sci. Technol. 2013, 47, 5336–5343. [Google Scholar] [CrossRef]
- Jeguirim, M.; Belhachemi, M.; Limousy, L.; Bennici, S. Adsorption/reduction of nitrogen dioxide on activated carbons: Textural properties versus surface chemistry–A review. Chem. Eng. J. 2018, 347, 493–504. [Google Scholar] [CrossRef]
- Chen, C.; Li, J.; Tan, X.; Wang, X. Comparative study of graphene oxide, activated carbon and carbon nanotubes as adsorbents for copper decontamination. Dalton Trans. 2013, 42, 5266. [Google Scholar] [CrossRef]
- Rong, H.; Liu, Z.; Wu, Q.; Pan, D.; Zheng, J. Formaldehyde removal by Rayon-based activated carbon fibers modified by P-aminobenzoic acid. Cellulose 2009, 17, 205–214. [Google Scholar] [CrossRef]
- Ma, C.; Li, X.; Zhu, T. Removal of low-concentration formaldehyde in air by adsorption on activated carbon modified by hexamethylene diamine. Carbon 2011, 49, 2873–2875. [Google Scholar] [CrossRef]
- Dunne, E.; Galbally, I.E.; Cheng, M.; Selleck, P.; Molloy, S.B.; Lawson, S.J. Comparison of VOC measurements made by PTR-MS, adsorbent tubes–GC-FID-MS and DNPH derivatization–HPLC during the Sydney Particle Study, 2012: A contribution to the assessment of uncertainty in routine atmospheric VOC measurements. Atmos. Meas. Tech. 2018, 11, 141–159. [Google Scholar] [CrossRef] [Green Version]
- Cui, L.; Zhang, Z.; Huang, Y.; Lee, S.; Blake, D.R.; Ho, K.F.; Wang, B.; Gao, Y.; Wang, X.; Louie, P.K.K. Measuring OVOCs and VOCs by PTR-MS in an urban roadside microenvironment of Hong Kong: Relative humidity and temperature dependence, and field intercomparisons. Atmos. Meas. Tech. 2016, 9, 5763–5779. [Google Scholar] [CrossRef] [Green Version]
- Yoo, J.Y.; Park, C.J.; Kim, K.Y.; Son, Y.-S.; Kang, C.-M.; Wolfson, J.M.; Jung, I.-H.; Lee, S.-J.; Koutrakis, P. Development of an activated carbon filter to remove NO 2 and HONO in indoor air. J. Hazard. Mater. 2015, 289, 184–189. [Google Scholar] [CrossRef]
- Henning, K.-D.; Schäfer, S. Impregnated activated carbon for environmental protection. Gas Sep. Purif. 1993, 7, 235–240. [Google Scholar] [CrossRef]
- Xu, Q.; Lei, W.; Li, X.; Qi, X.; Yu, J.; Liu, G.; Wang, J.; Zhang, P. Efficient removal of formaldehyde by nanosized gold on well-defined CeO2 nanorods at room temperature. Environ. Sci. Technol. 2014, 48, 9702–9708. [Google Scholar] [CrossRef]
- Liu, B.; Li, C.; Zhang, Y.; Liu, Y.; Hu, W.; Wang, Q.; Han, L.; Zhang, J. Investigation of catalytic mechanism of formaldehyde oxidation over three-dimensionally ordered macroporous Au/CeO2 catalyst. Appl. Catal. B Environ. 2012, 111, 467–475. [Google Scholar] [CrossRef]
- Li, H.-F.; Zhang, N.; Chen, P.; Luo, M.-F.; Lu, J.-Q. High surface area Au/CeO2 catalysts for low temperature formaldehyde oxidation. Appl. Catal. B Environ. 2011, 110, 279–285. [Google Scholar] [CrossRef]
- Jia, M.; Shen, Y.; Li, C.; Bao, Z.; Sheng, S. Effect of supports on the gold catalyst activity for catalytic combustion of CO and HCHO. Catal. Lett. 2005, 99, 235–239. [Google Scholar] [CrossRef]
- Suresh, S.; Bandosz, T.J. Removal of formaldehyde on carbon based materials: A review of the recent approaches and findings. Carbon 2018, 137, 207–221. [Google Scholar] [CrossRef]
- Sidheswaran, M.A.; Destaillats, H.; Sullivan, D.P.; Larsen, J.; Fisk, W.J. Quantitative room-temperature mineralization of airborne formaldehyde using manganese oxide catalysts. Appl. Catal. B Environ. 2011, 107, 34–41. [Google Scholar] [CrossRef] [Green Version]
- Sekine, Y. Oxidative decomposition of formaldehyde by metal oxides at room temperature. Atmos. Environ. 2002, 36, 5543–5547. [Google Scholar] [CrossRef]
- Liu, F.; Cao, R.; Rong, S.; Zhang, P. Tungsten doped manganese dioxide for efficient removal of gaseous formaldehyde at ambient temperatures. Mater. Des. 2018, 149, 165–172. [Google Scholar] [CrossRef]
- Li, J.-J.; Yu, E.-Q.; Cai, S.-C.; Chen, X.; Chen, J.; Jia, H.; Xu, Y.-J. Noble metal free, CeO2/LaMnO3 hybrid achieving efficient photo-thermal catalytic decomposition of volatile organic compounds under IR light. Appl. Catal. B Environ. 2019, 240, 141–152. [Google Scholar] [CrossRef]
- Scirè, S.; Fiorenza, R.; Gulino, A.; Cristaldi, A.; Riccobene, P.M. Selective oxidation of CO in H2-rich stream over ZSM5 zeolites supported Ru catalysts: An investigation on the role of the support and the Ru particle size. Appl. Catal. A Gen. 2016, 520, 82–91. [Google Scholar] [CrossRef]
- Genty, E.; Brunet, J.; Poupin, C.; Casale, S.; Capelle, S.; Massiani, P.; Siffert, S.; Cousin, R. Co-Al mixed oxides prepared via LDH route using microwaves or ultrasound: Application for catalytic toluene total oxidation. Catalysts 2015, 5, 851–867. [Google Scholar] [CrossRef] [Green Version]
- Han, K.H.; Zhang, J.S.; Guo, B. Toward effective design and adoption of catalyst-based filter for indoor hazards: Formaldehyde abatement under realistic conditions. J. Hazard. Mater. 2017, 331, 161–170. [Google Scholar] [CrossRef]
- Rengga, W.D.P.; Sudibandriyo, M.; Nasikin, M. Adsorptive removal of formaldehyde by chemically bamboo activated carbon with addition of Ag nanoparticle: Equilibrium and kinetic. MATEC Web Conf. 2016, 59, 4004. [Google Scholar] [CrossRef] [Green Version]
- Gorzkowska-Sobas, A.A.; Bjørgo, K.M. Adsorption Performance of Activated Carbon Towards Toxic Industrial Chemicals; Norwegian Defence Research Establishment (FFI): Kjeller, Norway, 2015; p. 59. [Google Scholar]
- Cortés-Arriagada, D.; Villegas-Escobar, N.; Miranda-Rojas, S.; Toro-Labbé, A. Adsorption/desorption process of formaldehyde onto iron doped graphene: A theoretical exploration from density functional theory calculations. Phys. Chem. Chem. Phys. 2017, 19, 4179–4189. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Baur, G.B.; Spring, J.; Kiwi-Minsker, L. Amine functionalized activated carbon fibers as effective structured adsorbents for formaldehyde removal. Adsorption 2018, 24, 725–732. [Google Scholar] [CrossRef]
- Deliyanni, E.; Bandosz, T.J. Effect of carbon surface modification with dimethylamine on reactive adsorption of NOx. Langmuir 2011, 27, 1837–1843. [Google Scholar] [CrossRef]
- Peterson, G.W.; Mahle, J.J.; Decoste, J.B.; Gordon, W.O.; Rossin, J.A. Extraordinary NO2 Removal by the metal-organic framework UiO-66-NH2. Angew. Chem. Int. Ed. 2016, 55, 6235–6238. [Google Scholar] [CrossRef]
- Schultz, L.; Andersson, M.P.; Dalby, K.N.; Müter, D.; Okhrimenko, D.V.; Fordsmand, H.; Stipp, S.L.S. High surface area calcite. J. Cryst. Growth 2013, 371, 34–38. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]




| Abbreviated Name | Material Description | Form | Synthesis Method/Company |
|---|---|---|---|
| CAC | AC beads | AC bead | Kureha Inc. |
| IAC_2 | CAC beads treated with para-aminobenzoic acid | AC bead | Rong, Liu, Wu, Pan and Zheng, 2010 |
| IAC_3 | CAC treated with dimethylamine | AC bead | Deliyanni and Bandosz, 2011 |
| IAC_4 | CAC treated with hexamethylene diamine | AC bead | Ma, Li and Zhu, 2011 |
| CCF_1 | Treated AC granules mounted on a High-efficiency particulate air (HEPA) style filter | Mounted AC granules | Mann + Hummel GbH |
| CCF_2 | Treated AC granules mounted on a HEPA style filter | Mounted AC granules | Mann + Hummel GbH |
| AC_COMP_1 | Treated AC pellets | AC composite pellets | Xaiomi |
| AC_COMP_2 | Primarily AC-based composite material | AC composite beads | Airlabs |
| CIF_1 | Treated AC beads mounted on to polymer foam | Mounted AC bead | Purafil Inc. |
| CIF_2 | Treated AC beads | AC bead | Airlabs |
| M_CAT_4 | Metal oxide catalyst based on MnO2 | Mounted Catalyst Powder | BASF SE |
| CAT_1 | Gold nanoparticle catalyst with a TiO2 support | Rounded grains | Astrea Materials |
| CAT_2 | Gold nanoparticle catalyst with a CeO2 support | Cylindrical grains | Astrea Materials |
| GO | Graphite oxide | Flakes | Hummers and Offeman, 1958 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russell, H.S.; Bonomaully, J.; Bossi, R.; Hofmann, M.E.G.; Knap, H.C.; Pernov, J.B.; Veld, M.i.‘t.; Johnson, M.S. Novel Materials for Combined Nitrogen Dioxide and Formaldehyde Pollution Control under Ambient Conditions. Catalysts 2020, 10, 1040. https://doi.org/10.3390/catal10091040
Russell HS, Bonomaully J, Bossi R, Hofmann MEG, Knap HC, Pernov JB, Veld Mi‘t, Johnson MS. Novel Materials for Combined Nitrogen Dioxide and Formaldehyde Pollution Control under Ambient Conditions. Catalysts. 2020; 10(9):1040. https://doi.org/10.3390/catal10091040
Chicago/Turabian StyleRussell, Hugo S., James Bonomaully, Rossana Bossi, Magdalena E. G. Hofmann, Hasse C. Knap, Jakob B. Pernov, Marten in ‘t Veld, and Matthew S. Johnson. 2020. "Novel Materials for Combined Nitrogen Dioxide and Formaldehyde Pollution Control under Ambient Conditions" Catalysts 10, no. 9: 1040. https://doi.org/10.3390/catal10091040
APA StyleRussell, H. S., Bonomaully, J., Bossi, R., Hofmann, M. E. G., Knap, H. C., Pernov, J. B., Veld, M. i. ‘t., & Johnson, M. S. (2020). Novel Materials for Combined Nitrogen Dioxide and Formaldehyde Pollution Control under Ambient Conditions. Catalysts, 10(9), 1040. https://doi.org/10.3390/catal10091040